Significant outcomes
-
∙ Unrecognised myocardial infarction (MI) may be more common in patients with schizophrenia.
-
∙ The number of patients receiving adequate treatment for previous MI is low.
Limitations
-
∙ This study was based on electrocardiogram diagnosis with no further confirmation of the acute myocardial infarction diagnosis.
-
∙ This study did not include a control group, which makes it difficult to interpret the findings.
Introduction
Schizophrenia affects ~0.5% of the population, with most patients having a chronic course illustrated by 10% being institutionalised and 90% receiving early retirement pension (Reference Uggerby, Nielsen, Correll and Nielsen1). In addition to the psychiatric symptoms, schizophrenia is also associated with increased mortality with up to 20 years reduction of the life span (Reference Correll and Nielsen2). Metabolic syndrome is more common in this patient group and is present in one-third to half of the patients, depending on the chronicity of the patient sample (Reference De Hert, van Winkel and Van Eyck3,Reference McEvoy, Meyer and Goff4). Patients with schizophrenia have poor eating habits and reduced level of physical activity (Reference Vancampfort, Knapen, Probst, Scheewe, Remans and De Hert5,Reference Roick, Fritz-Wieacker and Matschinger6). The antipsychotic medication adds to this risk by increasing the risk of somatic complications such as torsade de pointes arrhythmia (Reference Nielsen, Graff, Kanters, Toft, Taylor and Meyer7) and diabetes (Reference Nielsen, Skadhede and Correll8). In addition, patients with schizophrenia are less likely to follow screening programmes and regular health checks (Reference Nielsen, Munk-Jorgensen, Skadhede and Correll9,Reference Marder, Essock and Miller10). During the last few decades, the standardised mortality ratio has increased for patients with schizophrenia, which suggests that schizophrenia patients are less likely to benefit from the advances in the healthcare system, such as revascularisation of myocardial infarction (MI) (Reference Saha, Chant and McGrath11). This trend is supported by a Danish study by Laursen and Nordentoft, which found that patients with schizophrenia were less likely to receive invasive procedures for their ischaemic heart disease (Reference Laursen and Nordentoft12).
Silent or unrecognised MI is the presence of myocardial scar tissue without any clinical symptoms. Evidence from populations studies such as the Framingham study suggests that the average rate of silent MI is 35% in women and 28% in men (Reference Kannel13,Reference Kannel and Abbott14). An Icelandic study found the rate of unrecognised MI to be one-third, but other studies have reported rates as high as 64% (Reference Sigurdsson, Thorgeirsson, Sigvaldason and Sigfusson15) The prognosis of unrecognised and clinical MI is similar, and therefore unrecognised MI constitutes a significant healthcare problem (Reference Valensi, Lorgis and Cottin16). Diabetes is considered as a risk factor for unrecognised MI because of reduced pain sensitivity caused by neuropathic damage (Reference Valensi, Lorgis and Cottin16,Reference Arenja, Mueller and Ehl17). One study found that the percentage of unrecognised MI among diabetic patients was as high as 70% (Reference Niakan, Harati, Rolak, Comstock and Rokey18). Hypertension is also associated with an increased risk of unrecognised MI (Reference Valensi, Lorgis and Cottin16).
Several case reports have suggested that patients with schizophrenia may have reduced pain sensitivity – for example, lack of symptoms of bowel perforation (Reference Rosenthal, Porter and Coffey19), ruptured appendix (Reference Apter20), and peritonitis (Reference Fishbain21). Reduced pain sensitivity has also been found in otherwise healthy relatives of patients with schizophrenia (Reference Hooley and Delgado22). However, only one study has investigated the rate of unrecognised MI in patients with schizophrenia. In 1955, Marchand et al. reported that 85% of MIs that occurred in psychotic patients were unrecognised (Reference Marchand23). As diabetes is more common in patients with schizophrenia, this comorbidity may contribute to the high rate of unrecognised MI in patients with schizophrenia.
Although the mechanism of reduced pain sensitivity is poorly understood, a review by Bonnot et al. concluded that pain insensitivity is more related to a different mode of pain expression than to a real endogenous analgesia (Reference Bonnot, Anderson, Cohen, Willer and Tordjman24). It also remains unclear whether this pain insensitivity is a state or trait marker, one may suggest that this is only present in the psychotic phase and not in the maintenance phase (Reference Singh, Giles and Nasrallah25).
Aims of the study
With this study, we aimed to investigate the rate of unrecognised MI in patients with schizophrenia, and whether these patients received appropriate treatment for their condition, such as β-blockers and aspirin, by using the nation-wide Danish health registers.
