Published online by Cambridge University Press: 25 April 2005
Natural abundances of the stable isotopes, 15N/14N (δ15N) and 13C/12C (δ13C), were used to study temporal host-parasite relationships of the wild rabbit, Oryctolagus cuniculus (L.). During the 12-month sampling period, temporal isotopic shifts in δ15N were noted for dietary vegetation, host rabbit faeces and fur, but not for muscle or stomach contents. δ15N varied temporally for the parasitic cestode species, Mosgovoyia pectinata but not for Cittotaenia denticulata. Similarly, intestinal parasitic nematodes had apparent species-specific δ15N patterns. Only rabbit fur and intestinal parasitic nematodes did not exhibit temporal shifts in δ13C. Overall, host faeces and stomach contents were isotopically indistinct as a likely consequence of coprophagy. Relative to their host, parasitic nematodes were 15N-enriched, consistent with an increase in trophic level status. Conversely, cestodes were 15N-depleted. Isotopically, each parasite reflected a species-specific relationship with their rabbit host. This technique could be utilized to integrate parasites into food-web studies.
The European rabbit (Oryctolagus cuniculus) was most likely introduced into the United Kingdom in the late twelfth century (Lever, 1977). Due to its rapid reproductive cycle and changes in land management (Pollard, Hooper & Moore, 1974), the European rabbit is now one of the major agricultural pests with an estimated loss of revenue of >£100 million to UK agriculture (Mills, 1986). Consequently, the ecology of the rabbit has been well studied (Thompson & King, 1994). However, there is a paucity of data on both the temporal trophic dynamics of the rabbit and the trophic-level interactions between the rabbit host and its parasites.
Naturally occurring stable isotopes of carbon (C) and nitrogen (N) have been utilized by ecologists and zoologists as a tool to determine trophic relationships within a community (e.g. Tieszen et al. 1979; Hobson & Welch, 1992; Scrimgeour et al. 1995; Neilson et al. 1998; Neilson, Boag & Smith, 2000). Furthermore, hitherto unknown temporal changes in diet have also been identified using stable isotope analyses (Ramsay & Hobson, 1991; Ben-David, Flynn & Schell, 1997; Godley et al. 1998; Hobson, Drever & Kaiser, 1999). Natural abundances of stable isotopes are effectively an integrated record of assimilated elements such as C and N (Peterson & Fry, 1987; Robinson, 2001) and, as such, are a better representation of the recent biochemical and dietary past of an organism than traditional snapshot methods, e.g. gut content analyses. Changes in the ratios of 13C/12C and 15N/14N (expressed as δ13C and δ15N) in animal tissue are indicative of dietary sources and trophic grouping, respectively (DeNiro & Epstein, 1978, 1981; Wada, Kabaya & Kurihara, 1993). Animal tissues such as muscle, hair (fur) and liver have different turnover rates of carbon and nitrogen due to differences in their metabolic activity (Tieszen et al. 1983; Hobson & Clark, 1992; Pinnegar & Polunin, 1999) and have been used as a proxy to indicate short and long-term dietary sources in birds (Hobson & Sealy, 1991; Hobson & Clark, 1992; Hobson, 1993), mammals and reptiles (Tieszen et al. 1983; Ames, van Vleet & Sackett, 1996; Hilderbrand et al. 1996; Godley et al. 1998; Hobson et al. 1999). Previously, Boag et al. (1998) from a small dataset (n=10), reported isotopic differences between rabbit tissue (e.g. fur, muscle, embryos and faeces) and also noted that coprophagy had little effect on the isotopic discrimination between faeces and stomach contents.
Although parasites are an integral component of any trophic system, their inclusion in food-web studies is infrequent (Marcogliese & Cone, 1997). Similarly, the utilization of stable isotope techniques in host-parasite studies is rare although a few studies have investigated host-parasite interactions in plants (Neilson & Brown, 1999, 2000), chironomids (Doucett, Giberson & Power, 1999) and fish (Iken et al. 2001; Pinnegar, Campbell & Polunin, 2001; Deudero, Pinnegar & Polunin, 2002). Data from Boag et al. (1998) suggested that different trophic relationships existed between parasitic intestinal nematodes and parasitic cestodes and their host, the European rabbit.
The objective of this paper is to report for the first time, the temporal trophic dynamics of a host-parasite system using the natural abundances of δ13C and δ15N, and to compare the isotopic discriminations between tissues to that reported by Boag et al. (1998) from a different wild rabbit population.
