INTRODUCTION
Intensity-modulated radiation therapy (IMRT) has been approved to be effective for the treatment of nasopharyngeal cancer (NPC).Reference Xia, Fu and Wong 1 Although, IMRT showed superior tumour coverage when compared with all other conventional techniques,Reference Lee and Le 2 skin toxicity caused by IMRT tend to be higher. This mainly due to the use of multiple tangential beams that causes an increase of 19 and 27% in skin dose, with and without an immobilisation mask, respectively.Reference Lee, Chuang and Quivey 3 Skin toxicity magnifies in head and neck cases as many tumours are located closely to the surface and skin sparing is limited.
Other factors may contribute to the skin toxicity such as; planning target volume (PTV) contouring, using the inverse treatment planning and concomitant or previous chemotherapy.Reference Lee, Chuang and Quivey 3 In addition, numerous patient-specific parameters that include individual biological variation in radiation sensitivity and the presence of coexisting diseases like diabetes mellitus may affect the injury threshold.Reference Wagner, McNeese, Marx and Siegel 4 Due to previous factors, the actual threshold needed to cause skin injuries varies, therefore, the minimum dose that might cause a skin change should not be expressed as a single threshold dose, but preferably as a range of doses.Reference Balter, Hopewell, Miller, Wagner and Zelefsky 5
Exposure of the skin to high doses of ionising radiation leads to the development of erythematous skin changes.Reference Lee, Chuang and Quivey 3 , Reference Hoppe, Laser and Kowalski 6 In radiation therapy, a skin dose of 6–8 Gy with 200 kV is required for erythema to occur.Reference Mettler, Koenig, Wagner and Kelsey 7 Radiation of higher energies (6 MV) requires larger doses to produce the same of erythema, as in these cases the maximum dose is received in deeper tissues below the skin.Reference Mettler, Koenig, Wagner and Kelsey 7
Treatment planning system (TPS) may not give accurate dose values at the skin as it was not planned as an organ at risk. An overestimation of surface dose by TPS of upto 18·5% of the prescribed dose has been reportedReference Chung, Jin and Dempsey 8 and by 10–12% as measured on the mask of a patient treated with tomotherapy.Reference Cherpak, Studinski and Cygler 9 This inconsistency between doses calculated by IMRT TPS and in vivo measured dose on the surface is the main reason for this research into the measurement of skin dose and to identify patients at risk of skin erythema.Reference Chung, Jin and Dempsey 8
In 1982, the Radiation Therapy Oncology Group (RTOG) developed the radiation morbidity scoring criteria to classify radiotherapy effects from Grade 0 to Grade 4. RTOG score has widely been employed for more than 25 years and it was accepted and acknowledged by medical and nursing communities.Reference Cox, Stetz and Pajak 10
With the lack of sufficient information in the literature to probably measure the skin dose in patients treated by IMRT and the inability of TPS to accurately estimate skin dose, this study aimed to quantify skin dose at different regions inside the mask and to demonstrate the factors contributing to acute skin toxicity. This study was approved by Medical Research Ethics Committee of the Ministry of Health.
MATERIALS AND METHODS
Inclusion criteria and sampling technique
Cases with confirmed diagnosis of NPC regardless of their stage were selected from a convenience sample of prevalent patients attending the radiotherapy department. All patients were asked to participate in the research and after agreement they signed a consent form. During the study period 21 patients were eligible and accepted to enrol in this study.
Thermoluminescent dosimeters (TLDs) calibration
Chip-shaped LiF:Mg, Ti Thermoluminescent (TLD-100) (Bicron Harshaw, NE, USA) were used after annealing and read out by TLD reader (Harshaw Model 3500, Thermo Fisher Scientific, Inc. (NYSE:TMO), USA). Five subsequent calibration cycles were then performed to establish individual calibration factors.Reference Radaideh, Matalqah, Lee Luen, Tajuddin, Abdel Munem and Bauk 11
In vivo measurements
TLDs detectors were taped properly inside the thermoplastic mask as follows: four dosimeters at each side of the face on the buccal region, six at each lateral side of the neck and four at each eye (orbital region). The average dose of TLDs was used to represent the skin or eye lens dose at each position. An extreme caution was taken to maintain the same location of the detectors for every patient. Constant time gap of 24 hours was maintained between irradiation and read out. IMRT using linear accelerator with the gross tumour volume receiving 70 Gy and the clinical target volume 60 Gy (the prescription goal was 95% of the PTV receives at least 70 Gy) in 33 fractions with 2·2 Gy/fraction was used to treat NPC patients. An immobilisation mask covering the head, neck and shoulder was used during the irradiation procedure.
In vivo TLDs reliability test
Skin dose was measured for five NPC patients for two consecutive fractions using TLDs and IMRT technique. To detect the agreement between both readings of TLDs at each region, the interclass correlation coefficient (ICC) was conducted.Reference Rankin and Stokes 12 The uncertainty in the calibration procedure was also found to be <4%. All TLDs readings displayed a linear dose response with respect to the measured dose at surface and at d max from 0·05 to 1 Gy.