Material and methods
Sample and design
Only patients diagnosed with an ICD-10 F20 diagnosis of schizophrenia in the Danish Psychiatric Central Research Register (DPCRR) were included in this study. Diagnoses from emergency room visits were disregarded because of low validity. The DPCRR contains data on all psychiatric admissions in Denmark from 1969 and onwards and outpatient contacts from 1996 (Reference Mors, Perto and Mortensen26). An electrocardiogram (ECG) was routinely obtained from patients admitted to the psychiatric ward and the data are stored in the patient file. In the present study, ECGs were obtained from psychiatric outpatient clinics in the cities Aalborg, Brønderslev, and Viborg. Only the latest 12-lead ECGs were included, regardless whether they were obtained during outpatient or inpatient status. ECGs were obtained from the patients’ file and scanned using a flatbed scanner.
The ECGs were manually interpreted by authors J.J. and K.A. independently. We used the Third Universal Definition of Myocardial Infarction by Thygesen et al. (Reference Thygesen, Alpert and White27). Dates of recording and interpretation were entered in EPIDATA and later uploaded to Statistics Denmark for linkage to the national health registers. Linkage was carried out by the unique Danish identification number (CPR number). After upload to Statistics Denmark, all data analyses were carried out anonymously via the remote server.
The Danish National Patient Registry contains data on all somatic hospital admissions in Denmark from 1977 and onwards. Registry data from 1977 and until the time of ECG recording were examined to identify patients with MI diagnosis. MI was defined as a diagnosis of ICD-8 410 or ICD-10 I21. In addition, data were linked to the Danish Register of Medicinal Product Statistics in order to obtain data on the use of prescription medication such as β-blockers, statins, and aspirin.
A multivariate logistic model was developed to identify risk factors for unrecognised MI. The DPCRR provided data on schizophrenia subtype. Percentage of previous psychiatric hospitalisation and the patient’s age at onset of schizophrenia were used as markers of severity of schizophrenia.
Diabetes comorbidity was defined as either a diagnosis of ICD-8: 250 or ICD-10: E10, E11 in the National Patient Registry or redemption of at least one prescription of antidiabetic drugs including insulin (ATC: A10) before the time of the ECG recording. This definition did not differentiate between type 1 and type 2 diabetes because the ICD-10 system does not uniquely separate these diagnoses. Thus, patients with type 2 diabetes who were not receiving any antidiabetic medication were not identified by this definition.
As the diagnosis of hypertension is poorly registered in the health register, we used redemption of at least one prescription for hypertension as a proxy measure for the condition. Only medications for hypertension redeemed before the time of ECG recording were included. Hypertension medications included were as follows: antihypertensives (ATC: C02), thiazide diuretics (ATC: C03A), calcium antagonists (ATC: C08), and ACE inhibitors and AE antagonists (ATC: C09).
The study was approved by the Danish Data Protection Agency, and access to the case records were granted by the Danish Medicines and Health Board. As the study did not involve any direct patient contact, it did not warrant approval from any local research ethical committee.
Treatment
The treatment of patients with the ECG showing signs of MI was assessed using the Danish Registry of Medicinal Product Statistics. This register covers all prescription-based medications in Denmark from 1995 and onwards. Medication status during admission is not registered in this register. We looked for acetylsalicylic acid (ATC: B01AC06, N02BA01), beta-blockers (ATC: C07), and statins (ATC: C10AA). Treatment with these drugs was considered if the patient had redeemed at least one prescription from the pharmacy up to 180 days after the ECG recording date.
Statistics
All statistical analyses were carried out with STATA 13. All tests were two-sided and only p-values <0.05 were considered statistically significant. Continuous variables were compared using Student’s t-test. Log transformation was used to obtain normal distribution. In cases where an acceptable transformation not could be obtained, the non-parametric Wilcoxon rank sum test was used. Proportions were compared using Fischer’s exact test because of the low number.
A multiple logistic regression model was used to identify predictors for unrecognised MI. Predictor variables were included using a stepwise forward inclusion model of variables significant at the p<0.05 level. The following variables were included in the model: current age at the time of the ECG recording, age of onset of schizophrenia, sex, percentage of hospitalisation, diabetes, living in an institution, living alone, receiving early retirement pension and treatment with medications for hypertension. Age of onset of schizophrenia and current age were used as continuous variables in the regression model. In addition to the comparison of patients with electrocardiographically unrecognised MI and ECG-negative patients with no history of MI, the former group was also compared with patients with recognised MI using the multiple regression model described above.
Results
A total of 937 ECGs were interpreted, 538 men (57.4%) and 399 women (42.6%). Mean age at ECG sampling time was 40.6 years (95% CI: 39.7–41.5, range: 15.9–94.6). The distribution of patients was as follows: Aalborg 308 (32.9%), Brønderslev 162(17.3%), and Viborg 467 (49.8%).
