Introduction
The potential for many plant species to respond to climate change and other disturbances depends heavily on seeds. Seeds enable plants to regenerate and re-establish either in their current site or elsewhere and also to disperse germination over time. Seeds can persist in the soil for thousands of years (Shen-Miller et al., Reference Shen-Miller, Mudgett, Schopf, Clarke and Berger1995; Sallon et al., Reference Sallon, Solowey, Cohen, Korchinsky, Egli, Woodhatch, Simchoni and Kislev2008; Yashina et al., Reference Yashina, Gubin, Maksimovich, Yashina, Gakhova and Gilichinsky2012) and this dispersal over time assists plants to spread the risk of recruitment failure (Thompson, Reference Thompson2000; Ooi et al., Reference Ooi, Auld and Denham2009). This realized persistence in situ is a result of intrinsic seed longevity in concert with other biotic and abiotic factors (e.g. microclimatic conditions, granivory, disturbance). Measures of seed longevity therefore describe the potential lifespan of a mature seed underlain by complex interactions of physiological and physical traits (Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015).
Understanding variation in and drivers of longevity is important for effective ex situ seed banking because it underpins the selection of viability re-test intervals and re-collection strategies (Probert et al., Reference Probert, Daws and Hay2009). Direct measures of seed longevity and persistence, however, are hard to obtain in real time as it may take decades or even centuries (Walters et al., Reference Walters, Wheeler and Grotenhuis2005). The p 50 value, which is the time taken for seed viability to decline to 50% in artificial ageing, is now a well-established proxy for seed longevity in storage (Newton et al., Reference Newton, Hay and Probert2009) and in particular in conservation many studies have been undertaken at 45°C and 60% relative humidity (RH) to allow comparative ranking of species (Probert et al., Reference Probert, Daws and Hay2009; Merrit et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). P 50 serves as an index, although an approximate storage lifespan/longevity can be calculated using viability equations (if storage conditions and species constants are available) (http://data.kew.org/sid/viability/). A p 50 value might also be somewhat related to persistence in situ, for example Long et al. (Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008) demonstrate that a p 50 of 20 days in their study species and conditions is likely to indicate persistence of several seasons in situ. However, multiple factors can interact with longevity to affect persistence in situ (see Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). The controlled accelerated ageing test is a valuable technique because by applying a standard set of protocols it yields comparable results for understanding relative longevity across multiple species and biomes.
The longest-lived seeds tested thus far are Australian, with a maximum p 50 of 771 days and an average of 202 days under accelerated ageing conditions, which is much greater than longevity of seed from other biomes and regions (Probert et al., Reference Probert, Daws and Hay2009). Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014) confirm the relatively long lifespan of Australian species with an investigation of 172 species, which shows that p 50 ranges from 3 up to 588 days revealing that athough high on average, not all Australian species have long-lived seeds. Alpine seeds in general are known to be short-lived. Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010) report a mean p 50 of 20.5 days across 36 Italian alpine species. From the 36 species, six accessions were compared with lowland accessions of the same species and alpine seed were significantly shorter-lived than the lowland pairs, with mean p 50 values of 28.2 and 56.1, respectively (Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010).
Previous studies (Probert et al., Reference Probert, Daws and Hay2009; Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014; Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015) show that p 50 is associated with other seed or plant traits including seed mass, dormancy, embryo type, endospermy and life-form, and with environmental variables such as elevation and climatic variables. Maternal conditions can also affect seed longevity (Kochanek et al., Reference Kochanek, Buckley, Probert, Adkins and Steadman2010, Reference Kochanek, Steadman, Probert and Adkins2011; He et al., Reference He, de Souza Vidigal, Snoek, Schnabel, Nijveen, Hilhorst and Bentsink2014; Righetti et al., Reference Righetti, Vu, Pelletier, Vu, Glaab, Lalanne, Pasha, Patel, Provart, Verdier, Leprince and Buitink2015; Bernareggi et al., Reference Bernareggi, Carbognani, Mondoni and Petraglia2016). However, correlations or significant factors determining seed longevity vary depending on the species, biomes, the scale of study, as well as the combinations of variables included. Indeed, the only consistent pattern found multiple times across these studies is that non-endospermic seeds tend to be longer-lived. From a plant evolutionary biology perspective, endospermy, seed mass, embryo, dormancy type (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006), and the environment of origin (Feild and Arens, Reference Feild and Arens2005) would be expected to be related to longevity, but intriguingly, the variation of p 50 often seems to be independent of these traits and to change across environments and biomes. P 50 might also be expected to be correlated with phylogeny, as more closely related species could share mechanisms of longevity (Walters et al., Reference Walters, Wheeler and Grotenhuis2005; Probert et al., Reference Probert, Daws and Hay2009; Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). Lastly, given the significance of regeneration by seed for the persistence of species, p 50 might reflect differences in germination strategy but this is yet to be investigated. Thus in a search of predictors of p 50, one must consider a broad range of seed, plant and environmental traits.
Alpine ecosystems and alpine plant communities worldwide have been identified as particularly vulnerable to global warming (Coyne, Reference Coyne2001; Parolo and Rossi, Reference Parolo and Rossi2008; Thuiller et al., Reference Thuiller, Albert, Araújo, Berry, Cabeza, Guisan, Hickler, Midgley, Paterson, Schurr, Sykes and Zimmermann2008). Local extinction of plant taxa formerly restricted to high elevations has already been reported (IPCC, Reference Houghton, Ding, Griggs, Noguer, van den Linden, Dai, Maskell and Johnson2001). In Australia, the alpine region is distributed across just 0.15% of the continent's landmass but because of its high level of endemism is recognized as an alpine biodiversity hotspot, and is distinctive in many ways in relation to mountain areas worldwide (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000; Kirkpatrick, Reference Kirkpatrick2002; Hopper and Gioia, Reference Hopper and Gioia2004). The Australian Alps region maintains an assemblage of vegetation communities found nowhere else in the world (Costin et al., Reference Costin, Wimbush and Kirkpatrick2002), harbouring unique ecological communities (Department of Environment, 2015) and 212 plant species that include 30 exclusively alpine and 21 species endemic to Mt Kosciuszko (Costin et al., Reference Costin, Gray, Totterdell and Wimbush2000). In the face of climate change, the limited capacity of alpine plants to migrate to higher elevations may increase their extinction risk.
