Introduction
Upwards of 120 million people globally have depression, with 10–15% enduring a lifelong prevalence (Lepine & Briley, Reference Leuchter, Cook, Jin and Phillips2011). An estimated 15–35% of depressed patients have treatment-resistant depression (TRD), failing to reach remission (Nemeroff, Reference Nemeroff2007). TRD is associated with significant economic and medical burden: medical costs are six times more for patients with TRD compared with patients with non-TRD ($42 344 v. $6512) (Crown et al. Reference Crown, Finkelstein, Berndt, Ling, Poret, Rush and Russell2002).
Repetitive transcranial magnetic stimulation (rTMS) has emerged as an effective brain stimulation treatment for depression and TRD specifically (O'Reardon et al. Reference O'Reardon, Solvason, Janicak, Sampson, Isenberg, Nahas, McDonald, Avery, Fitzgerald, Loo, Demitrack, George and Sackeim2007; Connolly et al. Reference Connolly, Helmer, Cristancho, Cristancho and O'Reardon2012). Several large-scale studies have established the efficacy of this treatment (O'Reardon et al. Reference O'Reardon, Solvason, Janicak, Sampson, Isenberg, Nahas, McDonald, Avery, Fitzgerald, Loo, Demitrack, George and Sackeim2007; Schutter, Reference Schutter2009; Fitzgerald et al. Reference Fitzgerald, Hoy, Gunewardene, Slack, Ibrahim, Bailey and Daskalakis2011; George et al. Reference George, Taylor and Short2013). rTMS was approved as a treatment for TRD by the US Food and Drug Administration in 2008, and, since then, it has been used successfully for the treatment of depression in clinical practice, with response and remission rates of 53.4 and 31.5% (Connolly et al. Reference Connolly, Helmer, Cristancho, Cristancho and O'Reardon2012), as compared with those of 13.7 and 16.8% for additional sequential trials of pharmacotherapy after two failed medication trials (Rush et al. Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart, Warden, Niederehe, Thase, Lavori, Lebowitz, McGrath, Rosenbaum, Sackeim, Kupfer, Luther and Fava2006).
rTMS uses an electromagnetic coil placed on the scalp to create brief magnetic field pulses. The conventional approach for the treatment of depression directs the magnetic field pulses over the left dorsolateral prefrontal cortex (DLPFC) or the right DLPFC, or both in a sequential pattern. These magnetic fields penetrate the cortex unimpeded and induce an electrical current in the underlying cortex, altering the brain's activity (George et al. Reference George, Taylor and Short2013). Studies have investigated the effect of rTMS on neural circuits by investigating molecular, genetic, electrophysiological and imaging measures of brain function. Despite the large number of clinical trials that have been conducted on the efficacy of rTMS, there is a gap in the understanding of how rTMS exerts its antidepressant action and a summary of biological findings is lacking. Thus, the goal of this systematic review is to summarize and synthesize the literature on the neurobiological mechanisms of action of rTMS over the DLPFC in depressed patients.
Method
Search strategy
We searched three electronic databases: Medline, EMBASE and PsycINFO. Medline was searched from 1996 to March week 4 2015, EMBASE from 1980 to 2015 week 10, and PsycINFO from 1986 to March week 4 2015. The databases were searched using keywords and medical subject headings (MeSH). The search terms that were used to identify potentially relevant articles differed in the Medline, EMBASE and PsycINFO search and focused on terms related to rTMS, neurophysiology and neurobiology. The specific terms used in the search of the Medline database are presented in online Supplementary Table S1. The preliminary database search was conducted by one author (W.K.S.) and three authors (Y.N., W.K.S. and D.M.B.) independently extracted the data from all studies. Bibliographies of articles and review articles were manually searched to identify primary articles that may have been missed in the initial search. Only published, peer-reviewed articles of primary human studies available in English were considered for this review. These publications were identified in the initial stage of the search process, and articles with abstracts indicating relevance, which met the predetermined eligibility criteria, were retrieved to review for inclusion criteria.
Criteria for study selection
Inclusion criteria
We included studies evaluating any neurobiological mechanisms of rTMS in human subjects with depression. Only studies that utilized an rTMS protocol where the stimulation site was the DLPFC were included as this is the predominant target area for the treatment of depression. Studies of any design were included (i.e. experimental and observational) even when there was no comparison group of individuals who did not receive rTMS (i.e. sham rTMS). Studies had to include a neurobiological measure before and after a course of treatment.
Exclusion criteria
We excluded studies evaluating only the cognitive effects of rTMS. Studies with animal, healthy subjects and subjects with other disorders were also excluded. Clinical treatment studies without a concomitant investigation of neurobiological effects were also excluded. Conference abstracts, narrative reviews and editorials were excluded.
Data analysis
A meta-analysis on the effect of rTMS on the various neurobiological functions was planned. However, we were unable to do so as there were an insufficient number of studies per biological factor to effectively analyse the data quantitatively. Additionally, the large degree of heterogeneity in rTMS treatment protocols and the measures used to quantify the neurobiological factors prohibited a quantitative meta-analysis.
Quality assessment
The methodological quality of each study was assessed by first examining the sample size of the study. Those studies with 20 subjects or more were classified as being ‘strong’ studies, based on expert opinion that this is the minimum sample size needed for a study of biological mechanisms (Schoenfeld, Reference Schoenfeld1980; Birkett & Day, Reference Birkett and Day1994; Julious, Reference Julious2005; Moore et al. Reference Moore, Carter, Nietert and Stewart2011). To further classify the quality of the findings, those studies with a control condition of individuals who were not exposed to rTMS were considered stronger than studies without. A four-tiered classification system of strength was thus developed, whereby a score of 1, the strongest-quality study, had 20 or more subjects and control subjects, a score of 2 had 20 or more subjects without a control condition, a score of 3 had fewer than 20 subjects with a control condition, and a score of 4, the weakest-quality study, had fewer than 20 subjects without a control condition.