RTOG scale validity and reliability
Patients’ skin toxicities were then evaluated weekly and after completion of 7 weeks of radiotherapy, evaluated by two observers (physician and researcher). At this stage, skin reactions were classified using the RTOG scale.
According to RTOG scale, classifications were identified: Grade 0 (no reaction), Grade 1 (faint erythema, dry desquamation, epilation, diminished sweating), Grade 2 (moderate, brisk erythema, exudative dermatitis in plaques and moderate oedema), Grade 3 (exudative dermatitis, besides cutaneous folds and intense oedema) and Grade 4 (ulceration, haemorrhage, and necrosis).Reference Cox, Stetz and Pajak 10
An excellent inter-rater reliability for grades on the RTOG scale was reported.Reference Evans 13 Rosewall et al.Reference Rosewall, Yan and Bayley 14 stated that the RTOG grading system has shown high inter-rater reliability compared with other grading systems such as World Health Organization, Eastern Cooperative Oncology Group, Ajani and the Common Toxicity Criteria.
Statistical analysis
All data entry and analyses were conducted using Statistical Package for Social Sciences Software version 18, (SPSS Inc., Chicago, IL, USA).To detect the agreement between both readings of TLDs at each region, the ICC was obtained by SPSS reliability test.Reference Rankin and Stokes 12 Univariate logistic regression model was used in the statistical analyses to evaluate the significant factors associated with skin toxicity risk. Only factors with univariate significance level of <0·20Reference Greenland 15 were included in the multivariate conditional logistic regression model to assess multiple risk factors for skin toxicity simultaneously. For each factor in the model, the likelihood of skin toxicity was estimated by the odds ratios at 95% CI. A p value of<0·05 was considered significant in the statistical analyses.
Analysis of variance (ANOVA) test was applied to detect the difference between the three groups of toxicity grades in regards of skin dose.
Results
TLDs calibration and in vivo reliability test
All TLDs were selected with sensitivity ranging within ±5% and reproducibility of ±3% for calibration and measurement in the surface. Excellent agreement was found between both TLDs reading with ICC>0·9.
Patients’ skin toxicity risk factors
In total, 21 patients met the study criteria and completed the planned course of treatment. The NPC patients who participated in this study were either in stage 1 or stage 2. The mean age (±SD) of the overall study population was 52±11 (range 42–66) years. Only eight patients were treated with chemotherapy (Table 1).
Table 1 Demographic and clinical characteristics of nasopharyngeal patients (n=21)

Clinically, all patients were monitored and observed for skin toxicity in between fractions and after the 33 fractions were completed. Grade 1 reactions were seen in eight patients, Grade 2 in 11 patients, whereas Grade 3 only in two patients. By applying ANOVA test, it was found a significant difference in skin dose between the three toxicity grades with p value <0·05 (Table 2).
Table 2 Average skin and eye doses (Gy) per each fraction for 21 patients using intensity-modulated radiation therapy plan technique

Abbreviations: R, right; L, left.
Patients with Grade 2 and Grade 3 were combined in one group and compared with Grade 1 group, thus enabling the statistical analysis using binary logistic regression (Table 3). The results showed that patients with age older than 60 years old are more likely to have skin toxicity by 1·6 times as compared with younger patients (40–49 years old). Furthermore, if the accumulative dose received by the skin is >7 Gy, the probability of skin toxicity increased by 2·8 times.
Table 3 Binary logistic regression analysis of skin toxicity risk factors

Notes: Univariate logistic regression test was used in obtaining p values and the odd ratios (OR).
*p value<0·05, Bold values indicate significant ORs.
Abbreviation: CI, confidence interval.
In the multivariate logistic regression analysis and after controlling other confounders, the final model showed that only the accumulative skin dose is the only predictive factors for skin toxicity dose (odds ratio: 2·61, 95% confidence interval: 1·01–7·90) (Table 4).
Table 4 Final model of multivariate logistic regression

a Note: Multivariate conditional logistic regression was applied for all variables with p values<0·20 in the univariate logistic regression in order to obtain adjusted odd ratios (OR).Abbreviation: CI, confidence interval.