To ensure meaningful ex situ conservation of Australian alpine seeds, seed conservation programmes must be underpinned by data on seed longevity. This study aims to investigate whether Australian alpine seeds perform like other alpine species and are short-lived, or are more like other Australian species and are relatively long-lived. Secondly, we aim to understand important variables that underlie the variation of p 50 for Australian alpine seeds. Because previous studies have shown that correlations between p 50 and seed or plant traits and environmental variables vary, we took a comprehensive approach, taking account of as many plausible intrinsic and extrinsic variables as we could. In addition, ours is the first study to examine potential associations between germination strategy and p 50. The germination strategy of Australian alpine plants is associated with seed mass and endospermy (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015), hence germination strategy (or the associated traits mass and endospermy) could be expected to be related to longevity as well. We set out to determine the relative importance of both seed/plant traits and environmental variables in explaining variation in p 50 values, and predicted that Australian alpine seeds would be short-lived (mean p 50 values ~20 days) like alpine seeds elsewhere.
Materials and methods
Study species
Species of Australian native plants that occur predominantly in the alpine bioregion on the mainland (from 1330–2228 m elevation), and in any alpine vegetation communities, were selected for this study. The Australian Alps, based on the bioregion's physiographic elements, can be categorized as alpine (>1850 m), subalpine (1400–1850 m), montane (1100–1400 m), and tableland (<1100 m) (NSW NPWS, 1988). In this study, we considered alpine broadly, therefore seeds that were collected at elevations above 1330 m were termed alpine. Most seeds were collected from Kosciuszko National Park between 2007 and 2013, and were retrieved from the National Seed Bank, Australian National Botanic Gardens (ANBG) Canberra, Australia. These seeds were stored according to international gene bank standards at approximately –20°C after drying at 15°C and 15% RH (Table 1, supplementary Fig. 1).
Table 1. Details of 56 species analysed for their seed longevity (p 50) and the correlates used in the analysis

Further details, including geographic coordinates, dates of collection, germination treatment and categorization of germination strategy is presented in supplementary Table S1.
Germination assays
Assays were carried out to determine whether a species had high enough germination (>75% viability adjusted) to be included in the controlled ageing tests. The assays were also used to determine the species germination strategy. Germination of 121 accessions of 96 species was investigated following the methodology published by Hoyle et al. (Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). Species known to have immediate germination (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015) were germinated on 1% water agar at 25/15°C. Species identified as postponed, staggered, or not listed in Hoyle et al. (Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015) were germinated on both 1% water agar and 1% water agar infused with gibberrelic acid (GA3) to achieve a final concentration of 200 mg l–1 GA3 at 25/15°C. If germination was low in both agar and GA3 assays under summer temperatures (25/15°C) then stratification was applied to mimic the natural progression of seasons. For the stratification steps, a further three replicates of 25 seeds each were exposed to 6 weeks at 25/15°C, followed by 8 weeks at 5/5°C, and returned to 25/15°C for at least 6 weeks, to mimic seasonal change in the field (see Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). These temperatures mimic in situ soil temperatures and the main seasonal changes alpine seeds are exposed to, and respond to, in Australia (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015).
Germination assays were also used to determine the germination substrate and dormancy alleviation methods. Following controlled seed ageing, each species was germinated either on water agar, water agar with GA3 infusion, or with stratification. The selection was based on the substrate/treatment that gave the highest germination percentage for each species. For staggered and postponed strategies, if GA3 or stratification increased germination compared with germination on water agar then the most successful treatment was selected to be applied to that species throughout the ageing experiment (supplementary Table S1). If GA3 and stratification produced similar results then stratification was selected. At the end of the germination assay, we performed cut-tests to determine seed fill and death for ungerminated seeds. There were 57 species that had germination >75% and thus were amenable to Probit analyses (see below) and were included in the controlled ageing test (Table 1, supplementary Table S1).
Controlled ageing test
Seed longevity (represented by a p 50 value) is determined using a controlled artificial ageing test (also called a standard rapid ageing protocol; Newton et al., Reference Newton, Hay and Probert2009; Probert et al., Reference Probert, Daws and Hay2009). The basic principle of the controlled ageing protocol is to expose seeds to warm and humid conditions in order to decrease seed viability rapidly. The controlled artificial ageing test consists of two steps: rehydration and ageing. To first hydrate the seeds, 11 samples of 50 seeds for each species were placed in open glass vials for 14 days in air-tight polycarbonate electrical enclosure boxes (28 × 28 × 14 cm; NHP Fibox, Australia) above a non-saturated solution of LiCl (370 g l–1, anhydrous; Sigma, Australia) in an illuminated incubator (TRIL 396-1-SD; Thermoline Scientific, NSW, Australia), creating a RH of 47% at 20°C (Hay et al., Reference Hay, Adams, Manger and Probert2008). Overall, the design consisted of six boxes of seed vials. Each box contained a stratified random sample of multiple species with representatives of each of the different germination strategies. The 11 vials of a given species were kept in a single box.
To ensure seeds had equilibrated to 47% RH by the end of seed rehydration period their relative humidity was checked using a reference sample of Eucalyptus macrorhyncha seeds that accompanied the study species (supplementary Table S2). Unlike the study species, E. macrorhyncha seeds are relatively large and were available in sufficient quantity to fill in a hygrometer chamber for measurement of humidity. The seed equilibrium relative humidity was measured using a water activity measuring instrument that consisted of a hygrometer sensor housed in an AW VC-DI0 water activity probe, used in conjunction with a HygroPalm PD 2 dock-station display unit (Rotronic, Bassersdorf, Switzerland).
Following rehydration, the solution in the polycarbonate electrical enclosure boxes was replaced with a non-saturated solution of LiCl (this time 300 g l–1) at 45°C, which results in 60% RH in the incubator (Oven model TO-500 F, Thermoline Scientific) for seed ageing. The relative humidity was checked at 6-week intervals using a combined temperature and humidity data logger (T-TEC 7-1C, Temperature Technology, Adelaide, Australia) and the bulk solution adjusted as necessary to maintain the 60% RH, by adding distilled water (Hay et al., Reference Hay, Adams, Manger and Probert2008).
One vial of 50 seeds for each species was removed for germination testing after each of 11 times (1, 7, 14, 21, 28, 35, 42, 50, 75, 156 and 250 days). Seeds were sown on agar in 90 mm diameter Petri dishes and placed in an incubator (TRIL 396-1-SD, Thermoline Scientific) under the germination treatment previously demonstrated in the germination assays to be suitable for germination of each species (supplementary Table S1). Species that required stratification to germinate were subjected to stratification steps following removal from the electrical boxes. Germination was checked weekly and seeds scored as germinated once the radicle was visible, approximately >1 mm. Observation for each plate was considered completed when there were no further germinants for 6 weeks.