Results
Characteristics of included studies
Our search terms yielded an initial 1647 articles. Reference lists of relative articles and 11 relevant reviews were examined and five additional articles meeting inclusion criteria were identified. A total of 1252 were screened for eligibility with 76 full-text articles retrieved and reviewed. After further review, a total of 66 met the full inclusion criteria (Fig. 1). Of these, 14 studies included a control condition and 52 did not. Five studies investigated only the effects of low-frequency right-sided (LFR) rTMS, and 11 studies investigated the effects of LFR and high-frequency left-sided (HFL) rTMS combined. The remaining 50 studies examined the effects of HFL-rTMS alone. Data are presented qualitatively in Table 1 (for detailed information, see online Supplementary Tables S2–S4), and main findings are described in the text.

Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009).
Table 1. Summary of findings of the various studies, according to the biological function being examined a

rTMS, Repetitive transcranial magnetic stimulation; LFR, low-frequency right-sided; DLPFC, dorsolateral prefrontal cortex; THP, tetrahydroprogesterone; HFL, high-frequency left-sided; ACTH, adrenocorticotropic hormone; EEG, electroencephalography; BDNF, brain-derived neurotrophic factor; SEM, sleep electroencephalogram modulated; DHEA, dehydroepi-androsterone; 5-HIAA, 5-hydroxyindoleacetic acid; RMT, resting motor threshold; CSP, cortical silent period; ICF, intracortical facilitation; ICI, intracortical inhibition; ERP, event-related potential; sLORETA, low-resolution brain electromagnetic tomography; PNS, parasympathetic nervous system; SNS, sympathetic nervous system; BA, Brodmann area; FA, fractional anisotropy; rCBF, regional cerebral blood flow; PFC, prefrontal cortex; BOLD, blood oxygen level-dependent; LFL, low-frequency left-sided; HAM-D, Hamilton Depression Rating Scale; ACC, anterior cingulate cortex.
a Findings were categorized by neurobiological function in three tables; each table contained the neurobiological factors of molecular factors, genetic polymorphisms or gene expression, electrophysiology, and neuroimaging changes induced by rTMS treatment (see Supplementary Tables S2–S4). For each function, the effect of rTMS on the function was summarized. The authors’ names, number of subjects, study design (e.g. experimental, observational), rTMS protocol, the neurobiological parameter under study, and the findings were extracted from each included study. Studies were then categorized by the biological measure examined: molecular factors, genetic polymorphisms or gene expression, electrophysiology, and neuroimaging.
Effects of rTMS on molecular mechanisms
Neurotransmitters
The neurotransmitter hypothesis of depression postulates that the deficit of certain neurotransmitters such as serotonin, dopamine and/or norepinephrine in the synaptic clefts throughout the brain is responsible for the corresponding features of depression. In this context, the rTMS effect of dopamine is disputed; some studies found that HFL-rTMS did not induce dopamine level changes as measured with magnetic resonance spectroscopy (MRS), positron emission tomography (PET) scans or biochemical examinations (Miniussi et al. Reference Miniussi, Bonato, Bignotti, Gazzoli, Gennarelli, Pasqualetti, Tura, Ventriglia and Rossini2005; Yukimasa et al. Reference Yukimasa, Yoshimura, Tamagawa, Uozumi, Shinkai, Ueda, Tsuji and Nakamura2006; Kuroda et al. Reference Kuroda, Motohashi, Ito, Ito, Takano, Nishikawa and Suhara2006, Reference Kuroda, Motohashi, Ito, Ito, Takano, Takahashi, Nishikawa and Suhara2010), whereas other single photon emission computed tomography studies found increased dopamine levels (Pogarell et al. Reference Pogarell, Koch, Pöpperl, Tatsch, Jakob, Zwanzger, Mulert, Rupprecht, Möller, Hegerl and Padberg2006, Reference Pogarell, Koch, Pöpperl, Tatsch, Jakob, Mulert, Grossheinrich, Rupprecht, Möller and Hegerl2007). HFL-rTMS increased norepinephrine measured with biochemical examination (Yukimasa et al. Reference Yukimasa, Yoshimura, Tamagawa, Uozumi, Shinkai, Ueda, Tsuji and Nakamura2006), glutamate (Luborzewski et al. Reference Luborzewski, Schubert, Seifert, Danker-Hopfe, Brakemeier, Schlattmann, Anghelescu, Colla and Bajbouj2007), choline (Luborzewski et al. Reference Luborzewski, Schubert, Seifert, Danker-Hopfe, Brakemeier, Schlattmann, Anghelescu, Colla and Bajbouj2007) and myo-inositol (Zheng et al. Reference Zheng, Zhang, Li, Liu, Gao, Liu, Zou, Zhang, Liu, Zhang, Li and Men2010) levels measured with MRS. Specifically, one study showed a relative increase in glutamate levels (11%) in the left DLPFC in responders after HFL-rTMS (Yang et al. Reference Yang, Kirton, Wilkes, Pradhan, Liu, Jaworska, Damji, Keess, Langevin, Rajapakse, Lebel, Sembo, Fife and MacMaster2014). Another MRS study reported that the choline:creatine ratio increased post-HFL-rTMS (Zheng et al. Reference Zheng, Zhang, Li, Liu, Gao, Liu, Zou, Zhang, Liu, Zhang, Li and Men2010). Last, there were no significant differences in the levels of serotonin and 5-hydroxyindoleacetic acid measured with biochemical examination after bilateral rTMS, compared with sham-treated patients (Miniussi et al. Reference Miniussi, Bonato, Bignotti, Gazzoli, Gennarelli, Pasqualetti, Tura, Ventriglia and Rossini2005).
