INTRODUCTION
A variety of factors have limited the expansion of shrimp farming in coastal ecosystems. These factors include the impact of shrimp effluents on the water that receives them, environmental regulations, the high cost of land and the constant appearance of viral diseases (McIntosh and Fitzsimmons, Reference McIntosh and Fitzsimmons2003). This situation has stimulated the development of new technologies and innovations that have allowed the aquaculture industry to become more sustainable. Several alternatives have been considered for resolving or mitigating the impact of shrimp pond effluents on the water quality of adjacent waters; the innovations that have been investigated include recirculating systems, constructed wetlands, suspended solids removal and polyculture using pond water to feed other organisms in the effluent streams. Although each of these innovations can reduce or mitigate the load of nutrients and metabolites in the discharged effluent, none of them provides an additional exploitation for the water after its utilization in the aquaculture. In this context, Stevenson et al. (Reference Stevenson, Fitzsimmons, Clay, Alessa and Kliskey2010) suggest that the additional utilization of water constitutes an especially important option in arid and semi-arid regions around the world in which water availability is a limited resource; thus, integrating aquaculture production with traditional agriculture may be one solution to achieve a more efficient water use by maximizing farming production without increasing water consumption. Additionally, the potential benefit of having nutrient-enriched waters to irrigate field crops is that this strategy could be fundamental to reducing the demand on chemical fertilizers. Another advantage of integrated agro-aquaculture systems is the decrease of the environmental cost that applies individually to cultures; each culture separately imposes an environmental impact in terms of water use and the amounts of nutrients discharged, which may be reduced or totally removed when the two cultures are appropriately integrated.
In the case of inland aquaculture, effluents have been utilized for a number of agricultural products. Freshwater from fish cultures has been traditionally used for the irrigation of flowers and vegetables. Similarly, moderately saline groundwater from aquaculture has been used to irrigate cotton, barley, sorghum and wheat (McIntosh and Fitzsimmons, Reference McIntosh and Fitzsimmons2003; Stevenson et al., Reference Stevenson, Fitzsimmons, Clay, Alessa and Kliskey2010). Fluvial water from shrimp farming has been used for melon irrigation (Miranda et al., Reference Miranda, Lima, Crisóstomo and Santana2008). The present study describes the performance of shrimp (Litopenaeus vannamei)-tomato (Lycopersicon esculentum) culture system (STCS) where shrimp effluent is used for irrigation. Water quality variables and phytoplankton variation are also presented.
MATERIALS AND METHODS
Production system and experimental design
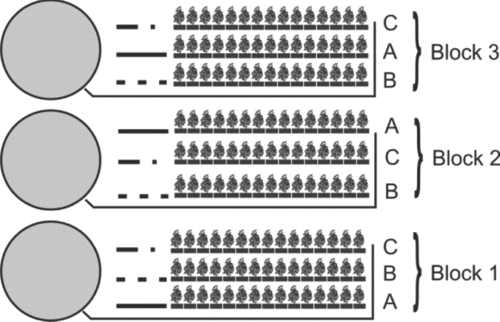
A 19-week field study was conducted at the Experimental Station of Centro de Estudios Superiores del Estado de Sonora (111o 08` W and 28o 18` N) in the Sonora state, Northwestern Mexico, from August 4 to December 12, 2008. Three circular tanks (6 m in diameter) covered with high-density (1.5 mm) polyethylene and subjected to zero water exchange were selected for the study. The tanks had a mean water depth of 1.1 m and a volume of 31 m3 per tank. To quantify the effect of the water used in shrimp culture on the growth of tomato plants, we used a randomized experimental design (McIntosh and Fitzsimmons, Reference McIntosh and Fitzsimmons2003) to compare the following alternatives: (1) irrigated tomato plants with water effluents from shrimp tanks, (2) tomato plants irrigated with groundwater directly from the well and (3) tomato plants irrigated with a hydroponic nutrient solution prepared for tomato (Samperio-Ruiz, Reference Samperio-Ruiz2005). Each module consisted of 45 plants with 3 lines of 15 plants each: the plants in three lines (A) were irrigated with shrimp effluents from each tank; three lines (B) were irrigated with a nutritive solution (Samperio-Ruiz, Reference Samperio-Ruiz2005); and the final three lines (C) were irrigated directly with groundwater (Figure 1). Each tomato plant was put into a pot with a zeolite-based (20 cm). Aeration in the shrimp tanks was maintained using a blower (½ HP). For the irrigation, the shrimp effluent from the tanks was transferred by gravity. A similar system was utilized for the irrigation with the nutritive solution and the groundwater. The effluent not consumed by plants and not retained by zeolite was recollected using a channel system and then returned to the shrimp tanks.

Figure 1. The experimental design of the shrimp-tomato culture: circles are the shrimp tanks; A, tomato plants irrigated with shrimp effluents; B, tomato plants irrigated with groundwater; C, tomato plants irrigated with nutritive solution.