To ensure that conditions were homogenous across boxes over the course of the experiment we placed seeds of Ficinia nodosa in each box and retrieved and germinated them at the same ageing time as the study species (supplementary Table S2). The variation of p 50 values of the Ficinia nodosa were small across boxes (mean p 50 = 60.61 ± 3.56 days). To ensure the experimental procedures were comparable to other longevity studies we included seeds from four of the same accessions used in the Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014) longevity study: Brachyscome tenuiscapa var. pubescens, Poa hiemata, Streptoglossa macrocephala and Wurmbeia dioica (supplementary Table S2). There were no significant differences in mean p 50 values for these species (paired t-test, d.f. = 3, P = 0.442), indicating that the conditions and thus results of the two studies, are comparable.
Potential correlates of seed longevity
Seed and plant traits and environmental data were gathered for the 57 study species (Table 1, supplementary Table S1). The plant and seed trait variables were: seed mass, embryo type, endosperm presence, germination strategy and plant life-form. Environmental data were: long-term mean annual temperature, temperature seasonality, mean annual precipitation and mean radiation. We also assessed elevation of collection site, average elevation for the species, and species elevation range. Specifics of each variable are discussed below.
Seed mass was measured on three replicates of 50 seeds per species, and average individual seed mass was calculated from this. Embryo type and endosperm presence were determined by dissecting the seeds, assessing presence/absence of endosperm, and categorizing embryo type. There were 38 species with endospermic seed and 18 species with non-endospermic seed. Embryo type was categorized as axile (31 species), basal (22 species), or peripheral (3 species) following the classification system of Martin (Reference Martin1946).
Previously, three germination strategies (immediate, staggered and postponed) were identified among Australian alpine species (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). Germination strategy represents germination timing and pattern over time and seasons (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). Based on the germination results we categorize species as having a particular germination strategy under the conditions tested (see germination assays above). Non-dormant seeds germinate immediately once the germination requirement is met. Seeds with staggered and postponed strategies require certain dormancy alleviation cues (e.g. cold stratification) and the presence of favourable germination conditions before germination will occur. Staggered and postponed strategies differ in the timing of germination. Species with a staggered strategy have seeds that germinate both before and after winter, whereas seeds of postponed species germinate almost entirely after winter. Of the 57 species, 28 species were also tested in Hoyle et al. (Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015) and almost all of these exhibited the same germination strategy as in our assays. Ranunculus acrophilus and Wahlenbergia ceracea were exceptions to this (details in supplementary Table S1). In total, 24 species were categorized as immediate, 15 species as staggered, and 17 species as having a postponed strategy. Details on the germination strategy categorization of the 57 species can be found in supplementary Table S1. Finally, plant life-form was categorized as monocotyledonous herbs (14 species), other herbs (36 species), and woody shrubs (6 species) to follow the plant functional types described by Costin (Reference Costin, Gray, Totterdell and Wimbush2000) for the alpine flora (Table 1, supplementary Table S1).
For seed collection and environmental variables, we retrieved the collection elevation data from the ANBG living collection database. Mean, minimum and maximum elevation of the species in Australia were gathered from the Australian National Herbarium Specimen Information Register (http://www.anbg.gov.au/cgi-bin/anhsir) or from Australia's Virtual Herbarium (http://avh.ala.org.au) to determine the elevation range spanned by each species. Mean temperature, temperature seasonality, precipitation and mean radiation were bioclimatic values estimated from interpolation of long-term climate data, 75 years of data for temperature and precipitation, and 25 years for solar radiation, at a resolution of 0.01 degrees, and were imported from the Atlas of Living Australia (http://spatial.ala.org.au).
Data analyses
Probit analysis of seed longevity
Probit analysis was carried out on the germination data for the study species as a function of ageing days using GenStat Release 17.1 (VSN International Ltd, Oxford, UK). P 50 was estimated by fitting a viability equations (Ellis and Robert, Reference Ellis and Roberts1980):
where v is the viability in normal equivalent deviates (NED) at time p days in storage, K i is the estimated initial viability, and σ is the standard deviation of the normal distribution of seed deaths over time.
Drivers of seed longevity
First, we checked for collinearity among explanatory variables using correlation tests, ANOVAs or χ2 tests, depending on the nature of the data (supplementary Table S3). Some variables were very strongly correlated (r > 0.85; supplementary Table S3), so we kept only one from each group. We kept collection elevation and elevation range but removed mean elevation. We kept mean temperature and temperature seasonality but removed mean precipitation and radiation. Finally, we kept endospermy type but removed embryo type and life form. These selections were made because mean annual temperature has previously been shown to be positively related to p 50 (Probert et al., Reference Probert, Daws and Hay2009), and longer-lived seeds to be non-endospermic (Probert et al., Reference Probert, Daws and Hay2009; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). After removing those variables, we performed preliminary explorations with all-subset regressions and found that seed mass and collection elevation were consistently significant predictors of p 50 (results not shown). We also tested family and genus as fixed factors and confirmed that the variation in p 50 was not related to family (P = 0.13) or genus (P = 0.37). Similarly, family and genus did not explain much variation of p 50 when assigned as random factor (supplementary Table S4).
To formally test which explanatory variable(s) best explained p 50, we performed model selection based on the goodness-of-fit of the data quantified by the corrected or second order Akaike Information Criterion (AICc) (Burnham and Anderson, Reference Burnham and Anderson2003; Johnson and Omland, Reference Johnson and Omland2004; Bolker et al., Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009; Grueber et al., Reference Grueber, Nakagawa, Laws and Jamieson2011) in R 3.2.4 (R Core Team, 2016). All continuous and binary explanatory variables were standardized to facilitate model convergence and comparison of effect sizes between models (Gelman, Reference Gelman2008; Merkling et al., Reference Merkling, Blanchard, Chastel, Glauser, Vallat-Michel, Hatch, Danchin and Helfenstein2017). Standardization was achieved by subtracting the mean of the all data points and dividing by 2 standard deviations (Gelman, Reference Gelman2008). Standardization does not influence the correlations of variables with the response variable and is undertaken to facilitate comparisons between the effects of variables that are on different scales. The p 50 values were log-transformed in all analyses to meet model assumptions. Following our data exploration (above), we also included the interaction term between seed mass and each of the two elevation variables in our list of models (supplementary Table S5). To avoid over-fitting and to limit the number of models in our list, each model contained a maximum of two explanatory variables and we did not consider models containing weakly correlated explanatory variables (supplementary Table S5).