Brain-derived neurotrophic factor (BDNF)
BDNF is a critical neurotrophic factor involved in neuronal homeostasis and neuroplasticity that is decreased in depressed patients (Lee & Kim, Reference Lee and Kim2010). Measures of BDNF using the enzyme-linked immunosorbent assay (ELISA) method before and after rTMS treatment have shown some conflicting results; some studies found that HFL-rTMS causes BDNF levels not to change (Lang et al. Reference Lang, Bajbouj, Gallinat and Hellweg2006; Gedge et al. Reference Gedge, Beaudoin, Lazowski, duToit, Jokic and Milev2012), whereas others found it to increase (Yukimasa et al. Reference Yukimasa, Yoshimura, Tamagawa, Uozumi, Shinkai, Ueda, Tsuji and Nakamura2006; Zanardini et al. Reference Zanardini, Gazzoli, Ventriglia, Perez, Bignotti, Rossini, Gennarelli and Bocchio-Chiavetto2006). However, it should be noted that brain regions stimulated by rTMS could potentially show localized and important changes in BDNF activity that are not detectable in peripheral blood samples.
Cortisol and other neurohormones
Hypothalamic–pituitary–adrenocortical dysregulation is a pathophysiological mechanism involved in depression. In three out of four studies that used a dexamethasone–corticotrophin-releasing hormone test, cortisol levels decreased significantly among subjects after HFL-rTMS (Reid & Pridmore, Reference Reid and Pridmore1999; Baeken et al. Reference Baeken, De Raedt, Leyman, Schiettecatte, Kaufman, Poppe, Vanderhasselt, Anckaert and Bossuyt2009a ; Mingli et al. Reference Mingli, Zhengtian, Xinyi and Xiaoping2009). In the fourth study, cortisol levels decreased only among subjects who did respond to HFL-rTMS treatment (Zwanzger et al. Reference Zwanzger, Baghai, Padberg, Ella, Minov, Mikhaiel, Schüle, Thoma and Rupprecht2003). However, one strong study showed significantly lower plasma adrenocorticotropic hormone and cortisol concentrations post-rTMS (Mingli et al. Reference Mingli, Zhengtian, Xinyi and Xiaoping2009). In another study, no changes in the quantity of dehydroepi-androsterone (DHEA), progesterone, allopregnanolones [3β,5α-tetrahydroprogesterone (THP), 3α,5β-THP and 3α,5α-THP] were detected following HFL-rTMS (Padberg et al. Reference Padberg, di Michele, Zwanzger, Romeo, Bernardi, Schüle, Baghai, Ella, Pasini and Rupprecht2002).
Gene expression
Leucocyte expression of c-FOS and DUSP1 is recognized as unique peripheral biomarker (Le-Niculescu et al. Reference Le-Niculescu, Case, Hulvershorn, Patel, Bowker, Gupta, Bell, Edenberg, Tsuang, Kuczenski, Geyer, Rodd and Niculescu2011) of anxiety and psychological stress. The expression of c-FOS and DUSP1 decreased in leucocytes post-LFR-rTMS (Teyssier et al. Reference Teyssier, Trojak, Chauvet-Gelinier and Bonin2013), which suggests a down-regulation in the expression of stress response genes.
Effects of rTMS on electrophysiological mechanisms
Electroencephalography (EEG) studies
Alpha band
HFL-rTMS causes significant changes in frontal regions of the alpha (about 8–13 Hz) and beta (about 14–30 Hz) bands of the EEG, where the effect is greater in the alpha band (García–Anaya et al. Reference García-Anaya, González–Olvera, Ricardo–Garcell, Armas, Miranda, Reyes and Adelina Otero2011). Specifically, HFL-rTMS was shown to increase alpha band power in the central and parietal regions (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012). In addition, HFL-rTMS increased the alpha 2 (high-alpha) power when eyes are open (Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008). Alpha power increases in the right hemisphere post-HFL-rTMS (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012) have been shown, whereas others found increases without electrode specificity in the prefrontal region after HFL-rTMS (Noda et al. Reference Noda, Nakamura, Saeki, Inoue, Iwanari and Kasai2013). Post-HFL-rTMS, alpha power significantly decreased over the left-DLPFC during rapid eye movement sleep (Pellicciari et al. Reference Pellicciari, Cordone, Marzano, Bignotti, Gazzoli, Miniussi and De Gennaro2013). rTMS administered in the alpha frequency band synchronized to patient's individual alpha frequency (i.e. synchronized rTMS) promoted immediate event-related synchronization followed by event-related desynchronization in the alpha band (Leuchter et al. Reference Lepine and Briley2013).
Beta band
One study found that LFR-rTMS caused changes in the alpha (8–13 Hz) and beta (14–30 Hz) bands of the EEG, particularly over the frontal, central and temporal regions, where the effect was more pronounced in the beta band (García-Anaya et al. Reference García-Anaya, González–Olvera, Ricardo–Garcell, Armas, Miranda, Reyes and Adelina Otero2011).
