Introduction
Pentastomida is a group of obligate parasites genetically related to crustaceans and is represented by approximately 131 species (Christoffersen & De Asis, Reference Christoffersen, De Asis, von Vaupel Klein, Charmantier-Daures and Schram2015). Reptiles, mainly snakes and crocodiles, are most frequently the definitive host for many of the species of pentastomids. These hosts are parasitized by species of the families Porocephalidae (snakes) and Sebekidae (crocodiles) (Christoffersen & De Asis, Reference Christoffersen and De Asis2013). Members of Porocephalidae use small mammals as intermediate host, while those of Sebekidae use freshwater fishes (Riley, Reference Riley1986).
In the Neotropical region, the genus Porocephalus (Porocephalidae) parasitizes the respiratory system of crotalid and boid snakes. Their immature stages have been found encysted in the lungs, liver and mesenteries of a variety of small mammals (Esslinger, Reference Esslinger1962; Riley & Self, Reference Riley and Self1979; Christoffersen & De Asis, Reference Christoffersen and De Asis2013). In Peru, three species of Porocephalus have been found in snakes (as definitive hosts): Porocephalus clavatus in Boa constrictor and Epicrates cenchria; Porocephalus crotali in Bothrops atrox; and Porocephalus stilessi in Lachesis muta (Tantaleán & Gozalo, Reference Tantaleán and Gozalo1985; Gárate et al., Reference Gárate, Naupay, Suyo, Colquichagua, Rodríguez and Yarlequé2007; Gomez-Puerta et al., Reference Gomez-Puerta, Lopez-Urbina and Gonzalez2011). However, reports of the occurrence of Porocephalus in intermediate hosts are scarce. In the present report, nymphal stages of Porocephalus spp. were collected from small- and medium-sized Peruvian mammals and identified using morphological and molecular methods. The results of this survey add new intermediate hosts for the genus Porocephalus.
Material and methods
The research protocol was approved by the Research Ethics Committee for Experimentation in Wildlife at the Dirección General de Flora y Fauna Silvestre from Peru (0350-2012-AG-DGFFS-DGEFFS) and by a Resolution of the Head of the National Reserve of Pucacuro (no. 03-2012-SERNANP-RN Pucacuro–Jef). Some parasite specimens from the parasite collection of the School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos (SERFOR RDG no. 023-201) were included in this study. Host data and collection date are summarized in table 1.
Table 1. Hook measurements of nymphal stages of Porocephalus spp. isolated from different wild mammals from the Peruvian Amazon.
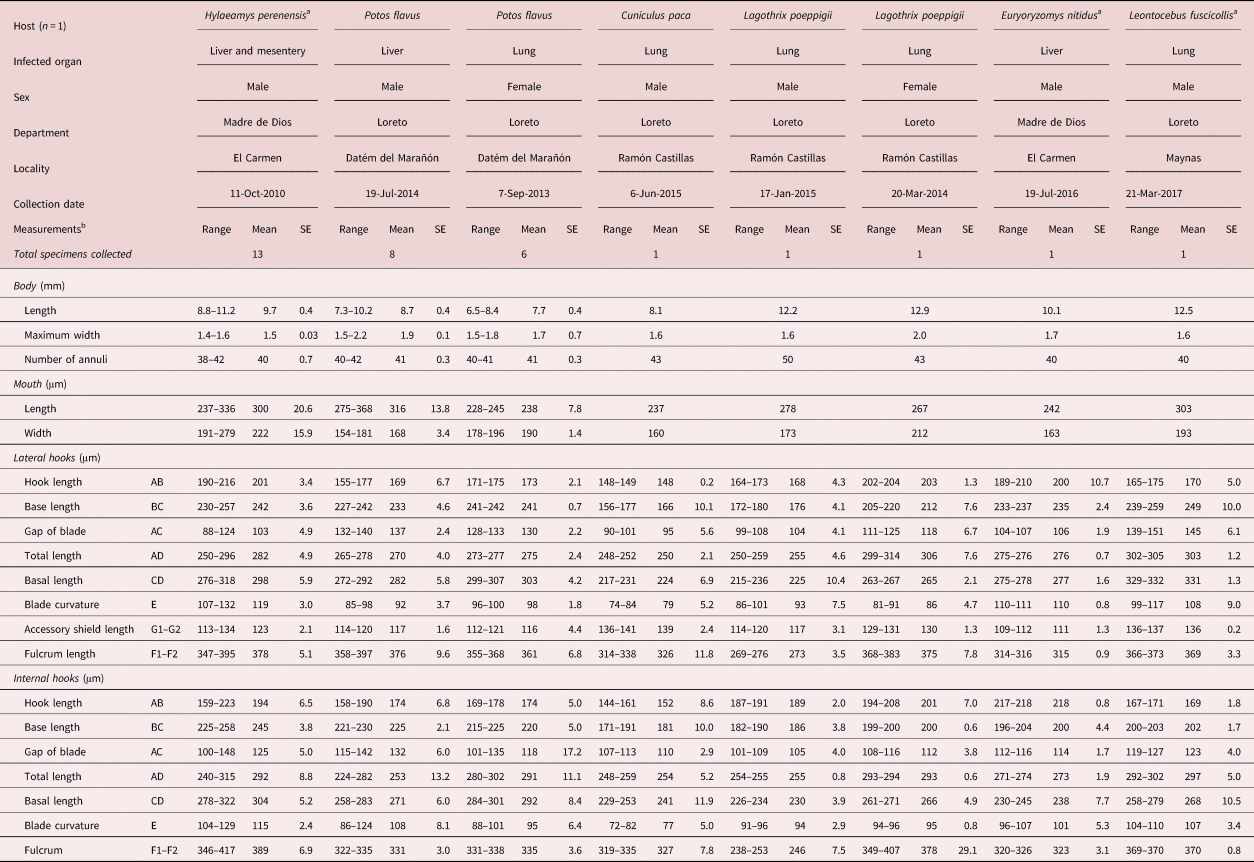