Ten wild rabbits were captured using box traps from a study site located in the Scottish Borders on 6 separate occasions (January, March, May, July, September and November) during a 12-month period. The rabbits were humanely dispatched and post-mortems undertaken to collect tissue samples and intestinal parasites (Boag, 1972). Samples of fur, muscle, stomach contents, faeces and when present, parasitic cestodes (Mosgovoyia pectinata and Cittotaenia denticulata) and parasitic nematodes (Graphidium strigosum, Passalurus ambiguus and Trichostrongylus retortaeformis) were collected from the rabbits. Samples of vegetation, predominantly graminaceous plants, from within the home range of the sampled rabbits were removed at ground level by secateurs on each sampling occasion. Samples were processed and analysed by continuous-flow isotope ratio mass spectrometry (CF-IRMS) as described by Boag et al. (1998).
Isotope natural abundances are reported as:

where Rsample and Rstandard are the heavy/light isotope ratios of sample and standard. Analytical precision was [les ]0·2‰ for δ13C and [les ]0·4‰ δ15N.
A one-way analysis of variance using Minitab (Minitab, Pennsylvania, USA) was done to identify significant temporal differences for host and parasite samples.
Muscle and stomach content δ15N varied little (P=0·365 and P=0·829, respectively) during the sampling period (Fig. 1A, C). In contrast, faeces, fur and vegetation δ15N exhibited statistically significant temporal trends (Figs 1B, D and 2A). Vegetation showed the greatest temporal variation (P=0·021) by becoming 2·9‰ less 15N-enriched during the period January to September, thereafter, becoming 2·5‰ 15N-enriched from September to November (Fig. 2A). Faeces also became less 15N-enriched between January and July, although by only 1·0‰ before following the same pattern as vegetation and becoming more 15N-enriched (1·5‰) towards the end of the sampling period (P=0·045; Fig. 1B). In contrast, between January and July fur became 2·1‰ more 15N-enriched, thereafter gradually becoming less 15N-enriched during the sampling period (P=0·000; Fig. 1D).

Fig. 1. Temporal mean δ15N (closed squares) and δ13C (open circles) for (A) rabbit muscle; (B) rabbit faeces; (C) rabbit stomach contents and (D) rabbit fur.

Fig. 2. (A) Temporal vegetation mean δ15N (closed squares) and δ13C (open circles) and (B) Temporal mean δ15N (closed symbols) and δ13C (open symbols) for the parasitic cestodes, Cittotaenia denticulata (squares) and Mosgovoyia pectinata (triangles).
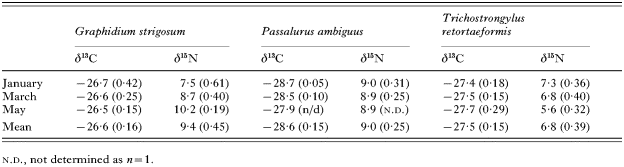
The 3 species of intestinal nematode were only found on the first 3 sampling dates, indicative of their known biology when their intensity is greatest (e.g. G. strigosum) or when they are prevalent (Boag, 1988). Consequently, it was not possible to statistically test any observed isotopic temporal differences. Intestinal parasitic nematode δ15N exhibited discordant temporal patterns (Table 1). Between January and May G. strigosum became 2·7‰ more 15N-enriched, whereas T. retortaeformis became 1·7‰ less 15N-enriched during the same period (Table 1). In contrast, both P. ambiguus and the cestode C. denticulata (P=0·988) exhibited no δ15N temporal patterns (Table 1; Fig. 2B). A second cestode species, M. pectinata, exhibited significant (P=0·013) temporal variation in δ15N initially becoming slightly less 15N-enriched during January–May and thereafter 1·6‰ 15N-enriched from May to November (Fig. 2B).
Table 1. Mean δ13C (‰) and δ15N (‰) values for 3 intestinal nematodes recorded from the European rabbit (Oryctolagus cuniculus) (Values in parentheses are standard errors.)
Muscle (P=0·000), faeces (P=0·000), vegetation (P=0·000) and stomach content (P=0·036) δ13C varied temporally during the sampling period (Figs 1A–C and 2). Between September and November, all three tissues became 1·1–1·4‰ more 13C-depleted (Figs 1A–C and 2). In contrast, fur δ13C was uniform throughout the sampling period (Fig. 1D).