Stocking, fertilization and feeding of shrimp tanks
Recent research on the shrimp L. vannamei has evaluated the potential for culturing this species in low-salinity water (Miranda et al., Reference Miranda, Lima, Crisóstomo and Santana2008; Roy et al., Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010). In the present study, this species was selected, and twelve-day-old postlarvae (PL12) of L. vannamei were stocked at a density of 50 PL m−2. Prior to stocking the tanks, the postlarvae were acclimated in a raceway (2 m3). The salinity in the tank was 35 g L−1, and freshwater was continuously added over a period of six days until the salinity in the raceway reached 0.6 g L−1. During the acclimatization period, the shrimp were fed with a 50% crude protein dry diet that was supplemented with newly hatched Artemia nauplii (100 nauplii/postlarvae/day). The diet was supplied five times daily. The animals were then stocked in the experimental system (initial weight: 82±10 mg). Initially, the three tanks were filled 10 days prior to the shrimp stocking. The tanks were filled with groundwater (salinity of 0.65 g L−1) to a level of 0.8 m. Next, the fertilizers KCl and MgNO3 were supplemented in amounts to increase the levels of K and Mg in the water tanks to the levels indicated in Table 1. These major ions are important for growth (Roy et al., Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010) and stimulate phytoplankton production. After three days, the water level in the tanks was increased to 1.0 m. The tanks were maintained at this water level during the culture cycle. During the trial, additional KCl and MgNO3 fertilizers were applied if the primary productivity in the pond water was low (when transparency was higher than 0.35 m). The shrimp were fed manually from the sides of the tanks. During the first 8 weeks, a commercially formulated feed composed of 40% protein was utilized (Nassa 1); an average of 4.2 kg of feed per tank was used during the cycle. The feed was changed at week 9 to feed with a protein content of 35% (Nassa pellets of 1/32¨), which was used until the end of the culture period (week 19). Depending on the demand, an average of 13.8 kg was used during the cycle. The amounts of feed were controlled using feeding trays (2 feeding trays per pond) three times daily (07:00, 13:00 and 20:00 h). The feeding procedure was based on the biomass, survival and consumption rate; the daily ration was adjusted according to Casillas-Hernández et al. (Reference Casillas-Hernández, Nolasco-Soria, Garcia-Galano, Carrillo-Farnes and Páez-Osuna2007).
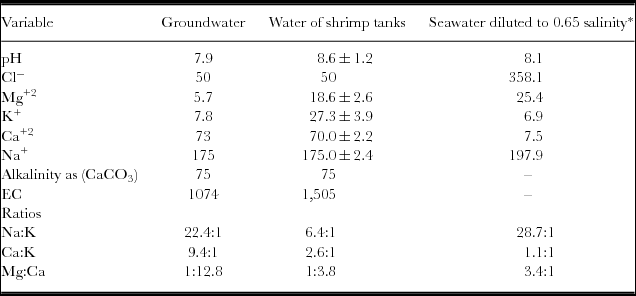
Table 1. Major chemical components (in mg/L), EC (in μS/cm) and pH of the groundwater used to fill the tanks and the water used in the cultures.
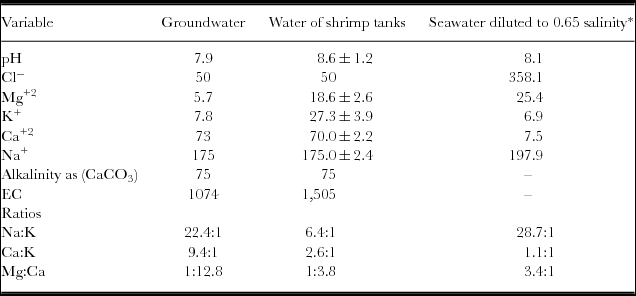
*calculated from factors of Roy et al. (Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010).
Planting and harvest of tomato
The tomato plant (L. esculentum Mill) was selected because it is a product with high demand, cultivated in the NW region of Mexico, and is tolerant of waters with moderate or relatively elevated levels of ions such as Cl−, Na+, K+ and Mg+2 (Magán et al., Reference Magán, Gallardo, Thompson and Lorenzo2008). The seeds were initially stocked in seed recipients utilizing peat-moss-perlite in a 1:1 ratio (v:v) as a substrate (Samperio-Ruiz, Reference Samperio-Ruiz2005). The tomato plants were transferred to pots when the first pair of leaves appear (20–30 days). A total of 135 plants were transplanted, with a density of 4.9 plants per m2 (15 plants per shrimp tank). The separation between the plants was 0.30 m, and the separation between adjacent rows was 1.0 m. The plants were vertically supported by a nylon cord guide and were pruned and managed according to local practices. The harvest of the tomato plants was initiated when their fruit first appeared red.