a Specimens from the parasite collection of the School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos (SERFOR RDG no. 023-201).
b The measurements are expressed in millimetres (mm) and micrometres (μm). SE, standard error.
Pentastomids were fixed and preserved in 10% formaldehyde or 70% ethanol. All pentastomids collected were analysed morphologically, measured and, finally, body rings or annuli were enumerated. Hooks were dissected using fine needles and were mounted directly onto glass slides in Berlese's medium to facilitate examination of their morphology. Morphometric examination of hooks included eight different dimensions (fig. 1a). Photographs and measurements were taken using a Carl Zeiss microscope, Axioskop 40 (Göttingen, Germany), and the software Leica IM50 version, 4.0 R117 (Leica Microsystems, Wetzlar, Germany).

Fig. 1. (a) Lateral hook of Porocephalus nymph from Hylaeamys perenensis. Hook measurements used in this study: AD, total length of the hook; AB, length of the hook measured in a straight line from point A to point B; AC, distance between the tip of the hook (point A) and the most prominent point of the base (point C); BC, length of the base, from point B to point C; CD, distance in a straight line between points C and D; E, hook curvature; F1–F2, fulcrum length; G1–G2, accessory shield length. (b) Porocephalus nymphs collected from H. perenensis (1), Leontocebus fuscicollis (2), Lagothrix poeppigii (3), Euryoryzomys nitidus (4), Lagothrix poeppigii (5) and Cuniculus paca (6). Scale bar: 5.0 mm.
Molecular analysis involved total DNA extraction from internal tissue samples of select specimens using the Chelex method (Gomez-Puerta et al., Reference Gomez-Puerta, Alarcon, Pacheco, Franco, Lopez-Urbina and Gonzalez2016) and a salting-out method (Kelehear et al., Reference Kelehear, Spratt, O'Meally and Shine2014). A total of three pentastomids were studied molecularly – one from the western Amazonian oryzomys, one from the elegant oryzomys and one from the brown-mantled tamarin. A polymerase chain reaction was performed to partially amplify the mitochondrial cytochrome c oxidase subunit I gene (cox1) using a previously published protocol (Kelehear et al., Reference Kelehear, Spratt, Dubey, Brown and Shine2011) and primers LCO1490 and HCO2198 (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994). Amplicons were sequenced using an ABI 3100 automated sequencer (Applied Biosystems, Foster City, CA, USA). The MEGA-X software (http://www.megasoftware.net/) was used to create a phylogenetic tree via the neighbour-joining method with Kimura two-parameter distance. Nucleotide sequences from this study were registered in the GenBank database.