Both cestode species became less 13C-depleted during the sampling period (P=0·000) although the temporal trend of both species differed (Fig. 2B). During the period January–May, intestinal nematode δ13C showed no obvious temporal trends (Table 1).
Figure 3 summarizes the isotopic data, based on an average value for each component or parasite group across the entire sampling period. Both rabbit tissues, muscle and fur, were 1·1 and 1·7‰ 15N-enriched, respectively, relative to the host (rabbit) diet (C3-grass). Similarly, stomach contents and faeces were 1·2 and 1·4‰ 15N-enriched relative to dietary material (Fig. 3). Compared to rabbit muscle, levels of 15N-enrichment differed among intestinal nematode species: G. strigosum (5·7‰), P. ambiguus (5·3‰) and T. retortaeformis (3·1‰)(Table 1, Fig. 3). It should be noted that the nematode data pertained only to the period January–May and, in contrast, the parasitic cestodes were less 15N-enriched than rabbit muscle and as with the nematodes this differed among cestode species, C. denticulata (2·7‰) and M. pectinata (1·5‰).

Fig. 3. Seasonal mean δ13C vs δ15N for all samples. Cd, Cittotaenia denticulata; Gs, Graphidium strigosum; Mp, Mosgovoyia pectinata; Pa, Passalurus ambiguus and Tr, Trichostrongylus retortaeformis. In some instances error bars are smaller than the symbols.
Mean faeces and stomach content δ13C were similar to vegetation δ13C (Fig. 3). Overall, both rabbit tissues sampled were less 13C-depleted than the dietary material by 1·7‰ (muscle) and 3·2‰ (fur), respectively (Fig. 3). Unlike δ15N, mean δ13C for G. strigosum and T. retortaeformis was similar to that for host muscle (Fig. 3). In contrast, P. ambiguus was 1·6‰ more 13C-depleted (Fig. 3). Similarly, both cestode species were more 13C-depleted than host muscle by 1·9‰ (C. denticulata) and 5·5‰ (M. pectinata), respectively (Fig. 3).
Information regarding the trophic dynamics of herbivores is maximized by isotopic analysis of a combination of tissue, faecal and stomach content samples (Tieszen et al. 1983), providing information on the average long-term, short-term and immediate diet. Dietary information derived from bone collagen is typically used as an indicator of long-term diet as it is considered to be an integrated value over the life-term of the animal (Stenhouse & Baxter, 1979; Tieszen et al. 1983; Hobson & Clark, 1992), although Mizutani, Hasegawa & Wada (1986) reported that collagen isotope values could be indicative of diet primarily during developmental stages.
In this study, sampling was done approximately every 60 days; therefore collagen analysis was considered to be ineffective in measuring any isotopic shifts in diet during the sampling period. Under controlled experiments, isotopic turnover in muscle has been reported as 12·4 days for quails (Hobson & Clark, 1992) and 27·6 days for gerbils (Tieszen et al. 1983). Consequently, in the context of this study, muscle was used as a potential indicator of any isotopic shifts in ‘long-term’ diet.
Samples of vegetation, predominantly grass species, taken from within the home range of the sampled rabbits exhibited temporal isotopic shifts in both δ13C and δ15N, likely to represent temporal water availability and internal translocation of plant N, respectively, similar to that previously reported for a range of plants (Farquhar & Richards, 1984; Stewart et al. 1995; Handley & Scrimgeour, 1997; Neilson et al. 1998; Chang & Handley, 2000) including Lolium perenne (perennial ryegrass) and Poa species (Neilson et al. 1998, 2002) typical of Scottish uplands such as the study site.
Similarly, rabbit faeces δ13C and δ15N varied temporally. Temporal shifts in rabbit faeces δ13C were opposite to that of dietary δ13C. Whilst the pattern of temporal variation of rabbit faeces δ15N mimicked that of dietary δ15N, the relative changes in 15N-enrichment at each sampling date were less than that measured for dietary material as a likely consequence of fractionation along biochemical pathways (Robinson, 2001). In contrast, rabbit muscle and sampled stomach contents only exhibited temporal isotopic shifts in δ13C but not in δ15N, whereas the opposite was true for rabbit fur, i.e. a temporal shift in δ15N but not δ13C. The temporal shift in fur δ15N may reflect the moulting and regenerating process that commences in March, peaks in July and terminates in October (Cowan, 1991). Selective assimilation of isotopically distinct compounds derived from the same dietary source (Macko et al. 1987; Hare et al. 1991) and/or differential metabolism of C and N between diet and muscle, fur, and stomach contents may have masked smaller temporal isotopic shifts (Boag et al. 1998).