Sampling and chemical analysis
During the culture cycle, weekly water samples were taken directly from the tanks (25 cm below the surface water) and from the output of the pots (recovery channels). The sampling time was between 12:00 and 13:00 hr. One sample (120 mL) was fixed with Lugol's solution for further phytoplankton analysis in the laboratory (Cronberg and Annadotter Reference Cronberg and Annadotter2006). Other filtered (Whatman GF/F) water samples were stored in clean plastic bottles (120 mL) and transported at low temperature (4 °C) to the laboratory after collection. The dissolved oxygen, temperature, salinity and pH were measured in situ twice a day (at 6:00 and 18:00) using a dissolved oxygen meter (YSI model 58, Ohio, USA) and a pH meter (Hanna). The filtered water samples were used to determine the concentrations of nutrients. A second aliquot was filtered, and the filters (Whatman GF/F) utilized were placed in black vials with acetone for chlorophyll a analysis. Measurements of the nutrients and the chlorophyll a were conducted using the procedures outlined by Grasshoff et al. (Reference Grasshoff, Ehrhardt and Krembling1990). The groundwater used in this trial was analysed in duplicate for major ions using standard techniques (APHA, 1989). With the exception of ammonia, for which the coefficient of variation was between 5 and 15%, the precision of other determinations was <10%. The estimated detection limits were 0.001 mg L−1 for N-ammonia, 0.001 mg L−1 for N-nitrite, 0.001 mg L−1 for nitrate + nitrite and 0.005 mg L−1 for phosphate.
Growth and statistical analyses
The shrimp were weighed at the time of harvest. The final survival and biomass were determined by individually measuring the weight at the time of harvest of shrimp. The feed conversion ratio FC (the total amount of feed delivered and apparently consumed by the shrimp relative to the wet weight gain in shrimp biomass) was calculated as FC = AFI/W, where AFI is the apparent feed intake on a dry matter basis (g) and W is the wet weight gain in shrimp biomass (g). At the time of harvest, the plants were weighed, and the fruits were also weighed and counted.
The water quality parameters (dissolved oxygen, temperature, pH, electrical conductivity (EC), and concentrations of ammonia, nitrite, nitrate, phosphates and chlorophyll a), the variables of production, the growth and the feed conversion were examined using one-way ANOVA. The Shannon-Weaver diversity index and the Wilcoxon test for composition, abundance and diversity of phytoplankton comparison between tanks were used. The comparison among the different treatments was made using a mean comparison of the harvests with Tukey's test.
RESULTS
Characterization of groundwater, growth rates, yield and water usage
The groundwater used to fill the tanks had a pH of 7.9 and an EC of 1074 μS/cm, which is equivalent to about 0.65 g L−1 of total dissolved solids (TDS). The major components of the TDS were carbonates, sodium, calcium and chloride. KCl and MgNO3 were added to increase the levels of K, Cl and Mg and to elevate the nitrogen content in the water and stimulate phytoplankton for the culture. The resulting composition after the addition of such fertilizers is presented in Table 1. These additions and the management of the tanks also provoked an increase in pH, which was probably associated with the fertilizers.
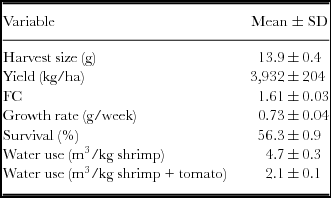
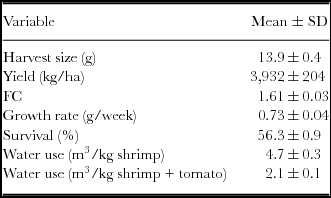
The shrimp harvests in tanks 1, 2 and 3 were 11.3 kg, 11.1 kg and 11.0 kg, respectively, corresponding to a FC of 1.6. The average growth rates observed in tanks 1, 2 and 3 were 0.76, 0.72 and 0.71 g week−1, respectively. The difference among the tanks was likely driven by the slight differences in the demand and the amount of food and fertilizer supplied. In tank 1, the amount of formulated food added with 40% protein was 4.1 kg, and the amount added with 35% protein was 13.7 kg; the corresponding values in tank 2 were 4.2 kg and 14.0 kg, respectively; in tank 3, these values were 4.2 kg and 13.8 kg, respectively. Table 2 presents the FC, yield and the water usage. A total of 4.7 m3 per kg of shrimp harvested was calculated in this STCS. When the two products were considered, shrimp and tomato, the water usage was 2.1 m3 per kg. The production of tomatoes during the culture cycle of the STCS utilizing groundwater, nutritive solution and shrimp effluent for irrigation was 8.48±0.80 kg (equivalent to 27.6±2.6 ton ha−1), 11.90±0.58 kg (38.7 ton ha−1) and 11.1±0.71 (36.1±2.3 ton ha−1), respectively, for 15 plants. The yield obtained from the tomato plants irrigated with the nutritive solution and those irrigated with the shrimp effluent were significantly (p<0.05) higher than those obtained with the tomato plants irrigated with groundwater.
Table 2. Harvest size, yield, feed conversion, growth rate, survival and water use (mean ± 1 s.d.) in the experimental shrimp tanks.