We did not have access to a phylogeny of the 56 species considered, so we accounted for the effect of relatedness by running linear mixed models with family or genus as a random effect. There were no significant effects of the random terms, therefore showing that phylogeny explained a negligible amount of the variance in p 50 (supplementary Table S5). Although we could probably have discarded the random effects and run linear models (supplementary Table S5), we decided to retain genus as a random effect to get more conservative estimates of the factors influencing p 50. Genus was selected as random because the AICcs were smaller compared with when we used family, or genus nested in family, as the random factors (supplementary Table S5).
Model selection was carried out using the lme4 package (Bates et al., Reference Bates, Mächler, Bolker and Walker2015; R Core Team, 2016). For each model, we calculated its AICc and its AICc difference (ΔAICc). ΔAICc provides a measure of information lost between the best model and a given model of a candidate set. We selected models with ΔAICc < 5 following Bolker et al. (Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009) and calculated a weight (ωAICc) for each model using the AICcmodavg package (Mazerolle, Reference Mazerolle2016) based on maximum likelihood estimation (supplementary Table S6). The calculated weight (ωAICc) represents the probability that a given model is the best of the subset of models. We then computed model-averaged parameter estimates, standard errors and confidence intervals to take model selection uncertainty into account.
Comparisons of seed longevity within Australia and between alpine regions
In order to determine whether Australian alpine longevity is similar to other alpine seeds or more similar to the seed longevity of Australian plants, we did two comparisons: Australian alpine versus alpine elsewhere (European species), and Australian alpine versus Australian non-alpine. To enable international and continental scale comparisons we selected families that were shared across the different studies. We compared the p 50 for the available paired families, i.e. families that were included in the present study as well as in the Italian alpine study of Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010). Likewise, we paired among families of Australian non-alpine species using data from Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). The Italian alpine p 50 values were selected from those species specifically indicated as alpine in Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010). For Australian seed longevity comparison we excluded five species from the Merritt study (Brachyscome tenuiscapa subsp. pubescens, Epilobium gunnianum, Poa hiemata, Podolepis robusta and Xerochrysum subundulatum) to ensure the analysed data were exclusively non-alpine accessions (collected at elevations <1300 m) (Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). We used linear mixed models, assigning p 50 as a response variable, the region of origin (either alpine versus non-alpine in Australia, or Australian alpine versus Italian alpine) as an explanatory variable, and paired family (six families for Australian alpine versus Italian alpine and 10 families for Australian alpine versus non-alpine comparisons) to control for potential effects of relatedness among species.