Theta band
The theta (4–7 Hz) band of the EEG was found not to change post-HFL-rTMS in two out of four studies (Loo et al. Reference Loo, Sachdev, Elsayed, McDarmont, Mitchell, Wilkinson, Parker and Gandevia2001; Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008). However, it was reported that theta power was lower post-HFL-rTMS when the subjects’ eyes were closed (Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008). Other studies demonstrated that theta power increased post-HFL-rTMS in the prefrontal (Noda et al. Reference Noda, Nakamura, Saeki, Inoue, Iwanari and Kasai2013), central, parietal and occipital regions, where the increase in power was seen across the whole brain (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012).
Delta band
Some studies demonstrated delta (0.5–3 Hz) band activity increased post-HFL-rTMS (Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008; Saeki et al. Reference Saeki, Nakamura, Hirai, Noda, Hayasaka, Iwanari and Hirayasu2013; Noda et al. Reference Noda, Nakamura, Saeki, Inoue, Iwanari and Kasai2013), particularly in the central and parietal regions (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012), which led to a subsequent theta power increase in the left hemisphere (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012). One study showed delta power was significantly increased without specificity at prefrontal electrode sites (Noda et al. Reference Noda, Nakamura, Saeki, Inoue, Iwanari and Kasai2013).
Cordance in delta and theta band power
Cordance, which is related to absolute and relative spectral powers derived from the resting EEG, increased after HFL-rTMS in both delta and theta bands at left frontal and all right electrodes, except for the F8 electrode, in responders, whereas it decreased in left frontal and right prefrontal regions in non-responder (Ozekes et al. Reference Ozekes, Erguzel, Sayar and Tarhan2014).
Sigma band
HFL-rTMS also did not cause significant changes in the sigma band (i.e. 11–15 Hz; spindle at stage 2 in sleep EEG) power (Saeki et al. Reference Saeki, Nakamura, Hirai, Noda, Hayasaka, Iwanari and Hirayasu2013).
Resting-state functional connectivity in gamma band
In a standardized low-resolution brain electromagnetic tomography (sLORETA) method, resting-state functional connectivity anti-correlation in the gamma band between the left DLPFC and precuneus was significantly modulated by HFL-rTMS (Kito et al. Reference Kito, Pascual-Marqui, Hasegawa and Koga2014).
Event-related potentials (ERPs)/rTMS-related potentials
rTMS also affects various different event-related potentials. Specifically, in the oddball ERP, the amplitude of P2 and P3 post-HFL-rTMS increased ipsilaterally, whereas the amplitude of N1 and N2 decreased ipsilaterally (Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008). A more recent study has replicated the result that P2 amplitude was significantly increased in the left middle frontal gyrus after 3 weeks of HFL-rTMS (Choi et al. Reference Choi, Jang, Jang, Um, Kim, Kim, Shin and Chae2014). Also, LFR-rTMS was shown to increase frontal alpha power asymmetry towards the right hemisphere (Valiulis et al. Reference Valiulis, Gerulskis, Dapšys, Vištartaite, Šiurkute and Mačiulis2012).
TMS neurophysiology studies in the motor cortex
There are conflicting findings as to what the effect of rTMS is on resting motor threshold (RMT); one study demonstrated that HFL-rTMS does not affect RMT (Bajbouj et al. Reference Bajbouj, Luborzewski, Danker-Hopfe and Lang2005b ) whereas other studies have shown that HFL-rTMS causes MT to decrease (Triggs et al. Reference Triggs, McCoy, Greer, Rossi, Bowers, Kortenkamp, Nadeau, Heilman and Goodman1999; Zarkowski et al. Reference Zarkowski, Navarro, Pavlicova, George and Avery2009) or increase (Shajahan et al. Reference Shajahan, Glabus, Steele, Doris, Anderson, Jenkins, Gooding and Ebmeier2002). HFL-rTMS causes P300 (Moller et al. Reference Moller, Hjaltason, Ivarsson and Stefansson2006; Spronk et al. Reference Spronk, Arns, Bootsma, van Ruth and Fitzgerald2008) and intracortical inhibition (Bajbouj et al. Reference Bajbouj, Brakemeier, Schubert, Lang, Neu, Schindowski and Danker-Hopfe2005a , Reference Bajbouj, Luborzewski, Danker-Hopfe and Lang b ) to increase, the latter suggesting that rTMS further activates the GABAergic system, whereas auditory threshold (Loo et al. Reference Loo, Sachdev, Elsayed, McDarmont, Mitchell, Wilkinson, Parker and Gandevia2001), and intracortical facilitation (Bajbouj et al. Reference Bajbouj, Brakemeier, Schubert, Lang, Neu, Schindowski and Danker-Hopfe2005a , Reference Bajbouj, Luborzewski, Danker-Hopfe and Lang b ) remain unchanged. The effect of HFL-rTMS on the cortical silent period (CSP) is not clear; one study has found that it does not change (Bajbouj et al. Reference Bajbouj, Luborzewski, Danker-Hopfe and Lang2005b ) whereas another shows it does (Bajbouj et al. Reference Bajbouj, Brakemeier, Schubert, Lang, Neu, Schindowski and Danker-Hopfe2005a ). In summary, these studies provide relatively inconclusive information on the neurobiological effects of rTMS in depression.