Water quality and phytoplankton
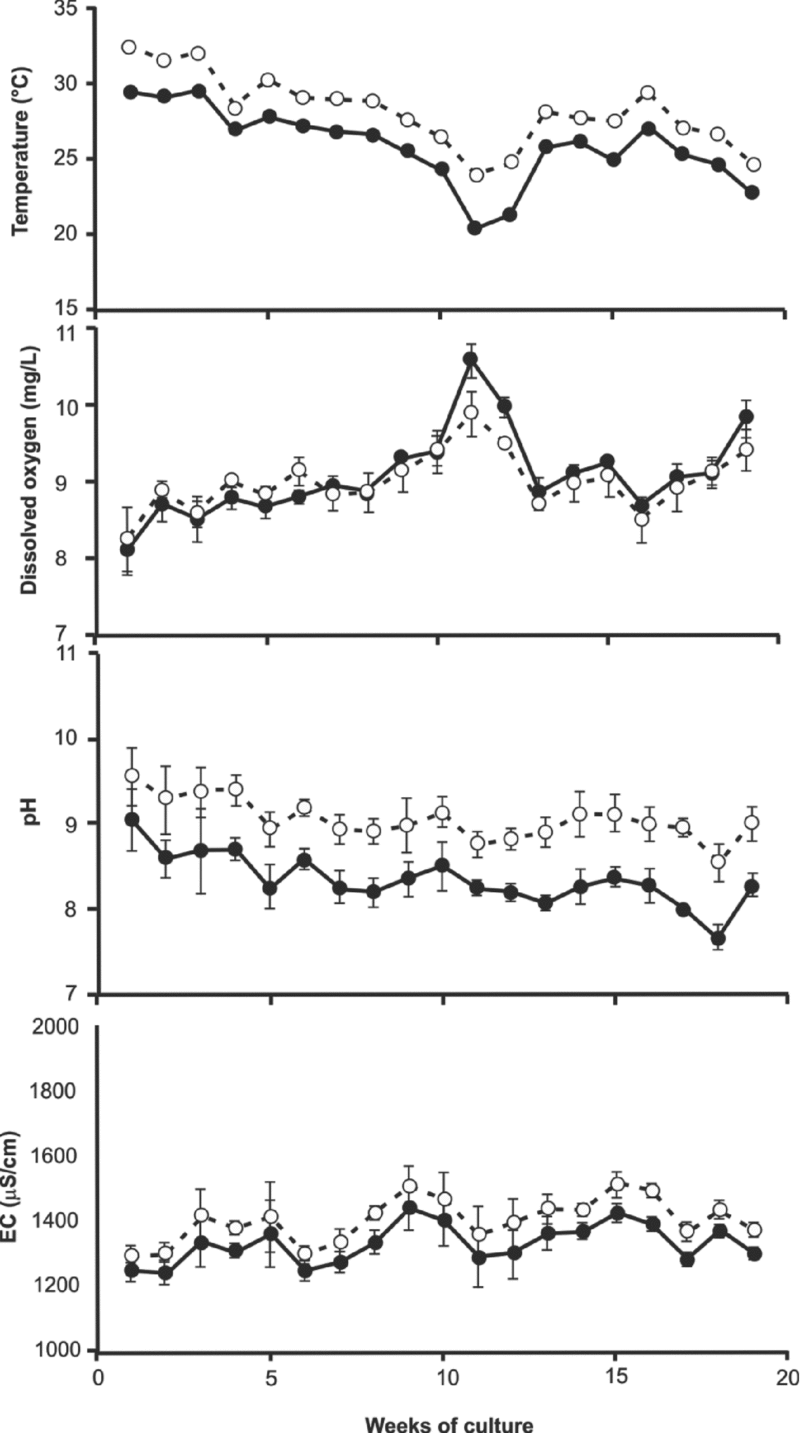
The water temperature of the tanks fluctuated between 20.4 and 32.3 °C at 6:00 and 18:00 during the 19 weeks of culture (Figure 2); the maximum was registered in week 1 during the afternoon, and the minimum occurred in week 11 during the morning. The dissolved oxygen (DO) was between 8.1 and 10.6 mg L−1 during the culture period. At the beginning of the trial, the DO in the morning (8.1 mg L−1) and in the afternoon (8.3 mg L−1) was at a minimum level in the three tanks. This concentration increased slightly throughout the cycle, reaching a maximum in the morning of week 11. The pH values varied between 7.7 and 9.6 according to the culture period in the three tanks, with measurements in the morning and afternoon. The lowest pH was registered at week 18, and the highest value was measured during the first week. The EC of the three tanks was maintained between 1,248 and 1,505 μS cm−1. The salinity estimated from the EC varied throughout the cycle from 0.80 g L−1 to 0.97 g L−1 at the time of harvest.

Figure 2. Fluctuations of temperature, dissolved oxygen, pH and EC in the three shrimp tanks: mean (± 1 s.d.) at 6:00 (filled circles) and 18 hr (unfilled circles).