Results
Australian alpine seed longevity
Across the 57 species considered, all but one (Melaleuca pityoides) declined in viability such that p 50 could be calculated (supplementary Table S1). Hereafter, we will discuss the 56 species for which p 50 could be calculated. Our index of longevity (p 50) ranged from 2.28 days for Gingidia algens (Apiaceae) to 81.59 days for Epacris celata (Ericaceae), with a mean of 19.8 ± 15.5 days. Nearly 60% of the species tested had seeds with p 50 values <13.7 days (<13.7 days = short-lived; Newton, Reference Newton, Hay and Probert2009, Probert et al., Reference Probert, Daws and Hay2009), and so can be considered to be relatively short-lived. Four species had p 50 > 50 days, relatively long lived compared with other floras. The seeds of seven of the nine Kosciuszko alpine zone endemic plants tested were short-lived (p 50 values <13.7 days), whereas Ranunculus dissectifolius, Brachyscome stolonifera and Craspedia costiniana had relatively intermediate p 50 values of 17.17, 24.3 and 34.7 days, respectively.
Among Australian alpine species, the variation of seed longevity among species and across families was large (Fig. 1). Sometimes, the variation within a single genus was larger than variation between genera within a family (Fig. 2). For example, within Ericaceae, the p 50 values of two Epacris species differed by 59.19 days (Epacris celata and E. paludosa; Fig. 2). Among the ecologically dominant Poaceae species we found most were short- to intermediate-lived (seven species), with the exception of one, Poa phillipsiana. Apiaceae (five of six species short-lived, one intermediate) and Ranunculaceae (five of seven species short-lived, two intermediate) were shown to have consistently short-lived seeds compared with other Australian alpine families (Fig. 2). Conversely, Myrtaceae had relatively intermediate- to long-lived seeds (p 50 45.34 and 27.08 for Baeckea gunniana and B. utilis, respectively); germination of Melaleuca pityoides did not decline sufficiently over the 250 days of ageing time and therefore we could not calculate a p 50 value for this species (supplementary Table S1).
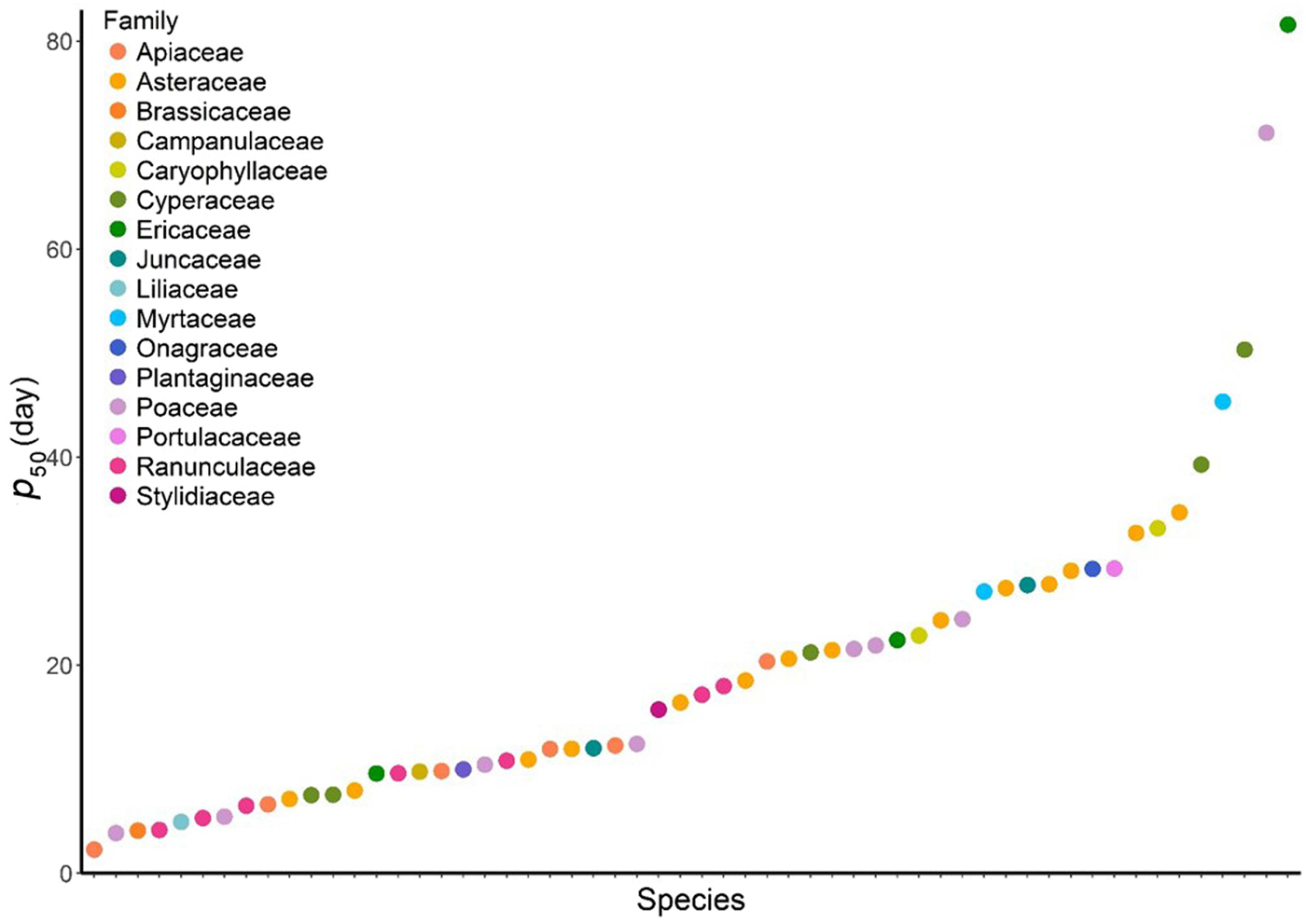
Figure 1. Variation in seed longevity index (p 50) across 56 Australian alpine species from 16 families. Each dot represents one species, colours indicate family.
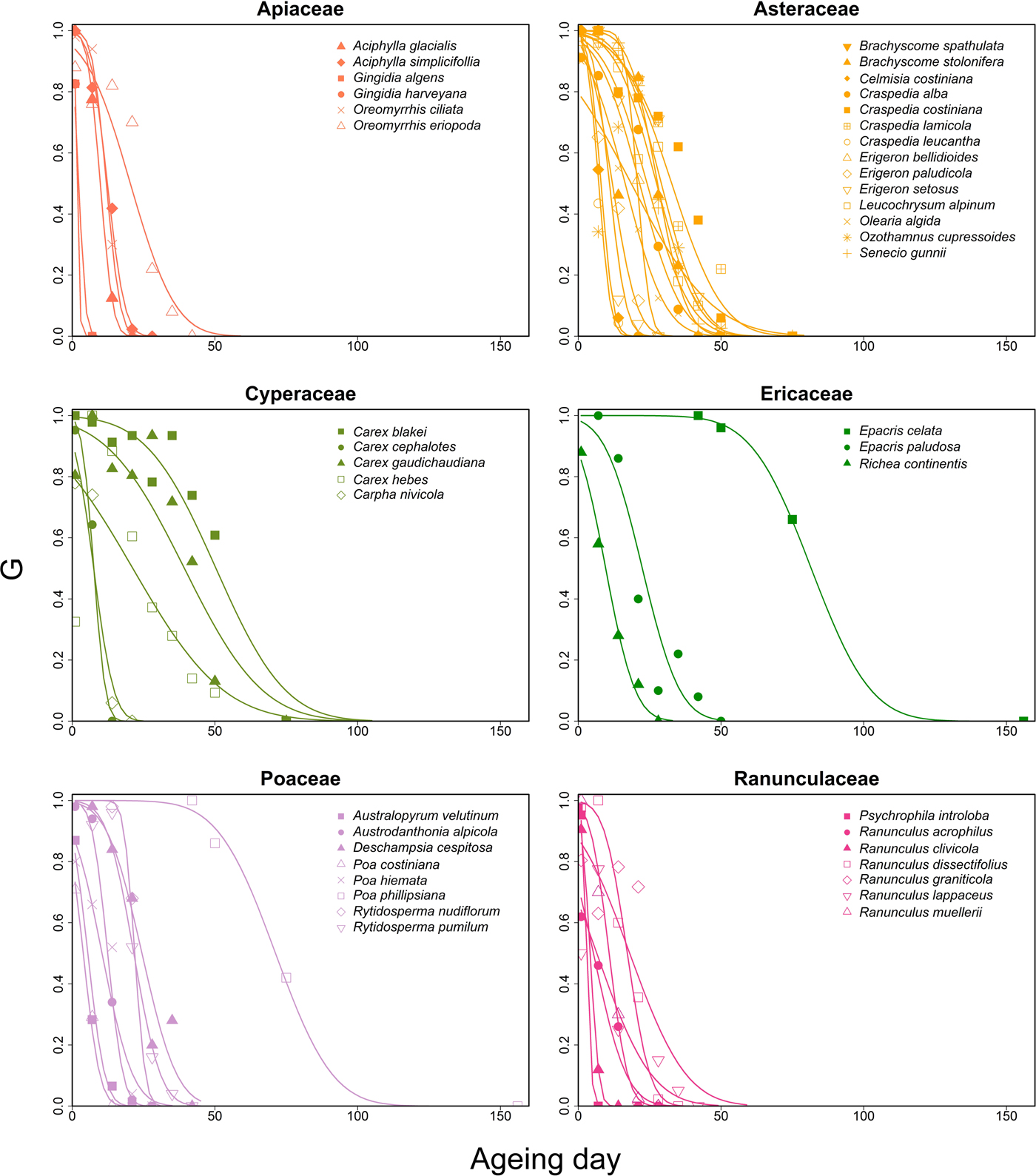
Figure 2. Seed longevity varies among families and among genera within a family. G (y-axis) is germination proportion at an ageing day (x-axis). For every species (each represented by a different line), p 50 is the day when G was 0.5. Families shown here are those for which more than two species were included in our study, family colour-matched to Fig. 1.