It is unclear why faecal δ15N should have a temporal trend similar to dietary δ15N but stomach content δ15N did not. Rabbits are known to produce two faeces types, soft and hard (Madsen, 1939; Taylor, 1939), the soft being immediately re-ingested by the rabbit directly from the anus during excretion, thus faeces in this study refers to hard faeces that are excreted at night. A detailed description of faeces production by rabbits has been presented by Björnhag (1994) and Hirakawa (2001). Hirakawa (2001) reported that rabbits not only re-ingest soft faeces but also regularly re-ingest hard faeces. Thus a mix of isotopic sources derived from raw dietary material, soft and hard faeces in the stomach may have diluted the overall isotopic value for stomach content.
Although temporal trends of δ15N differed between faecal and stomach content, there was no overall statistical seasonal difference. Based on similar results, Boag et al. (1998) suggested that the discriminating branch-point for nitrogen metabolism for lagomorphs occurred within the animal not the gut. Given that in this study there was a clear temporal trend in faecal δ15N reflecting dietary material, it is unclear whether this is true. It is possible that the discrepancy in δ15N between faecal material and stomach content indicates that coprophagy may be an isotopic fractionation process contrary to that noted by Boag et al. (1998). However, the assertion made by Boag et al. (1998) was based on a limited dataset (single sampling date, n=10) and it is clear from our data that if sampling of the rabbits used in that study had occurred between May and September, the interpretation by Boag et al. (1998) was valid.
The lack of any significant 15N-enrichment in muscle during the sampling period suggests that the sampled rabbit population were not under any nutritional stress (Hobson, Alisaukas & Clark, 1993; Scrimgeour et al. 1995).
Intestinal nematodes were recovered from their rabbit hosts only between January and May. Consequently, no comment can be made about temporal isotopic variation during the complete sampling period. However, during the period January–May it was clear that the three parasitic nematode species exhibited different isotopic trends. This may reflect differences in their epidemiology, derivation of dietary sources and/or bioavailability of certain compounds (Neilson & Brown, 1999). Both cestode species were recovered throughout the sampling period. Cittotaenia denticulata δ15N was consistent throughout whereas M. pectinata δ15N became less 15N-enriched during the first three sampling dates and thereafter became 15N-enriched during the latter half of the sampling period. Similarly, there were different trends in δ13C for both cestode species during the sampling period. The isotopic variation between both cestode species may reflect inter-specific differences in (a) metabolic pathways, (b) lipid content that is derived directly from the host and/or (c) membrane digestion that directly effects which molecules are absorbed (Smyth, 1994). Such differences between nematode species may also indicate differing metabolisms, selective absorption of isotopically distinct compounds derived from either dietary material or the host (Neilson & Brown, 1999).
Averaged over the sampling period, fur was less 13C-depleted than muscle, consistent with that reported by Boag et al. (1998) but contradictory to Hilderbrand et al. (1996) who noted that a range of rabbit tissues including ‘hair’ (=fur) were isotopically similar for both δ13C and δ15N, although the rabbits in that study were domesticated and fed a constant diet. Muscle and fur of wild rabbits in this study had similar δ15N values consistent with that previously reported for wild rabbits (Boag et al. 1998).
The trophic relationships between intestinal parasites and their host rabbits reported from a small dataset (Boag et al. 1998) from an upland pasture in north central Scotland was similar to that reported here from a more extensive dataset from an upland pasture in southern Scotland. All three parasitic intestinal nematode species were 15N-enriched relative to the rabbit host (muscle) with a mean 15N-enrichment of 4·8‰ similar to that reported by Boag et al. (1998) and within the range (0–6‰) of a single step increase in putative trophic level (Minagawa & Wada, 1984; Wada et al. 1993; Scrimgeour et al. 1995; Neilson et al. 2000). A similar increase in putative trophic level between parasitic chironomid larvae and stonefly nymphs was reported by Doucett et al. (1999), whereas Neilson & Brown (1999) noted that putative trophic level increases appeared to be species specific when 5 different longidorid plant-parasitic nematode species were studied. In contrast, nematode parasites of fish have been reported to be less 15N-enriched relative to their respective hosts (Iken et al. 2001; Pinnegar et al. 2001; Deudero et al. 2002). Furthermore, Deudero et al. (2002) reported species-specific δ15N values for nematode parasitic taxa from 10 different fish hosts. Similarly, our nematode δ15N data suggest specific host-parasite relationships that may reflect the derivation of N from different host sources, for example, T. retortaeformis from villi, G. strigosum from blood and P. ambiguus from intestinal bacteria (Barker & Ford, 1975) that in turn affect the bioavailability of certain compounds and/or bio-molecules (Neilson & Brown, 1999).