Results
A total of 31 pentastomids collected from eight hosts were studied morphologically. These pentastomids corresponded to the immature stage (nymphs) of Porocephalus spp., and they were found encysted mostly in the hosts’ liver (13 nymphs), followed by the lung (ten nymphs) and then the mesentery (nine nymphs) (fig. 1b). All specimens had numerous annuli in the body (38 to 43) and the cephalic region contained two pairs of oral hooks (lateral and internal). Morphological analysis showed the occurrence of at least five Porocephalus morphospecies: type 1 were collected from one western Amazonian oryzomys (Hylaeamys perenensis) and one elegant oryzomys or elegant rice rat (Euryoryzomys nitidus); type 2 from two kinkajous (Potos flavus) and one brown-mantled tamarin (Leontocebus fuscicollis); type 3 from one lowland paca (Cuniculus paca); type 4 from one silvery woolly monkey (Lagothrix poeppigii); and, finally, morphospecies type 5 were collected from the second silvery woolly monkey.
Three Amazonian rodents were infected by more than one pentastomid. One western Amazonian oryzomys was infected with 13 Porocephalus nymphs found in the mesentery and the liver. One elegant oryzomys or elegant rice rat was infected with one Porocephalus nymph in the liver. One Porocephalus nymph was collected from the lung of a lowland paca (C. paca). The morphological parameters of these nymphs are tabulated in table 1.
Two kinkajous (P. flavus), a carnivorous mammal of the family Procyonidae, were examined post-mortem and both were parasitized with pentastomids. One kinkajou was infected in the hepatic parenchyma and the other in the pulmonary parenchyma. A total of eight and six Porocephalus nymphs were collected from the liver and lungs, respectively. The morphological dimensions are mentioned in table 1.
The pulmonary parenchyma of a brown-mantled tamarin (L. fuscicollis) and two silvery woolly monkeys (L. poeppigii) was each infected with one Porocephalus sp. nymph. The nymph from the brown-mantled tamarin was 12.5 mm long and 1.6 mm wide, with 40 annuli over the entire body length. Porocephalus sp. nymphs from each silvery woolly monkey varied morphologically. The morphological measurements are shown in table 1.
A total of 673 base pairs of the cox1 gene were amplified in three Porocephalus nymphs from the western Amazonian oryzomys, one from the elegant oryzomys and one from the brown-mantled tamarin (GenBank accession numbers MK903613–MK903617). Analysis of nucleotide sequences of the cox1 gene showed three distinct sequences with differences ranging from 2.3 to 4.2%. Differences occurred at 29 alignment positions, 27 transitions (19 C–T and eight A–G) and two transversions (one A–T and one A–C). In addition, the nucleotides G + C content of these sequences ranged from 37.44 to 38.34%.
Nucleotide sequences from this study were specific to each host, which formed three clades on the neighbour-joining tree (fig. 2). Also, these sequences were compared with sequence references from GenBank and showed an identity of 95.8–97.9% to P. crotali collected from the Burmese pythons (Python bivittatus) and the cottonmouth snakes (Agkistrodon piscivorus) (GenBank accession numbers MG559647–MG559655).

Fig. 2. Phylogenetic tree of nucleotide sequences of cox1 gene of Porocephalus spp. from wild mammals from the Peruvian Amazon and other pentastomids used as references from GenBank (accession numbers KX686568, MG559648, MG559650-2 and MG559655). The scale bar indicates 2 substitutions per 100 nucleotide positions.
Discussion
All pentastomids collected and studied were identified as nymphal stages of the genus Porocephalus, based on the morphology of mouth, hooks and the number of annuli (Esslinger, Reference Esslinger1962; Vargas, Reference Vargas1970). This was confirmed by molecular analysis, the cox1 gene had more than 95% similarity to P. crotali collected from the Burmese pythons and the cottonmouth snakes (GenBank accession numbers MG559647–MG559655) (Miller et al., Reference Miller, Kinsella, Snow, Hayes, Falk, Reed, Mazzotti, Guyer and Romagosa2018).