Correlates of Australian alpine seed longevity
Seed mass and elevation were the only two of the ten variables tested in this study that were in all the top-ranked models of our model list and therefore explained most of the variance of p 50 for the Australian alpine species considered (supplementary Table S6). Across species, seeds with lower seed mass were longer-lived than seeds with higher seed mass (Fig. 3a, Table 2), and seeds collected at lower elevation were longer-lived than those from higher elevation (Fig. 3b). The model with the interaction between seed mass and elevation was the second best of the models considered (supplementary Table S6). However, its AICc was only 0.59 higher than the best (additive) model, indicating that the interaction had a very low explanatory power. The interaction suggests that if anything, at higher elevation seed mass was more negatively correlated with p 50 than at lower elevation (Fig. 3d). Also, species elevation range had a positive effect on p 50, but the relationship was much weaker compared with the effects of seed mass and collection elevation, as the confidence interval did slightly overlap with zero (Table 2, Fig. 3c).

Figure 3. (a) Seeds with lower seed mass were longer lived than seeds with higher seed mass. (b) Seeds collected at higher elevation are shorter lived than seeds collected from lower elevation. (c) Species with wide elevation range are longer lived, but the significance was borderline. For panels a, b, and c, the continuous line represents model-averaged predictions from the model, dashed lines are the model-averaged standard errors, and points are standardized raw data. (d) Three-dimensional graph depicting the interaction of seed mass (standardized) and collection elevation (standardized); the plane represents the p 50 value predictions from the interaction model.
Table 2. Model-averaged estimates of the variables explaining the seed longevity of 56 Australian alpine species (p 50, log-transformed)

Continuous and binary input variables were standardized to facilitate model comparisons. Bold type indicates estimates in which CI (confidence interval) does not overlap with zero and are therefore significant.
The climatic variables and other seed traits such as germination strategy, endospermy and embryo type, did not explain significant variation in p 50; these other variables only appeared in a top model also containing seed mass and elevation or they did not appear in any of the best models (supplementary Table S6). Most of the models containing just one of these variables were actually worse than the null model (supplementary Table S5).
Comparisons of the Australian alpine flora with other floras
The longevity of Australian alpine seeds was relatively similar to alpine seeds in Italy, across the six families, i.e. 19.8 and 19 days, respectively (P = 0.741; Fig. 4a). In contrast, Australian alpine seeds were relatively short-lived compared with non-alpine Australian flora (P < 0.000; Fig. 4b). The difference in p 50 between alpine and non-alpine families was large and varied across the ten families. For example, the difference between mean p 50 values for alpine and non-alpine Australian Poaceae was 9 days, whereas for Myrtaceae the difference was 139.8 days. Despite large geographic separation there was a greater similarity among species and families from the same bioregion (i.e. alpine) than there was among species that occur within Australia in different bioregions (alpine or not).

Figure 4. Comparison of longevity (p 50) of Australian alpine seeds with (a) Italian alpine species (Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010) and (b) Australian non-alpine seeds (Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014). The comparisons were made across paired families; family colour-matched to Fig. 1.
Discussion
We aimed to understand patterns of seed longevity among Australian alpine flora and the important variables influencing it. Our index of seed longevity (p 50) for 56 Australian alpine species ranged from ~2 days to more than 81 days with a mean of 19.8 days; these values were relatively small compared with the wider Australian flora in which the p 50 can exceed 750 days and averages 105 days, and comparable to other alpine flora. The present results indicate that seed mass and collection elevation are important drivers of seed longevity in the Australian Alps. Germination strategy and endospermy, although predicted to be important intrinsic traits, were not associated with longevity of Australian alpine seeds. Here we explore intrinsic traits and environmental drivers of Australian alpine seed longevity, as well as the significance longevity may hold for ex situ plant conservation and in situ seed bank persistence, particularly in the context of a changing climate.
Comparative longevity and drivers thereof
The p 50 variation across the 56 species considered here is rather large (2.28 to 81.59 days), and twice that reported for another alpine region (Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010). This might be attributed to the diverse combinations of plant and seed traits, and collection locations or habitats that we sampled in our study. In contrast to some previous studies (Probert et al., Reference Probert, Daws and Hay2009; Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014), we found that the p 50 of Australian alpine species was positively associated with seed mass. Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014) also found that Australian seed longevity generally increased with seed mass, but no correlation was found across 195 species worldwide (Probert et al., Reference Probert, Daws and Hay2009). Although seed mass is often a significant factor in longevity studies, the direction of the relationship seems to vary across biomes and geographic scales. Seed mass is dependent on environmental variables. For instance, larger seeds are generally found at lower latitudes and this relationship co-varies with higher long-term temperature (Moles et al., Reference Moles, Ackerly, Webb, Tweddle, Dickie, Pitman and Westoby2005, Reference Moles, Ackerly, Tweddle, Dickie, Smith, Leishman, Mayfield, Pitman, Wood and Westoby2007; Soper Gorden et al., Reference Soper Gorden, Winkler, Jahnke, Marshall, Horky, Hudelson and Etterson2016). Furthermore, within the Australian Alps, the seed mass of Aciphylla glacialis, Oreomyrrhis eriopoda, Ranunculus gunnianus, Richea continentis and Wahlenbergia ceracea increased with elevation (Segal et al., unpublished data). Such within-species variation may potentially have a counter-balancing effect on the p 50 decline among species at higher elevation. Some of the species in our study (e.g. Carex gaudichaudiana, Neopaxia australasica, Oreomyrrhis eriopoda, Ranunculus lappaceus and Stylidium graminifolium) have cosmopolitan distributions, and thus one could examine whether the effect of elevation and seed mass on seed longevity is consistent across regions.
Endosperm has been shown to have a consistent positive association with p 50 in other species elsewhere, but was not directly related to the p 50 of the species tested here. Across Australian alpine plants, non-endospermic species have a low seed mass, are non-dormant at dispersal, and thus have an immediate germination strategy, but endospermy has not been shown to be associated with SLA (Specific Leaf Area), plant height or elevation (Sommerville et al., Reference Sommerville, Martyn and Offord2013; Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). We found the same correlation patterns among non-endospermy, seed mass, dormancy class and germination strategy as in the previous studies (Sommerville et al., Reference Sommerville, Martyn and Offord2013; Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015), yet endospermy and germination strategy were not associated with longevity. Endospermic seeds in Australian alpine species are generally associated with dormant seed and a postponed germination strategy (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015). One can expect that for a dormant seed, high longevity is more relevant than for a non-endospermic seed with an immediate germination strategy that, if the required conditions are met, presumably germinates soon after natural dispersal. These patterns may indicate that across Australian alpine plants seed longevity, though influenced by some of the same traits as germination strategy, may be an ecologically important trait that evolves somewhat independently of germination strategy.