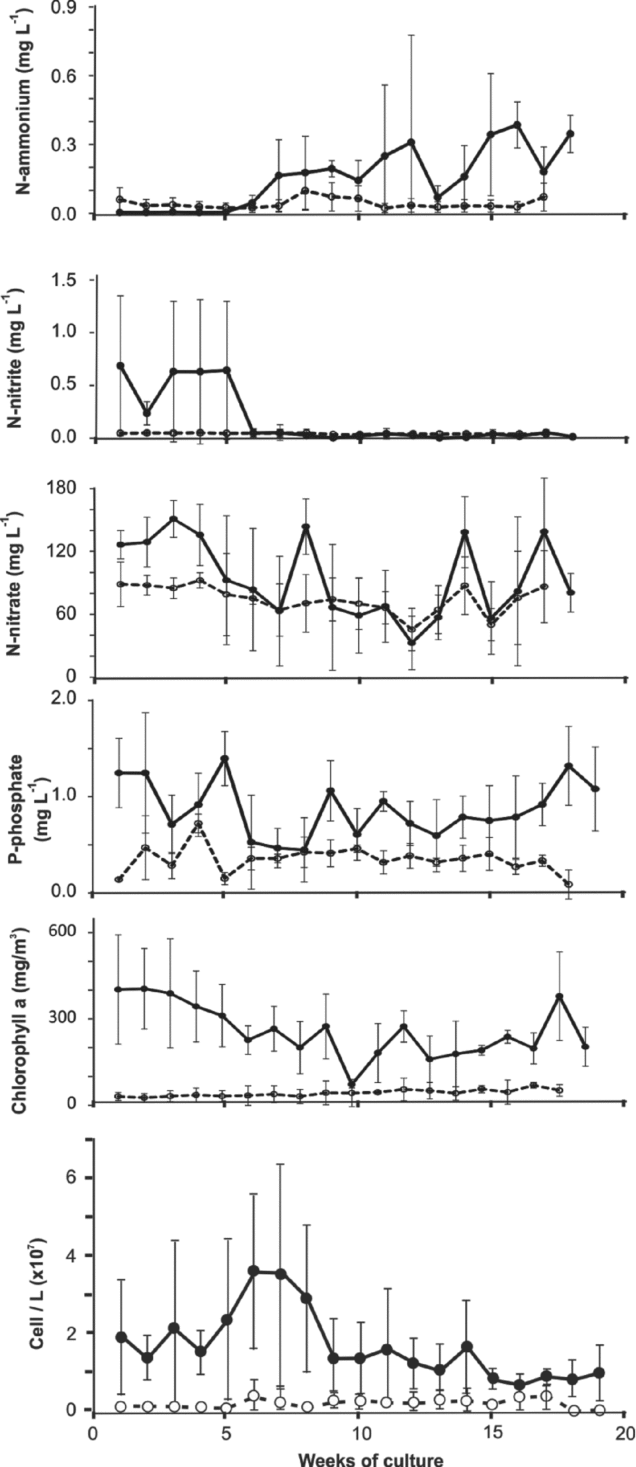
The N-ammonia concentration fluctuated significantly in the shrimp culture tanks (<0.001–0.848 mg L−1) during the culture cycle, with a clear tendency to increase in the three tanks throughout the cycle. In the output water from the tomato plants, the N-ammonia levels were between 0.022 and 0.097 mg L−1. There were no significant (p<0.05) differences among the three tanks and among the output water from the plants. The concentration of N-nitrites in the shrimp culture tanks (<0.001–1.450 mg L−1) during the first 5 weeks was relatively elevated. Subsequently, from week 6 to 19, the levels were between <0.001 and 0.14 mg L−1. The output water of the tomato plants fluctuated between 0.032 and 0.050 mg L−1. The mean concentrations of N-nitrites among the tanks were not significantly (p>0.05) different; however, the means among the output waters of the plants and the tank waters exhibited significant (p<0.05) differences. The N-nitrate concentrations in the shrimp culture tanks varied from 5.25 to 172.2 mg L−1, whereas the N-nitrate concentrations in the output waters from the plants fluctuated from 45.4 to 92.4 mg/L. The most elevated levels were observed in week 3, which was clearly related to the addition of fertilizer (MgNO3), and the levels in the week 12 were the lowest. No significant (p>0.05) differences were observed among the mean concentrations of the three tanks, whereas the mean levels of nitrates in the output water from the plants and the tank waters showed significant (p<0.05) differences. The concentrations of dissolved P-phosphates throughout the culture cycle were generally low; the concentrations in the shrimp tanks were <0.005 to 0.343 mg L−1, and a more elevated mean level was observed in week 10 (Figure 3). In the output water from plants, the P-phosphate levels fluctuated only slightly (of <0.005 to 0.023 mg L−1). There were significant (p>0.05) differences among the mean phosphate concentrations of the shrimp tanks and among the output water from the plants.