DISCUSSION
TLDs are widely used for in vivo dosimetry.Reference Radaideh, Matalqah, Lee Luen, Tajuddin, Abdel Munem and Bauk 11 , Reference VanDam and Marinello 16 – Reference Mayles, Heisig and Mayles 18 TLDs were used in this study as it gives an accurate point dose, easy to manipulate, it is multiuse and it gives a comparable skin dose to metal-oxide-semiconductor field-effect transistor MOSFET.Reference Huyskens, Bogaerts and Verstraete 19
The severity of radiation effects depends on the patient and on exposure specific parameters; dose fractionation, total dose and irradiation field size.Reference Lee, Chuang and Quivey 3 Excluding patient-specific factors (age, stage of cancer, having other diseases, etc.), the severity of the injuries found to be dependent on radiation dose absorbed by the skin. Although, fractionation over multiple sessions can reduce the possibility of erythema, radiation effects tend to be accumulative.Reference Koenig, Wolff, Mettler and Wagner 20
Although monitoring the study participants, all patients have suffered from skin toxicity especially on the neck area, ranging from minor to major toxicity. The severity of skin burns related obviously to the total accumulative dose and it became serious after the dose of 7 Gy. Only, two patients were found to have a sever skin erythema on the neck. Not surprisingly, the total accumulative doses at neck region were >7 Gy for both cases (Figures 1 and 2). In agreement with previous literature that revealed that erythema occurred at skin dose of 6–8 Gy.Reference Mettler, Koenig, Wagner and Kelsey 7
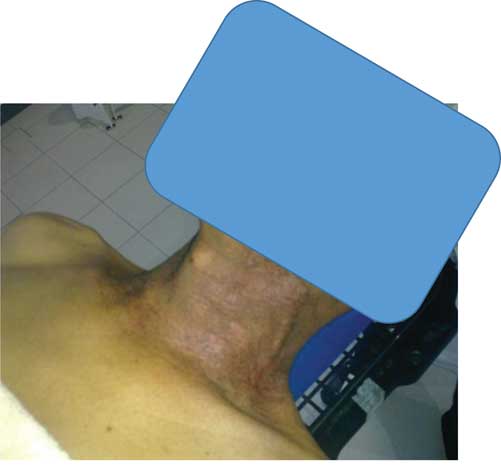
Figure 1 Grade 2 skin reaction after 25 fraction using three-dimensional conformal radiation therapy treatment for nasopharyngeal cancer.
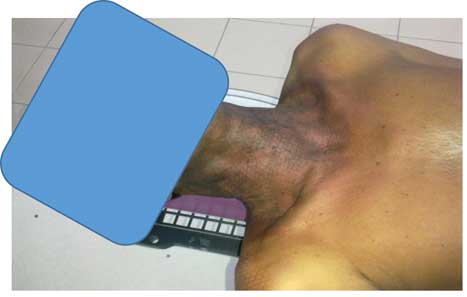
Figure 2 Grade 3 skin reaction after 25 fraction using intensity-modulated radiation therapy treatment for nasopharyngeal cancer.
Literature showed that radiotherapy is well tolerated in older patients as they present smaller mitosis indices, they would be less sensitive to radiation that destroys cells mainly in the mitosis phase and, consequently, would cause weaker skin reactions.Reference Porock 21 , Reference Shih, Miaskowski, Dodd, Stotts and MacPhail 22 This was contradictory with our results where increasing age was found to be a predictor of skin toxicity. However, it was not possible to show significance due to the small sample size.
Increased skin sensitivity following radiotherapy was observed when chemotherapy is given concomitantly with radiotherapy and the skin turned red and can become itchy several months after the end of radiotherapy.Reference Fiets, Van Helvoirt, Nortier, Van der Tweel and Struikmans 23 , Reference See, Wright and Denham 24 However, this study failed to find any association between previous or concomitant chemotherapy with radiotherapy with increasing risk of skin injuries. Only eight patients were treated by chemotherapy before radiotherapy, and they did not show significance in relation with skin toxicity.
When the results were jointly analysed, they revealed low incidence of Grade 3 skin reactions in head and neck patients, and also that, when they occurred, the neck region was the most frequently affected area. Consequently, the neck skin was identified as a sensitive structure for dose optimisation. It was found earlier that when the skin of the neck was contoured as an organ at risk for dose optimisation, the volume of skin that received >45 Gy was further reduced by about 20%.Reference Lee, Chuang and Quivey 3
This study also showed that skin dose is the most important factor for the severity of skin reactions in patients treated by IMRT. Despite the low incidence of severe skin reaction, care with the irradiated skin is a relevant factor for radiotherapy in head and neck patients.
CONCLUSION
Multiple factors contributed to the observed acute skin reaction for head and neck cancer patients including age and the accumulative skin dose. We highly recommend considering skin as an organ at risk during the planning technique, this may reduce the skin dose to a tolerable level without compromising tumour target coverage. Interventional precautions should be taken for the neck region in NPC patients treated with IMRT. Furthermore, in vivo dosimetry measurements are highly recommended for quality assurance of individual treatment planning and before starting therapy especially for IMRT technique where high skin dose is expected and TPS cannot accurately estimate the dose to the skin.
Limitation
First, the RTOG scoring system only measures the appearance of the skin from the point of view of the physician regardless of patient’s view of their skin reactions, which may cause underestimation of the severity of skin toxicity. The Radiation-Induced Skin Reaction Assessment ScaleReference Noble-Adams 25 that addresses this problem may be considered in future works. Second, the study sample size limits the generalisability of the results. Finally, even though the researchers tried to keep TLDs at the same positions for all patients, however, there may be slight deviation from the correct position.
Acknowledgement
The authors gratefully acknowledge Qassim University, represented by the Deanship of Scientific Research, on the material support for this research under the number (3324) during the academic year 1436 AH/2015 AD.