Other neurophysiology studies
Post-HFL-rTMS, sympathovagal balance was found to be reduced as it causes an increase in parasympathetic nervous system activity, and a decrease in sympathetic nervous system activity (Udupa et al. Reference Udupa, Sathyaprabha, Thirthalli, Kishore, Raju and Gangadhar2007). Last, HFL-rTMS suppressed unwanted saccade behavior, which is thought to represent decreased psychomotor inhibition (Crevits et al. Reference Crevits, Van den Abbeele, Audenaert, Goethals and Dierick2005).
Effects of rTMS on cerebral activity, blood flow, volume and functional connectivity as measured by neuroimaging
Global brain function
HFL-rTMS does not appear to affect the integrity of the blood–brain barrier (Li et al. Reference Li, Nahas, Lomarev, Denslow, Shastri, Bohning and George2003). LFR-rTMS decreased global blood flow, but HFL-rTMS also caused blood flow to increase ipsilaterally (Mottaghy et al. Reference Mottaghy, Keller, Gangitano, Ly, Thall, Parker and Pascual-Leone2002; Kito et al. Reference Kito, Hasegawa and Koga2011a ). HFL-rTMS caused glucose metabolism to decrease in the left fusiform gyrus and left middle temporal cortex (Baeken et al. Reference Baeken, De Raedt, Van Hove, Clerinx, De Mey and Bossuyt2009b ; Li et al. Reference Li, Wang, Hirvonen, Hsieh, Bai, Hong, Liou and Su2010) and increase in the middle cingulum, bilateral somatosensory areas, precuneus, bilateral Brodmann area (BA) 32, and right BA24 (Baeken et al. Reference Baeken, De Raedt, Van Hove, Clerinx, De Mey and Bossuyt2009b ; Li et al. Reference Li, Wang, Hirvonen, Hsieh, Bai, Hong, Liou and Su2010). White matter fractional anisotropy (FA) in the left prefrontal and middle frontal gyrus increased post-HFL-rTMS (Kozel et al. Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al. Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012).
Cerebral cortex
Prefrontal cortex
HFL-rTMS caused regional cerebral blood flow (rCBF) to increase in most studies (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Catafau et al. Reference Catafau, Perez, Gironell, Martin, Kulisevsky, Estorch, Carrió and Alvarez2001; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004), although in one study it did not cause any rCBF change (Nahas et al. Reference Nahas, DeBrux, Chandler, Lorberbaum, Speer, Molloy, Liberatos, Risch and George2000) in the prefrontal cortex. In the DLPFC, HFL-rTMS increased rCBF (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001) and LFR-rTMS decreased it (Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007). Other studies have also found that HFL-rTMS increased rCBF (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004; Kito et al. Reference Kito, Fujita and Koga2008a ) and LFR decreased rCBF in the DLPFC (Kito et al. Reference Kito, Fujita and Koga2008b , Reference Kito, Hasegawa and Koga2011a ). HFL-rTMS also caused blood flow and activity to increase in the dorsomedial frontal cortex (Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003; Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007). HFL-rTMS also caused blood flow and activity to consistently increase across several studies in the inferior frontal lobe (Teneback et al. Reference Teneback, Nahas, Speer, Molloy, Stallings, Spicer, Risch and George1999; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003; Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007). In the orbitofrontal cortex, low-frequency left-sided rTMS increased rCBF in one study (Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004), and decreased it in others (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003), whereas LFR-rTMS only decreased rCBF (Kito et al. Reference Kito, Fujita and Koga2008b , Reference Kito, Hasegawa and Koga2011a ). In the ventrolateral prefrontal cortex, activity and rCBF decreased post-LFR-rTMS (Kito et al. Reference Kito, Hasegawa and Koga2011a ), while in the ventromedial prefrontal cortex, post-HFL-rTMS, activity and rCBF decreased (Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004). In frontal white matter, activity and rCBF decreased post-LFR-rTMS (Kito et al. Reference Kito, Hasegawa and Koga2011a ). White matter FA in the left prefrontal cortex and middle frontal gyrus increased post-HFL-rTMS (Kozel et al. Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al. Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012).
Primary motor and somatosensory cortex
HFL-rTMS increased rCBF (Kito et al. Reference Kito, Fujita and Koga2008a ) and LFR-rTMS decreased rCBF in the premotor (Kito et al. Reference Kito, Fujita and Koga2008b ) and somatosensory regions (Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003; Kito et al. Reference Kito, Fujita and Koga2008b ). HFL and bilateral rTMS caused blood flow and activity to be consistently increased in the precentral gyrus (Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007; Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013).
Parietal, temporal, and occipital lobes
In the parietal lobe, HFL-rTMS caused rCBF to either increase (Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004) or decrease (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001), whereas LFR-rTMS decreased rCBF (Kito et al. Reference Kito, Fujita and Koga2008b ) and bilateral rTMS increased it in the parietal region (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). Bilateral rTMS consistently increased rCBF in the angular gyrus (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). Bilateral rTMS consistently increased rCBF in the bilateral anterior gyrus (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). HFL-rTMS increased rCBF (Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003) and LFR-rTMS decreased rCBF in the precuneus (Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007; Dumas et al. Reference Dumas, Richieri, Guedj, Auquier, Lancon and Boyer2012). In the temporal lobe, in one study, HFL-rTMS increased rCBF (Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004), whereas in other studies, HFL-rTMS caused rCBF to decrease (Teneback et al. Reference Teneback, Nahas, Speer, Molloy, Stallings, Spicer, Risch and George1999; Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001). Bilateral rTMS also caused rCBF to decrease in the temporal lobe (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). In the uncus, HFL-rTMS increased rCBF (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000) in certain studies, and it decreased it in others (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003). HFL-rTMS caused glucose metabolism to decrease in the left fusiform gyrus and left middle temporal cortex (Loo et al. Reference Loo, Sachdev, Elsayed, McDarmont, Mitchell, Wilkinson, Parker and Gandevia2001). Responders presented with significant decreases in activity and rCBF post-bilateral rTMS, as compared with non-responders, in the perirhinal cortex (Richieri et al. Reference Richieri, Boyer, Padovani, Adida, Colavolpe, Mundler, Lançon and Guedj2012). In the occipital lobe, activity and rCBF decreased post-bilateral rTMS (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013).