Figure 3. Fluctuations of ammonium, nitrite, nitrate, phosphate, chlorophyll a and abundance of phytoplankton: mean (±1 s.d.) in the three shrimp tanks (filled circles) and the output water from the module of tomato plants (unfilled circles).
The chlorophyll a concentrations in the shrimp tanks were 62.3 to 615 mg m−3 during the culture cycle. The highest and lowest values were observed in weeks 2 and 11, respectively (Figure 2). In the output water from the plants, the chlorophyll a levels fluctuated from 1.9 to 100.3 mg m−3 during the culture cycle. Qualitative and quantitative phytoplankton analyses were performed weekly. A total of 35 taxa belonging to three different algal classes were observed. In the cropping tanks, the phytoplankton abundance was low in the first five weeks (12.3 to 22.7 × 107 cell L−1) and peaked at six to eight weeks from 27.8 to 32.6 × 107 cell L−1. The abundance remained low throughout the remainder of the cycle (less than 13.8 × 107 cell L−1). In the outputs of the tomato module systems, the phytoplankton abundance was very low and did not reach more than 37.2 × 107 cell L−1. The general group composition consisted of Chlorophyta (87 to 98% of relative abundance), Bacilliariophyta (2 to 9%) and Cyanophyta (0–3%) in the culture tanks. In the effluent, the following change in the composition was observed: Chlorophyta (63 to 93%), Bacillariophyta (5 to 26%) and Cyanophyta (2–12%). The genera more representative of the phytoplankton in the tanks were Coelastrum (73%) in tank 1 and Golenkinia (56 and 71% in tanks 2 and 3, respectively), whereas the genera in the effluent from the tomato module were Scenedesmus and Coelastrum.
DISCUSSION
Considering the duration of the culture cycle (19 weeks), the mean harvest (3932 kg ha−1) and the FC (1.6) obtained in the STCS, these are comparable to those obtained from commercial monoculture of shrimp ponds in NW Mexico (Lyle-Fritch et al., Reference Lyle-Fritch, Romero-Beltrán and Páez-Osuna2006). The mean tomato production obtained by irrigating with shrimp effluent, the nutritive solution and groundwater, were equivalent to 36.1, 38.7 and 27.6 ton ha−1, respectively. When these three harvests are compared with outfield commercial agricultural systems from the NW Mexico (27.2–48.7 ton ha−1), our results are intermediate but obtained without fertilizers and pesticides.
A plausible explanation of the relatively high yield of the tomato irrigated with groundwater is that part of nutrients was justly provided by the groundwater and partially by zeolite. Although that zeolite had a low concentration of nutrients (0.03% N and 0.295 mg P/g), this could to be potentially available for the plants. Considering 180–200 L of groundwater (15.4 mg N/L and 0.02 mg P/L) per plant used during the irrigation, this provide about 71–100% of nitrogen required by one complete plant (Brito-Solano, Reference Brito-Solano2010). Therefore, the main fraction was derived from groundwater though we cannot rule out a small fraction from the zeolite.
Due to the increasing demand to produce more per unit volume of water, the quantification of water requirement is a topic of great importance. Till date, no work has been developed on water productivity, water quality and quantification of requirement for grow-out culture of white shrimp (L. vannamei) coupled to tomato. Aquacultural water productivity is an index of the value of water used (Boyd, Reference Boyd2005), and an indicator of efficient water management used to define the relationship between crop produced and the amount of water involved in crop production (Ali and Talukder, Reference Ali and Talukder2008). Here are involved two species (shrimp and tomato); consequently, an adaptation of such a definition is necessary. Here was used this same relationship combining the two harvests and the amount of water involved in the two crops, explaining the proportion of the two harvests reached. The term water usage considering the two products implies how best we can improve the outcome or yield of one or more crops with the water currently in use.
Depending on the management, evaporation, precipitation and the duration of the culture cycles, the amount of water consumed varies in shrimp farms. During the 19-week trial for this study, a smaller amount of water was used (to fill and maintain levels) in comparison with traditional shrimp commercial farms of the NW region of Mexico, which consume from 17 to 113 m3 kg−1 shrimp harvested (Lyle-Fritch et al., Reference Lyle-Fritch, Romero-Beltrán and Páez-Osuna2006). The range for shrimp culture in brackish, marine and hypersaline waters worldwide is 100–200 m3 per kg of shrimp (Hopkins and Villalón, Reference Hopkins, Villalón and Wiban1992). A total of 4.7 m3 per kg of shrimp harvested was consumed in this study, and the water usage considering both products (shrimp and tomato) was 2.1 m3 per kg (0.47 kg of shrimp + 0.53 kg tomatoes) (Table 2).