Seed longevity has also previously been shown to be associated with phylogeny (Probert et al., Reference Probert, Daws and Hay2009; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014), particularly when a given family possesses a distinctive character, e.g. hard-seededness (physical dormancy) in Fabaceae. However, this pattern was not detected among the alpine species studied here. A more thorough analysis applying actual phylogenetic data would be needed to formally assess the relationship between p 50 and phylogeny of Australian alpine seeds. Variation in seed longevity at family and genus level has previously been demonstrated in the Asteraceae (Walters et al., Reference Walters, Wheeler and Grotenhuis2005), Australian Cyperaceae (Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014), and the genus Plantago (Plantaginaceae; Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010). We suggest that the results presented here, in conjunction with the results of other studies that show high levels of variation within phylogenetic groups (and even species), phylogenetic data seem unlikely to reveal strong associations. Rather, longevity appears to be a fairly labile trait.
Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010) demonstrated that seeds of alpine plants are short-lived compared with seeds of the same species collected from lowland plants. Our study is the first to provide evidence that even within the higher elevation (sub-alpine and alpine), elevation still affects seed longevity; even though the climate gradient was relatively narrow and did not itself directly explain variation in p 50. Interestingly, climate was a significant correlate of seed longevity in other studies (Probert et al., Reference Probert, Daws and Hay2009; Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010; Merritt et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014) but was not significant in this study, possibly indicating that climate affects longevity at bioregional scales but that other drivers may be more important within a region. That elevation matters for p 50 but climate variables do not in our study may seem contradictory, but most likely indicates that a narrow amplitude of long-term climate variability recorded by alpine climate stations may not reflect conditions at plant or soil level. The long-term temperature (used to model bioclimate values), for instance, are calculated from temperature data measured at 1.2 m above the ground and there could be as much as a 7°C discrepancy between the temperatures measured at this height and at the ground (www.bom.gov.au). Seed development (post-zygotic and pre-zygotic) conditions, instead of long-term mean climate variables, were the significant factor explaining p 50 variation in Italian flora (Mondoni et al., Reference Mondoni, Probert, Rossi, Vegini and Hay2010), and maternal conditions may increase and decrease the p 50, again depending on the species and origin (Kochanek et al., Reference Kochanek, Buckley, Probert, Adkins and Steadman2010, Reference Kochanek, Steadman, Probert and Adkins2011; Bernareggi et al., Reference Bernareggi, Carbognani, Petraglia and Mondoni2015). Hence in addition to broad-scale elevation patterns, microclimatic and maternal conditions may explain subtler patterns of variation in p 50 and deserve further investigation.
Ex situ and in situ conservation implications in a climate change context
Of the species assessed here, nearly half had seeds with p 50 values lower than 13.7 days, a commonly applied threshold to define seeds to be short-lived (Newton et al., Reference Newton, Hay and Probert2009; Probert et al., Reference Probert, Daws and Hay2009), or if we refer to the classification by Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010), 18 species would be considered short-lived (p 50 < 10 days). The low p 50 values might mean years of viability under ex situ conditions, where relative humidity and temperature are kept low, but this still needs to be empirically proven.
Climate model studies have demonstrated that alpine plants are unlikely to be able to adapt or disperse quickly enough to keep up with rapid climate change, and thus there is a high risk of local extinction (Cotto et al., Reference Cotto, Wessely, Georges, Klonner, Schmid, Dullinger, Thuiller and Guillaume2017). Our data provide some indication that longevity was higher for species with wide elevation ranges than for species with short elevation ranges; such patterns could indicate an advantage for these species compared with those with restricted alpine distribution, and this effect thus may warrant further consideration.
The Australian alpine region is relatively low-lying and permanent snow is absent, leading to an even more constrained potential for upward migration. Thus, ex situ plant conservation might be the best option to provide a safeguard for many alpine plants, particularly in the Australian context. From an ex situ conservation perspective, p 50 values provide a convenient and reliable proxy to estimate the storage life. For native species where seed availability is often limited, determination of species constants and calculation of a full viability equation to determine storage lifespan, which requires many more seeds, cannot be performed. Thus p 50 derived from controlled ageing is the most practical approach to determine seed management in ex situ storage. Ex situ seed bank collections consist of ‘active’ collection (10–20 years term storage) for research use, and planting materials and ‘base’ collections (long-term storage; FAO/ IPGRI, 1994). The original FAO/ IPGRI (1994) genebank standards recommend for 5 to 10 years interval for viability testing but the more recent FAO (2013) protocol advised for an interval one third of the time predicted for viability to fall from its initial value. Our finding advocates for a more considered handling of alpine seeds in ex situ seed conservation – alpine seeds should be stored and utilized relatively quickly (i.e. base may be only 10–20 years) with ~3 year intervals of viability monitoring (see Hay and Probert, Reference Hay and Probert2013).
The relatively short longevity of Australian alpine seeds could also influence in situ persistence. Australian alpine soils contain a substantial seed bank of viable seeds that germinate well (Venn and Morgan, Reference Venn and Morgan2010; Hoyle et al., Reference Hoyle, Venn, Steadman, Good, McAuliffe, Williams and Nicotra2013). Alpine communities elsewhere also have sizeable soil seed banks (Arroyo et al., Reference Arroyo, Cavieres, Castor and Humaña1999, Reference Arroyo, Cavieres and Humaña2004), and can survive 5 years in a burial experiment in situ (Schwienbacher et al., Reference Schwienbacher, Marcante and Erschbamer2010). More than 50% of alpine species present in the standing vegetation may exist in the soil seed bank (McGraw and Vavrek, Reference McGraw, Vavrek, Leck, Parker and Simpson1989). Australian alpine seeds in contrast are relatively small (1.25 ± 0.2 mg across our study species, which was small compared with Australian seed mass tested in Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014): 8.84 ± 1.29 mg). Small size makes them more likely to be buried and incorporated into the soil. Seeds that are small and round underlie the strategy for longer persistence and a form of seed predator avoidance (Moles et al., Reference Moles, Hodson and Webb2000). Further, Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2010) suggested that seeds buried in the substrate at alpine sites are likely to deteriorate more slowly in alpine compared with lowland sites because of the low temperatures. Small-seeded species usually persist longer (e.g. Thompson et al., Reference Thompson, Band and Hodgson1993; Cerabolini et al., Reference Cerabolini, Ceriani, Caccianiga, Andreis and Raimondi2003; Funes et al., Reference Funes, Basconcelo, Díaz and Cabido2003; Peco et al., Reference Peco, Traba, Levassor, Sánchez and Azcárate2003).