As with the parasitic nematode species, the cestodes C. denticulata and M. pectinata were isotopically different. In contrast to nematodes, cestodes were 15N-depleted relative to host, concurring with previous studies on rabbit and fish hosts (Boag et al. 1998; Pinnegar et al. 2001; Deudero et al. 2002) but in complete disagreement with that expected of a consumer relative to its host.
With few exceptions, total lipid content of cestodes (20–35% vs 5–12% of dry tissue) is greater than that of nematodes (see Table 1, Frayha & Smyth, 1983; Köhler & Voight, 1988) and consequently cestodes are typically significantly more 13C-depleted than nematodes (DeNiro & Epstein, 1978; Focken & Becker, 1998; Pinnegar & Polunin, 1999). This was true for M. pectinata but not for C. denticulata. The former being 3·9–5·9‰ more 13C-depleted than any of the three parasitic nematode species recovered from the rabbit host, whereas the latter had a similar δ13C value to the parasitic nematode, P. ambiguus. The apparent species-specific difference in δ13C between C. denticulata and M. pectinata noted both in this study and Boag et al. (1998) may be as a result of either selective absorption of fatty acids and subsequent lipid fraction incorporation (Jacobsen & Fairburn, 1967; Barrett, 1981) or the source of fatty acid (Smyth, 1994), as studied extensively in the cestode, Hymenolepis diminuta (Frayha & Smyth, 1983).
This study provides additional information on the trophic relationships of a closed host-parasite system and strongly suggests that there are both temporal and species-specific differences between parasites and their relationship with the host. Furthermore, isotopic analyses can provide opportunities to characterize the role of parasites within food webs (Marcogliese & Cone, 1997) and compound-specific stable isotope analysis (Evans et al. 2003) could be utilized to study specific metabolic pathways in parasites, e.g. lipid metabolism by analysing isotopic variation in cholesterol (Chamberlain et al. 2004).
The authors thank Charlie Scrimgeour and Winnie Stein for isotopic analyses, and Jim Sutherland for access to the study site. Research at the Scottish Crop Research Institute is grant-aided by the Scottish Executive for Environment and Rural Affairs Department.

Fig. 1. Temporal mean δ15N (closed squares) and δ13C (open circles) for (A) rabbit muscle; (B) rabbit faeces; (C) rabbit stomach contents and (D) rabbit fur.
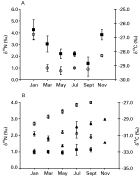
Fig. 2. (A) Temporal vegetation mean δ15N (closed squares) and δ13C (open circles) and (B) Temporal mean δ15N (closed symbols) and δ13C (open symbols) for the parasitic cestodes, Cittotaenia denticulata (squares) and Mosgovoyia pectinata (triangles).
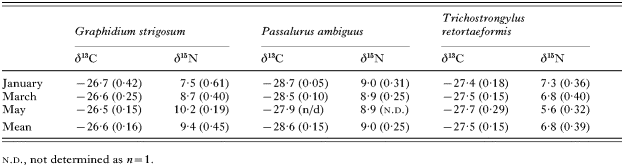
Table 1. Mean δ13C (‰) and δ15N (‰) values for 3 intestinal nematodes recorded from the European rabbit (Oryctolagus cuniculus)
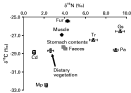
Fig. 3. Seasonal mean δ13C vs δ15N for all samples. Cd, Cittotaenia denticulata; Gs, Graphidium strigosum; Mp, Mosgovoyia pectinata; Pa, Passalurus ambiguus and Tr, Trichostrongylus retortaeformis. In some instances error bars are smaller than the symbols.