With the exception of nitrates, the values of the water quality variables found in this study were generally comparable to those found in previous studies of shrimp farming in commercial farms in the northwestern region of Mexico (Casillas-Hernández et al., Reference Casillas-Hernández, Nolasco-Soria, Garcia-Galano, Carrillo-Farnes and Páez-Osuna2007; Lyle-Fritch et al., Reference Lyle-Fritch, Romero-Beltrán and Páez-Osuna2006). Some variables exhibited levels that suggested caution for the shrimp culture. The pH level in the shrimp tanks fluctuated between 7.7 and 9.6. Boyd (Reference Boyd1989) indicated that an adequate pH interval for better growth and for the highest conversion of food is from 6 to 9. The highest values could be critical in combination with total ammonia. However, the levels of total N-ammonia found during the 19 weeks of culture were below 0.85 mg L−1. Conversely, high pH values (>7) result in nutrients (macro and micro) being captured for the plants in smaller percentages causing low growth and reduced production (Samperio-Ruiz, Reference Samperio-Ruiz2005).
The N-nitrite levels were relatively low (<0.14 mg L−1); however, during the first 5 weeks, these reached concentrations of up to 1.45 mg L−1 in tank 1, which could be a cause for concern. However, such levels are very low in comparison to the median lethal concentration for penaeids (Páez-Osuna, Reference Páez-Osuna2001). Nitrate levels in the shrimp tanks were relatively elevated (5.2–172.2 mg N-NO3− L−1) clearly associated with the fertilization. These levels are more elevated than those found in commercial shrimp farms from the NW region of Mexico (Lyle-Fritch et al., Reference Lyle-Fritch, Romero-Beltrán and Páez-Osuna2006). Such levels are also high compared with those for shrimp farms in which toxicity in shrimp have been documented; levels of 10–100 mg L−1 NO3− affect the hypodermis and the digestive gland of the Penaeus monodon protozoea (Páez-Osuna, Reference Páez-Osuna2001). From these results, it is likely that the nitrate levels observed here resulted in sublethal damage in L. vannamei; however, such effects in this species require further investigation. The ammonia levels observed in this study in the shrimp tanks (<0.001–0.85 mg N-NH3 L−1) were lower or comparable to those reported for the L. vannamei cultivated in saline (Casillas-Hernández et al., Reference Casillas-Hernández, Nolasco-Soria, Garcia-Galano, Carrillo-Farnes and Páez-Osuna2007; Lyle-Fritch, Reference Lyle-Fritch, Romero-Beltrán and Páez-Osuna2006), low-salinity (Roy et al., Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010) and river waters (Miranda et al., Reference Miranda, Lima, Crisóstomo and Santana2008). The essential reason for these low values of ammonia in our culture is related to the permanent aeration maintained throughout the culture period.
The groundwater from Hermosillo, like that from other regions worldwide (Boyd and Thunjai, Reference Boyd and Thunjai2003) was low in magnesium and chloride and high in calcium relative to the concentrations expected in seawater diluted to the same salinity (Table 1). L. vannamei is native to coastal waters (brackish waters) in which the proportion of major ions resemble those of normal seawater. It has been established that L. vannamei can survive and grow well at salinities as low as 1 g L−1. However, the exact requirements for individual ions are not known exactly (Roy et al., Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010). With the addition of MgNO3, the levels of Mg were mitigated and approached those of seawater. The optimum concentrations of major ions, the alkalinity and the ratios of Na:K, Ca:K and Mg:Ca in the salinity of the water used for the trial should theoretically produce the best growth and the greatest harvest. However, this study used groundwater with the addition of two supplementary salts, with levels of magnesium, potassium and sodium reaching close to the optimum. Calcium was in excess of the theoretical levels for the initial salinity (Table 1). The alkalinity (as CaCO3) observed in this study was equivalent to the recommended level (total alkalinity of 75 mg L−1) (Roy et al., Reference Roy, Davis, Saoud, Boyd, Pine and Boyd2010). Appropriate values of alkalinity and calcium are required to allow the exoskeleton to develop properly and to promote adequate growth and survival (McGraw and Scarpa, Reference McGraw and Scarpa2003). The Na:K ratio observed and that considered to be optimal were somewhat different, in that the Ca:K ratio in this shrimp culture was slightly high and the Mg:Ca ratio was low relative to those assumed to be optimal. This scenario suggests that the levels of individual major ions and the ratios cited may be related to the decreased survival and relatively low growth rates. Conversely, the high levels of nitrates observed in this study may also contribute, or such survival may result from a combination of both factors. However, a more relevant conclusion is difficult to establish from these results.
In the three tanks, Chlorophyta (or green algae) was the dominant form of algae during most of the culture cycle; these algae are considered non–toxic, but they have a lower nutritional value for shrimp culture systems (Boyd and Gautier, Reference Boyd and Gautier2000). The diatom was the second group in abundance. Bacillariophyta (diatoms) have been reported to be beneficial algae in shrimp culture ponds because they form large floc aggregates that can be ingested by shrimp (Boyd, Reference Boyd1989). The Cyanophyta (cyanobacteria or blue-green algae) species were present at the beginning and end of the growing season in the tanks and in the first half of the cycle in the effluent; some species are nitrogen fixers and toxin producers, although other species improve growth, protein digestibility, carcass quality and early maturation (Ju et al., Reference Ju, Forster and Dominy2009).