The genus Porocephalus includes nine species, of which six are exclusive to the Americas (Christoffersen & De Asis, Reference Christoffersen and De Asis2013), and three have been recorded in Peru: P. crotali, P. clavatus and P. stilesi (Gomez-Puerta et al., Reference Gomez-Puerta, Lopez-Urbina and Gonzalez2011). For many years, Porocephalus nymphs have been reported in various species of wild mammals and the great majority of them were identified as P. crotali due to the morphological parameters of the hooks (Vargas, Reference Vargas1970; Rego, Reference Rego1980; Martinez, Reference Martinez1982). However, these parameters have not been consistent in scientific works (Vargas, Reference Vargas1970; Riley & Self, Reference Riley and Self1979; Rego, Reference Rego1980). For example, Vargas (Reference Vargas1970) used measurements of total length (AD), gap of blade (AC), basal length (CD), blade curvature (E) and fulcrum length (F1–F2), while Rego (Reference Rego1980) used measurements of hook length (AB), base length (BC) and fulcrum length (F1–F2). For this reason, in our study, we included all the parameters used in these studies in order to have a standard for the morphological description of adult stage of Porocephalus hooks, which will serve for future studies.
Adult stages of Porocephalus show a dimorphism among species. This dimorphism occurs mainly in the hook parameters and the number of annuli (Riley & Self, Reference Riley and Self1979). However, this has not been demonstrated in larval stages (Vargas, Reference Vargas1970; Miller et al., Reference Miller, Kinsella, Snow, Hayes, Falk, Reed, Mazzotti, Guyer and Romagosa2018). In our study, all nymphs studied corresponded to the sixth stage of Porocephalus according to annuli and hook morphology (Esslinger, Reference Esslinger1962). Some morphological parameters of these Porocephalus nymphs were very similar to one another (table 1). However, morphological analysis indicated the occurrence of at least five species. Only the Porocephalus nymph collected from a silvery woolly monkey had a greater morphological difference compared with the others. This would indicate that larval stages of some Porocephalus species share similar morphological characteristics. However, molecular analysis indicated that at least two Porocephalus species were identified (fig. 2).
Procyonids such as raccoons and coatis (Christoffersen & De Asis, Reference Christoffersen and De Asis2013) and other mammals may act as intermediate hosts for Porocephalus species. This study is the first record of Porocephalus nymphs in kinkajous.
Porocephalus nymphs also occur in several species of cricetid rodents (Christoffersen & De Asis, Reference Christoffersen and De Asis2013). However, this is the first report of Porocephalus nymphs found in the western Amazonian oryzomys, the elegant oryzomys and the lowland paca. Likewise, some reports mentioned infection of immature stages of Porocephalus in non-human primates such as monkeys from the families Cebiidae and Callitrichidae (Christoffersen & De Asis, Reference Christoffersen and De Asis2013). In the current study, a brown-mantled tamarin (Callitrichidae) and a silvery woolly monkey (Atelidae) were infected with nymphal Porocephalus in the lung. Pulmonary infection by Porocephalus had been previously reported in a Rio Tapajós saki (Pithecia irrorata) from Brazil that experienced sudden death (Pereira et al., Reference Pereira, Benigno and Silva2010). Of the few studies about pentastomids in New World monkeys, there are two reports that mention cerebral infection by Porocephalus nymphs in the common squirrel monkey (Saimiri sciureus, Cebiidae) (Fox et al., Reference Fox, Diaz and Barth1972; Hall et al., Reference Hall, Haines and Frederickson1985). For this reason, it is necessary to be thorough and consider the brain in the inspection of mammals, mainly monkeys, at the time of necropsy.
The mitochondrial cox1 gene has been used in many studies regarding the phylogeny and taxonomy of invertebrates (Hu et al., Reference Hu, Chilton, Zhu and Gasser2002; Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014). The cox1 gene has proven to be a good marker to differentiate closely related species (Hu et al., Reference Hu, Chilton, Zhu and Gasser2002; Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014). For example, the cox1 gene is able to distinguish species of the genus Taenia. Nucleotide sequences showed a difference of up to 18% in one study (Zhang et al., Reference Zhang, Chen, Yang, Liu, Jiang, Gu, Wang and Wang2014). In our report, the nucleotide sequences from pentastomids had a difference of up to 5%. The morphological analysis supported by the molecular analysis indicates that the specimens studied are members of the genus Porocephalus.
Sequence analysis demonstrated two clades for the nymphs of this study (fig. 2), one for the western Amazonian oryzomys, and the other for the elegant oryzomys and the brown-mantled tamarin. Apparently, the elegant oryzomys and the brown-mantled tamarin were parasitized by the same species of Porocephalus (1.7% nucleotide difference). However, futures studies will be necessary to develop molecular analysis on existing Porocephalus species; this will help in molecular epidemiological studies regarding diagnosis and taxonomical investigations on Porocephalus nymphs.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.