Limbic cortex
Cingulate gyrus and insula
In the cingulate cortex, HFL-rTMS has demonstrated conflicting results regarding increased (Zheng, Reference Zheng2000; Shajahan et al. Reference Shajahan, Glabus, Steele, Doris, Anderson, Jenkins, Gooding and Ebmeier2002; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003) and decreased rCBF (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004). In the subgenual cingulate cortex, activity and rCBF was decreased post-bilateral-rTMS (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013) and post-HFL-rTMS (Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003); LFR-rTMS decreased rCBF in the subgenual cingulate in some studies (Kito et al. Reference Kito, Fujita and Koga2008b , Reference Kito, Hasegawa and Koga2011a ), but increased it in another (Kito et al. Reference Kito, Hasegawa, Okayasu, Fujita and Koga2011b ). In the insula, HFL-rTMS increased rCBF in some studies (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004), and decreased it in others (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001; Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002). LFR-rTMS decreased rCBF in the insula (Kito et al. Reference Kito, Fujita and Koga2008b , Reference Kito, Hasegawa and Koga2011a ).
Amygdala
HFL-rTMS increased blood flow in one study (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000), but in another study it decreased (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002). HFL-rTMS caused a near-significant increase in amygdala volume in responders to rTMS (Furtado et al. Reference Furtado, Hoy, Maller, Savage, Daskalakis and Fitzgerald2013).
Hippocampus
HFL-rTMS increased rCBF and activation (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004); one study showed a decrease in rCBF post-HFL-rTMS (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001). Bilateral rTMS decreased blood flow in the hippocampus (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). HFL-rTMS caused a decrease in hippocampal non-responders (Furtado et al. Reference Furtado, Hoy, Maller, Savage, Daskalakis and Fitzgerald2013). HFL-rTMS caused blood flow and activity to be consistently increased in the parahippocampal region across several studies (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003).
Basal ganglia and thalamus
There were inconsistent results of rCBF change after HFL-rTMS in the basal ganglia (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000) whereas activity and rCBF decreased post-LFR-rTMS in the globus pallidus (GP) (Kito et al. Reference Kito, Hasegawa and Koga2011a ). Activity and rCBF decreased post-bilateral rTMS in the lentiform nucleus (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). HFL-rTMS caused blood flow and activity to increase in the putamen (Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004). In the thalamus, HFL-rTMS caused rCBF to increase in some studies (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Li et al. Reference Li, Nahas, Kozel, Anderson, Bohning and George2004), and to decrease in another (Nahas et al. Reference Nahas, Teneback, Kozel, Speer, DeBrux, Molloy, Stallings, Spicer, Arana, Bohning, Risch and George2001). Bilateral rTMS and LFR-rTMS decreased rCBF in the thalamus (Kito et al. Reference Kito, Hasegawa and Koga2011a ; Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013).
Other brain areas
Cerebellum and midbrain
In the cerebellum, HFL-rTMS increased blood flow (Speer et al. Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000; Loo et al. Reference Loo, Sachdev, Haindl, Wen, Mitchell, Croker and Malhi2003) whereas bilateral rTMS decreased it (Takahashi et al. Reference Takahashi, Ukai, Tsuji, Kose, Shoyama, Yamamoto, Okumura and Shinosaki2013). Last, activity and rCBF decreased post-LFR-rTMS in the midbrain (Kito et al. Reference Kito, Hasegawa and Koga2011a ).
Functional connectivity
HFL-rTMS increased functional connectivity significantly in the neuroanatomical networks in the dorsolateral frontal loop (i.e. among DLPFC, caudate and GP) in the left hemisphere and the limbic loop [i.e. between the medial orbitofrontal cortex to the ventral striatum (VS)] on both sides within 1 h of stimulation (Shajahan et al. Reference Shajahan, Glabus, Steele, Doris, Anderson, Jenkins, Gooding and Ebmeier2002). However, in the limbic loop, connectivity of the amygdala to the VS increased on the left, whereas that of the VS to the GP decreased post-HFL-rTMS (Shajahan et al. Reference Shajahan, Glabus, Steele, Doris, Anderson, Jenkins, Gooding and Ebmeier2002). Further, a recent study has reported that successful accelerated HFL-rTMS (i.e. five HFL treatments per day spread over 4 days) inverted the anti-correlation between the subgenual anterior cingulate cortex (ACC) and parts of the left superior medial prefrontal cortex significantly to a mild positive correlation (Baeken et al. Reference Baeken, Marinazzo, Wu, Van Schuerbeek, De Mey, Marchetti, Vanderhasselt, Remue, Luypaert and De Raedt2014). In addition, another study has demonstrated that HFL-rTMS selectively modulated functional connectivity both within and between the central executive network and default mode network (DMN), resulting in an attenuation of abnormal hyperactivity in the DMN (Liston et al. Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014).