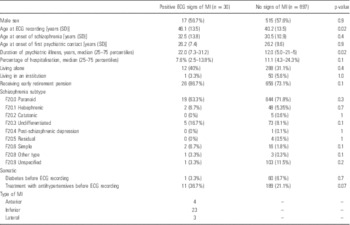
We identified 32 patients with positive ECG signs of MIs as shown in Table 1. Only two of these individuals had a diagnosis of MI in the National Patient Registry. An additional number of eight patients had a diagnosis of MI in the Danish National Patient Registry, but with no ECG signs of previous MI. This means that 30 out of 40 MIs were unrecognised (75%). The mean age of the 10 patients (four men, six women) with recognised MI in the National Patient Registry was 53.4 years (95% CI: 43.8–63.1, range: 38.8–83.5). The mean age of the 30 patients with unrecognised MI was 46.1 years, as shown in Table 2. Mean age of one-third of the unrecognised MI patients was <35 years.
Table 1 Distribution of ECG signs of AMI, and the diagnosis of MI in the patient register

ECG, electrocardiogram; AMI, acute myocardial infarction; MI, myocardial infarction.
Table 2 Demographics
ECG, electrocardiogram; MI, myocardial infarction.
Only increasing age was associated with unrecognised MI in the stepwise multiple logistic regression model compared with patients with no history of MI, OR: 1.03 per year of age, 95% CI: 1.00–1.06, p=0.021. Treatment with medication for hypertension before the ECG recording was associated with an increased risk of unrecognised MI, OR: 2.2, 95% CI: 1.1–4.6, p=0.046 in a univariate logistic regression, but remained non-significant in the multiple logistic regression model. No association with schizophrenia subtype was found. When comparing unrecognised MI with recognised MI, no variable reached statistical significance in the multiple logistic regression model.
Of the 30 patients with positive ECG signs of previous MI and no historical diagnosis of MI, only five patients received treatment with aspirin, two patients were treated with statins, and six patients received treatment with a β-blocker. Of the eight patients with a historical diagnosis of MI but no current ECG signs of previous MI, eight patients received treatment with aspirin, six patients received treatment with statins, and eight patients received treatment with a β-blocker.
Discussion
The major finding of this study was that a large portion of patients with schizophrenia had positive ECG signs of previous MI but no record of MI diagnosis in the Danish National Patient Registry. We found that 75% of patients fulfilled the ECG criteria for unrecognised MI. This is in line with previous findings by Marchand, who found the rate of unrecognised MI to be as high as 85% (Reference Marchand23). Increasing age was the only predictor of unrecognised MI, which is in line with previous findings and is attributable to the general increased risk of MI with increasing age.
In addition, this study clearly shows that schizophrenia patients with unrecognised MI do not receive adequate treatment such as aspirin, β-blockers, or statins. Although the compliance of patients with schizophrenia may be poorer compared with other patients, more efforts should be taken to reduce this gap of under treatment.
This study should be interpreted within its limitations. The major limitations of this study are the lack of a control group and the rather low number of patients with recognised and unrecognised MI, which makes it difficult to identify clinically relevant predictors. With the current design, patients may have had the unrecognised MI a long time ago, and for this reason we were not able to determine whether the MI occurred during a psychotic phase.
This study used the assumption that patients did not have any symptoms of MI because they did not receive treatment. However, some patients may have had symptoms but did not seek treatment because they may have been afraid of using the healthcare system – for example, due to risk of involuntary admission (Reference Andersen, Kappers, Sneider, Uggerby and Nielsen28). On the other hand, patients with schizophrenia may have experienced the symptoms but interpreted them in a psychotic context, such as radiation from outer space. This is the reason why we preferred the term unrecognised MI rather than silent MI. Unfortunately, we were not able to interview the patients about symptoms of their previous MI.
A screening test is only as good as its diagnostic performance – that is, its sensitivity and specificity. We used ECG as the screening test in order to define whether the patients had previous MIs. ECG is a cheap and suitable screening method especially in this population because it is a non-invasive technique and many patients have ECG performed as part of their monitoring of the treatment with antipsychotic drugs. Some patients may have had Q waves for other reasons such as cardiomyopathy. Supporting this is the rather low age of first MI occurrence found in this study. Although the specificity of ECG for detecting previous MI varies between 64% and 99%, most studies report specificity of more than 95% (Reference Ammar, Kors, Yawn and Rodeheffer29). On the other hand, the sensitivity of the ECG to detect previous MIs is rather poor, with a sensitivity varying from 3% to 50% (Reference Ammar, Kors, Yawn and Rodeheffer29). With the high specificity and the low sensitivity of ECG, it means that more patients may have had unrecognised MI and our findings, therefore, underestimated the true rate. Echocardiography would have provided essential information on the presence of previous MI. Unfortunately, we were not allowed to contact the patients to carry out the process.