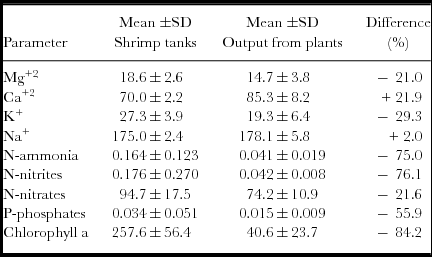
Regarding the concentrations of nutrients found in the waters of the shrimp tanks and the output from the module of tomato plants (Table 3), it is evident that for these tanks (31.1 m3), it is necessary to integrate a major area of tomato plants. Typically, in the NW region of Mexico, tomato production requires fertilization at a rate between 325 and 370 kg/ha for nitrogen to optimize production under outfield conditions in soil culture (13,000 tomato plants per ha). The mean concentrations of dissolved nitrogen measured as the fraction available for tomato plants, i.e., the dissolved inorganic nitrogen (DIN) was an average of 95.0 mg L−1 throughout the cycle in the three tanks. Assuming that it is necessary to maintain a concentration of 0.55–1.0 μg atom N L−1 for the sustainability of phytoplankton in shrimp tanks (half-saturation constant for tropical algae) (Valiela, Reference Valiela1995), the DIN required by phytoplankton in tanks is very low in comparison to the stock of DIN available in the shrimp tanks. Therefore, the DIN that is potentially available (final water effluent of shrimp tanks: 31.1 m3/tank) for the tomato plants is 104.5 g DIN m−2 of the shrimp tank. Under a traditional outfield soil culture system, the DIN of one shrimp tank can be utilized to fertilize 104–118 plants, i.e., 1 ha of a shrimp tank for 2.8–3.2 ha of tomato plants.
Table 3. Mean (± 1 s. d.) of water quality parameters (in mg L−1 except chlorophyll a in mg m−3) of shrimp tanks and output water from tomato plants module.
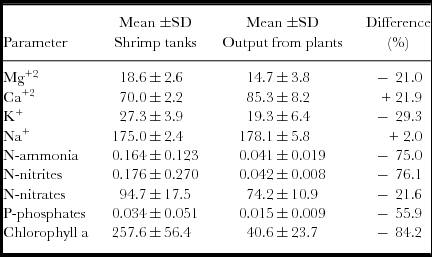
+Increase; − Decrease.
Alternatively, for the scenario studied here (4.9 plants/m2 in one plant per 3.1 kg of zeolite), 13.9 g N per plant are presumably required if the environmental losses (9.0 g N per plant coupled to shrimp tank) and the nitrogen retained per zeolite (0.475 g N per kg of zeolite) are considered. The available DIN of 1 m2 of shrimp tank can be used to fertilize 1.5 m2 of tomato plants, i.e., 1 ha of a shrimp farm can be coupled to 1.5 ha of tomato plants. From such calculations, considering the number of plants cultured in a zeolite substratum, one shrimp tank may be used to fertilize 213 plants. As expected, these calculations demonstrate that the soil-less culture (with a zeolite substratum) of tomato coupled to shrimp culture is more efficient in the use of nitrogen.
CONCLUSIONS
From this work, it is evident that the culture of shrimp-tomato is feasible, and that this system (STCS) could harvest more than 3.9 ton/ha of shrimp and 36 ton/ha of tomato, with a relatively low water consumption (≤2.1 m3 per kg harvested of both products). In general, the phytoplankton and components of water quality represented by nutrients, major ions and dissolved oxygen remained stable and within a range moderately acceptable throughout the trial. However, the relatively low weight gain and survival in shrimp can be attributed to a combination of ionic effects and those provoked by the relatively elevated levels of nitrates. From these results, it is clear that the integrated culture shrimp-tomato system have several advantages: (a) optimization of water usage; (b) efficiency in the use of nutrients; (c) elimination of chemical fertilization for the tomato plants; and (d) reduction or elimination of the impact of discharge of effluents with nutrients and salts. Future research on the optimal enforcement of the integrated shrimp-tomato system should consider: (a) the precise concentrations and ratios of major ions; (b) an examination of what species of phytoplankton from those found here constitute an adequate source of complementary food for the optimal growth of shrimp; and (c) more precise definition of the optimum area of tomato plants coupled with the number of shrimp (stocking density).
Acknowledgements
This project 26-2006-1031 was supported by Fundación Produce Sonora. We thank H. Bojórquez-Leyva, C. Ramírez-Jáuregui and G. Ramírez-Reséndiz for their collaboration in the field, chemical analysis, bibliographic services and statistical support. To hatchery Maricultura del Pacífico, who provide the postlarvae for the trial. This research forms part of the Ph. D. dissertation of MMML at Universidad de Occidente.