Neurobiological mechanisms associated with treatment response
As summarized in Table 2, there are several studies that have investigated the neurobiological mechanisms associated with treatment response. Six studies out of 11 are strong studies; however, the findings in these studies have not been replicated. Only one finding, in a relatively weak study, shown in Table 2 has been replicated. In addition, the finding showing a significant increase in glutamate levels in the left DLPFC in MRS after rTMS has not been replicated in strong studies. Therefore, further studies in larger samples and with a control condition are needed to validate these results.
Table 2. Neurobiological mechanisms associated with treatment response
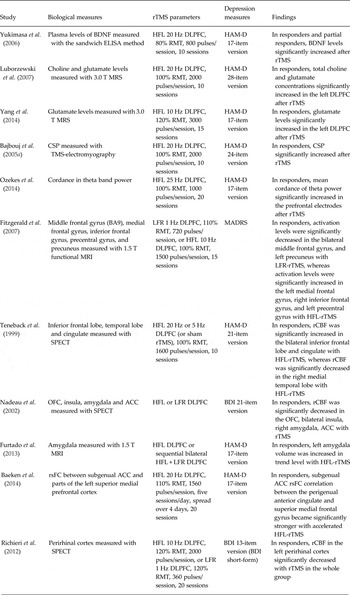
rTMS, Repetitive transcranial magnetic stimulation; brain-derived neurotrophic factor; ELISA, enzyme-linked immunosorbent assay; HFL, high-frequency left-sided; dorsolateral prefrontal cortex; RMT, resting motor threshold; HAM-D, Hamilton Depression Rating Scale; MRS, magnetic resonance spectroscopy; CSP, cortical silent period; BA, Brodmann area; MRI, magnetic resonance imaging; LFR, low-frequency right-sided; MADRS, Montgomery-Åsberg Depression Rating Scale; SPECT, single photon emission computed tomography; rCBF, regional cerebral blood flow; OFC, orbitofrontal cortex; ACC, anterior cingulated cortex; BDI, Beck Depression Inventory; rsFC, resting-state functional connectivity.
Discussion
The purpose of this systematic review was to summarize and synthesize all of the known neurobiological effects of rTMS applied to the DLPFC in patients with depression. To our knowledge, this is the first attempt to review the literature on the neurobiological effects of rTMS. Our review identified 66 studies evaluating the effects of rTMS on a variety of neurobiological measures in depression.
rTMS alters levels of various neurochemical and electrophysiological parameters including: mRNA expression of particular genes as well as blood flow and activity in brain regions involved in the etiopathogenesis of depression. However, there were conflicting findings on the effect of HFL-rTMS on cortisol levels. The effects of rTMS on BDNF and dopamine are still unclear due to inconsistent results. The effects of rTMS on cortical excitability measured by RMT are unclear; two strong studies showed that LFR and HFL-rTMS did not change RMT, but another strong study showed that LFR-rTMS decreased RMT. Moreover, strong studies demonstrated EEG spectral power increases in the delta, theta, alpha and beta bands as well as CSP increase post-rTMS; however, these findings were not replicated in some weaker-quality studies. An observable trend is that high-frequency rTMS increases rCBF, whereas low-frequency rTMS decreases rCBF, though some findings conflict with this trend. Further, rTMS was shown to increase rCBF in a certain brain region in responders whereas non-responders showed opposite effects, and vice-versa. There is conflicting data on the clinical correlations between blood flow or activity and improvement in depressive symptoms, as several studies suggest that a correlation exists while others do not. Thus, it is crucial that further studies be conducted to resolve conflicting findings regarding the effect of rTMS on regional brain functioning and to what extent stimulation frequency determines circuit activity.
Advances in functional neuroimaging are beginning to identify the potential etiopathogenic regions and neural circuits of depression. In addition, researchers have begun to apply these techniques to understand the circuit changes induced by rTMS treatment over the DLPFC (Baeken et al. Reference Baeken and De Raedt2011). Specifically, the frontal gyrus (Teneback et al. Reference Teneback, Nahas, Speer, Molloy, Stallings, Spicer, Risch and George1999; Fitzgerald et al. Reference Fitzgerald, Sritharan, Daskalakis, de Castella, Kulkarni and Egan2007), amygdala (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002; Furtado et al. Reference Furtado, Hoy, Maller, Savage, Daskalakis and Fitzgerald2013), ACC (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002), perirhinal cortex (Richieri et al. Reference Richieri, Boyer, Padovani, Adida, Colavolpe, Mundler, Lançon and Guedj2012), orbitofrontal cortex (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002), insula (Nadeau et al. Reference Nadeau, McCoy, Crucian, Greer, Rossi, Bowers, Goodman, Heilman and Triggs2002) and precuneus are involved in these regions. Further, resting-state functional connectivity of affective circuitry between the subgenual ACC and left superior prefrontal cortex is altered after successful rTMS treatment (Baeken et al. Reference Baeken, Marinazzo, Wu, Van Schuerbeek, De Mey, Marchetti, Vanderhasselt, Remue, Luypaert and De Raedt2014). Neurochemical factors that affect neuroplasticity such as BDNF and glutamate underlie dysregulation of the corresponding neural networks in depression and these seem to be modified by rTMS treatment (Yukimasa et al. Reference Yukimasa, Yoshimura, Tamagawa, Uozumi, Shinkai, Ueda, Tsuji and Nakamura2006; Luborzewski et al. Reference Luborzewski, Schubert, Seifert, Danker-Hopfe, Brakemeier, Schlattmann, Anghelescu, Colla and Bajbouj2007; Yang et al. Reference Yang, Kirton, Wilkes, Pradhan, Liu, Jaworska, Damji, Keess, Langevin, Rajapakse, Lebel, Sembo, Fife and MacMaster2014). In addition, neurophysiological findings such as CSP increase (Bajbouj et al. Reference Bajbouj, Brakemeier, Schubert, Lang, Neu, Schindowski and Danker-Hopfe2005a ) as well as prefrontal cordance increase in the theta band (Ozekes et al. Reference Ozekes, Erguzel, Sayar and Tarhan2014) following successful rTMS may reflect the improvement of the cortical GABAB receptor-mediated inhibitory functioning, and increased metabolic activity and blood flow perfusion in frontal regions, seen with rTMS of the DLPFC. More comprehensive studies that include multimodal biological markers are needed to better understand the antidepressant mechanism of DLPFC-rTMS treatment. By further elucidating these mechanisms the researchers may be able to individualize parameters of stimulation to enhance efficacy.