Although, the ECG criteria for MI recognition have proven to be essential for the outcome, no standard ECG definition exists (Reference Ammar, Kors, Yawn and Rodeheffer29). We used the latest version of the Universal definition of MI, (Reference Thygesen, Alpert, Jaffe, Simoons, Chaitman and White30) whereas other studies investigating unrecognised MI from ECG were conducted 10–25 years ago and used a variety of Minnesota codes (Reference Sigurdsson, Thorgeirsson, Sigvaldason and Sigfusson15). Although the width and the amplitude of the Q wave have been redefined, we think that the Universal definition of MI is more conservative because two consecutive leads are required compared with only one with the Minnesota codes. Using the Universal definition of MI may have underestimated the number of unrecognised MI.
In total, 10 patients had a diagnosis of MI in the Danish National Patient Registry, of whom only two patients had positive ECG signs of a previous MI. Several reasons for this may exist. Some patients with MI present with less or no ECG signs, such as MI related to occlusion of the circumflex artery (Reference Rasoul, de Boer and Suryapranata31). Long-term regression of the Q wave may also have occurred in some of these patients and this time-dependent ECG change may have slurred the ECG diagnosis of a previous MI (Reference Yano and MacLean32). The latter may also have influenced the sensitivity of ECG as a method to detect previous MI.
Some of the patients may have erroneously been registered with MI diagnosis, but we believe this is less likely because the validity of the Danish National Patient Registry and DPCRR has proven to be high for MI and schizophrenia diagnoses (Reference Uggerby, Ostergaard, Roge, Correll and Nielsen33,Reference Madsen, Davidsen, Rasmussen, Abildstrom and Osler34). Unfortunately, we did not have access to case records, which may have given valuable information on whether the diagnoses of MI were correct.
Unfortunately, we also did not have access to clinical variables or laboratory data such as body mass index, blood pressure, and fasting glucose levels. The association between unrecognised MI and diabetes is not clear, with some studies identifying diabetes as a risk factor while other did not (Reference Valensi, Lorgis and Cottin16). We did not find any association with diabetes. This may be explained by the low sample size or the fact that diabetes may be under-reported in the schizophrenia population (Reference Nasrallah, Meyer and Goff35). This may also indicate that unrecognised MI occurs as an independent phenomenon in the schizophrenia population. Larger studies are warranted to determine this causality. We did not find any association between severity or chronicity of schizophrenia and unrecognised MI. Nevertheless, we recommend that psychiatrists should monitor this association in all patients with schizophrenia.
Interestingly, one-third of the patients with unrecognised MI were younger than 35 years of age, which may suggest that patients with schizophrenia have their first cardiovascular event earlier than the general population. Unfortunately, the sample size was not large enough to display incidences by age groups. We did not find any association with sex, which is in line with previous findings (Reference Burgess, Hunt and Li36). Male sex is otherwise identified as a risk factor for recognised MI but not for unrecognised MI (Reference Burgess, Hunt and Li36).
The results of this study warrant replication in a larger sample, and if confirmed more studies should be carried out to screen schizophrenia patients for physical illness such as unrecognised MI. Whether routine ECG monitoring is useful for such screening and consequent long-term treatment with aspirin, statins, and β-blockers remains controversial, because of the sensitivity and specificity of the ECG test for MI. Instead, it has been suggested that patients with ECG signs of MI should be referred for cardiac imaging before the final diagnosis of MI is made (Reference Scirica37).
Unrecognised MI should receive more attention and larger studies including a healthy control group should investigate risk factors for unrecognised MI among patients with schizophrenia. Especially the combination of diabetes and unrecognised myocardial ischaemia seems to be associated with a poor prognosis (Reference Sejil, Janand-Delenne and Avierinos38). As patients with schizophrenia may have reduced pain sensitivity, psychiatrists and physicians should pay extra attention to the physical status of this vulnerable patient group.
Acknowledgements
J.N. designed the study and wrote the first draft of the manuscript. R.F. scanned the ECGs. J.J. and K.A. interpreted the ECGs. C.G., J.K., and S.J. assisted in the writing process. All authors approved the final manuscript.
Financial Support
This study was supported by TRYG Foundation (grant number 7-09-2011) and the Danish Strategic Research Foundation – (HEARTSAFE grant number: 10-092799). None of the foundations had any influence on the study design or reporting of this study.
Conflicts of Interest
The authors report no conflicts of interest.