Soil seed banks are likely to be important in maintaining the alpine communities in changing and variable climatic conditions and can potentially act as a buffer against variable weather conditions, and extreme fluctuations plants are not adapted to. Our data at least indicate that the seed longevity of many Australian alpine species may facilitate soil seed bank formation and persistence, but how seed longevity may interact with other biotic and abiotic factors to affect soil seed bank dynamics remains unknown. Actual persistence in the soil depends not only upon potential longevity, but also on other factors (Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015). In the Australian Alps, soil warming reduces germination from the soil seed bank, but increases the diversity of species and thus alters species composition of germinants (Hoyle et al., Reference Hoyle, Venn, Steadman, Good, McAuliffe, Williams and Nicotra2013). Nevertheless, Long et al. (Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008) show that p 50 values are positively correlated to realized seed persistence for 27 north-western European species. Long et al. (Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008) also estimate the association between p 50 and seed persistence for 13 Australian weed species, and suggest that p 50 values <20 days are equivalent to approximately 1 year persistence in situ and p 50 values of 20–50 days correspond to 1–3 years field persistence in their study region. Further efforts to refine estimates of in situ seed persistence from p 50 in different regions will further assist in developing approaches for habitat management and species conservation.
Conclusions
Recent comparative longevity studies using controlled accelerated ageing protocols show that the Australian flora generally has long-lived seed. Our study reveals that Australian alpine seeds are relatively short-lived just like alpine seeds elsewhere, and also demonstrated that elevation combined with seed mass were the strongest determinants for Australian alpine seed longevity (p 50) and suggest that even within the relatively narrow topographic range, the elevation of collection location is a strong determinant of seed longevity, although, at this scale we found little indication that specific climate variables were directly associated with elevation. This study is also the first study of longevity, globally, to examine the patterns and drivers of seed longevity by including regeneration processes such as germination strategy as explanatory variables. Germination strategy is particularly important for alpine plant recruitment. Interestingly, we found that seed longevity was not associated with germination strategies or with other seed traits known to be ecologically important for alpine plants. This suggests that although longevity may be influenced by some of the same seed traits that influence germination strategy, longevity may be an ecologically important process that operates independently from germination. The variables shown to be important for p 50 in Australian alpine species differed from other comparative longevity studies. In part, this reflects differences in approach. For example, we analysed 56 species which all had relatively low seed mass in a global context and were collected in one bioregion, and we took into account 12 potential correlates (including five seed/plant traits, see supplementary Table S1). Other studies have included more species with broader variation in seed mass and climatic gradients or bioregions, but often fewer correlates. Because the controlled ageing protocol has been shown to be robust and is a standardized method, a study looking at global p 50 is highly feasible and would bring even more comprehensive insights into the role of plant traits, seed structure and climate in explaining the evolution of seed longevity. Seed persistence and seed burial studies in the field, to complement the p 50 data, would also increase our confidence to use p 50 to predict seed persistence in various habitats and may be useful for informing in situ and ex situ conservation of priority species. Australian alpine regions are valuable yet vulnerable, and the success of ex situ as well as in situ plant conservation efforts will in part rely on information on seed longevity, soil seed bank persistence and germination of the alpine flora; the present study is an important step towards that knowledge.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258518000090.
Details of Australian alpine species collection, p 50 values, plant and seed traits as well as climate variable used in the analysis, and model averaging results can be found in the supplementary material online.
Supplementary Information
Supplementary Table S1. Collection information, details of ageing test (box number and germination treatment), p 50 result and intrinsic and environmental variables for each of the 57 accessions, ordered by p 50 values. Asterisk (*) indicates species endemic to the Australian Alps. Accession numbers are preceded by herbarium code CANB. KNP, Kosciuszko National Park, NSW; NNP, Namadgi National Park, ACT. During the controlled ageing each box contained at least one representative of each germination strategy [Immediate, Staggered, Postponed (Hoyle et al., Reference Hoyle, Steadman, Good, McIntosh, Galea and Nicotra2015)]. Treatment is the germination condition used for each germination screen where S = stratification (12 h/12 h photoperiod, 6 weeks 25/15°C, 8 weeks 5/5°C, 6 weeks 25/15°C), G = 1% water agar with 200 mg l–1 GA3 (12 h/12 h photoperiod 25/15°C), and A= 1% water-agar (12 h/12 h photoperiod 25/15°C). Embryo type follows Martin (Reference Martin1946). Details on climate data can be accessed from http://spatial.ala.org.au/layers. Melaleuca pityoides was not included in the probit or further analyses and hence the environmental correlates were not shown.
Supplementary Table S2. Additional seed accessions used as box-effect controls for rehydration and ageing and to ensure results of this study were comparable with Merritt et al. (Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014).
Supplementary Table S3. Correlations among the variables considered (seed/plant traits, climate variables and elevations).
Supplementary Table S4. Family (n = 16), genus (n = 33), and genus nested within family were assigned as either fixed term or random term, with p 50 (log-transformed) as response variable to tease apart the effect of phylogeny on p 50 values. F-statistics (fixed term) and estimated variance component (random term) values indicate the absence of phylogeny signal in the variation of our alpine seed longevity.
Supplementary Table S5. List of model selections ordered by second-order Akaike Information Criterion (AICc) values – better models are indicated by smaller AICc. Response variable p 50 was log-transformed, explanatory variables were standardized, and random variable was genus. Within a model, explanatory variables were independent from one another.
Supplementary Table S6. AICc model selection tables for models explaining p 50 in relation to the variables. ‘AICc’ is the AIC corrected for finite sample size, ‘ΔAICc’ is the difference between AICc of a given model to that of the best model, ‘ωAICc’ is the probability of each model being the best model given the data and the model set. These are models with the lowest ΔAICc (ΔAICc < 5) among all possible models listed in supplementary Table S5.
Supplementary Fig. S1. Map of the collection sites of the 56 species.
Acknowledgements
Australian alpine seeds were part of the collections of the National Seed Bank, the Australian National Botanic Gardens (ANBG), Canberra. Seeds for control species were kindly provided by Dave Merritt (Kings Park Botanic Gardens, WA) and the Australian Botanic Gardens, Mt Annan, NSW. We thank Terry Neeman (Stats Consulting Unit ANU) for advice on all subset regression, Tom North for facilitation at ANBG, Nicotra Lab members for providing constructive feedback, and Toton Liantoro for technical assistance.
Financial support
A.S. was supported by an Australian Government Research Training Program (RTP) Scholarship. T.M. was supported by an Endeavour Research Fellowship.
Conflicts of interest
None.