Study limitations
While this review summarized the neurobiological effects of rTMS in patients with depression, there are some limitations. First, this study only reviewed primary studies in English; our search terms identified several studies that examined the neurobiological effect of rTMS in depression that were either not written in English or presented at scientific conferences as abstracts. These studies may alter the scope of understanding of the effect of rTMS. In addition, our review only examined the effect of rTMS when delivered to either the left or right DLPFC. Including studies that only examined rTMS stimulation over these areas also limits our understanding of the full breadth of the biological effect of rTMS because stimulation of different areas of the brain may elicit alterations in other circuits (Conca et al. Reference Conca, Di Pauli, Beraus, Hausmann, Peschina, Schneider, König and Hinterhuber2002; Baeken & De Raedt, Reference Baeken and De Raedt2011). It is also important to note that results from the studies included in this review may not solely reflect the effect of rTMS; as many of the participants were on antidepressant medications. These pharmacological agents also affect neurobiological function (Oberlander et al. Reference Oberlander, Gingrich and Ansorge2009). Thus, the effect of rTMS treatment may be confounded by concomitant antidepressant use in these studies. As well, the relatively small number of studies that examined individual biological mechanisms prevented a quantitative meta-analysis. Last, the heterogeneity, including the time of the post-assessment, in the studies reviewed prevents definitive conclusions from being drawn on the effect of rTMS on neurobiological parameters.
Conclusion
There was a large degree of heterogeneity in the rTMS parameters of treatment, study approach, and these variations in procedures probably affected the ability to coherently summarize findings of the various studies. Despite this, the findings of this review will be able to direct future research focused on the effect of rTMS on various neurobiological factors. Overall, there were very few findings that were replicated in multiple strong studies. While there have been some corroboration and replication of some of the stronger findings, many mechanisms demonstrated conflicting findings. As rTMS continues to be adopted by more treatment providers, the mechanism of its neurobiological effects needs to be better understood. Well-designed rTMS studies, using a constant set of parameters, that include multi-modal measurements of putative neurobiological mechanisms, are still greatly needed.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715001609
Acknowledgements
Y.N. has received a postdoctoral fellowship through the Centre for Addiction and Mental Health (CAMH) Foundation.
W.K.S. does not have any financial disclosures.
M.S.B. receives research support from the Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator Grant and a Schizophrenia Junior Faculty Grant from the CAMH Foundation.
F.V.-R. does not have any financial disclosures.
J.D. has received research support from the Canadian Institutes of Health Research (CIHR), the US National Institutes of Health (NIH), the Klarman Family Foundation, the Buchan Family Foundation, and the Toronto General and Western Hospital Foundation, as well as a travel stipend from Lundbeck and from ANT Neuro, and in-kind equipment support for an investigator-initiated study from Tonika/Magventure.
T.K.R. receives research support from Brain Canada, the Brain and Behavior Research Foundation, the Canadian Foundation for Innovation, the CIHR, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the NIH and the W. Garfield Weston Foundation. T.K.R. reports no competing interests.
P.B.F. is supported by a National Health and Medical Research Council (NHMRC) Practitioner Fellowship (606 907). He has received equipment for research from MagVenture A/S, Medtronic Ltd, Cervel Neurotech and Brainsway Ltd and funding for research from Cervel Neurotech.
B.H.M. currently receives research support from the CIHR, the NIH, Brain Canada, the CAMH Foundation, Bristol-Myers Squibb (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He directly owns stocks of General Electric (less than $5000). Within the past 5 years, he has also received some travel support from Roche.
S.N.V. has received funding from the Ontario Mental Health Foundation (OMHF), the CIHR, SickKids Foundation and the Ontario Ministry of Long Term Care.
Z.J.D. received external funding through Brainsway Ltd and a travel allowance through Pfizer and Merck. Z.J.D. has also received speaker funding through Sepracor Inc., AstraZeneca and served on the advisory board for Hoffmann-La Roche Limited. Z.J.D. has received funding from the OMHF, CIHR, the Brain and Behavior Research Foundation and the Temerty Family and Grant Family and through the CAMH Foundation and the Campbell Institute.
D.M.B. receives research support from the CIHR, Brain Canada, NIH, Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He receives non-salary operating funds and in-kind equipment support from Brainsway Ltd for an investigator-initiated study. He is the site principal investigator for several sponsor-initiated clinical trials from Brainsway Ltd. He receives in-kind equipment support from Tonika/Magventure for an investigator-initiated study.
This material is based upon work supported by the Temerty Family through the CAMH Foundation and the Campbell Research Institute.
Declaration of Interest
None.





