Introduction
Insecticide resistance is associated with intensive insecticide exposure due to frequent insecticide use for insect pest control (Georghiou & Taylor, Reference Georghiou and Taylor1977; Roush & McKenzie, Reference Roush and McKenzie1987; McKenzie, Reference McKenzie1996). Insecticide resistance is usually associated with adaptative costs in the absence of insecticides (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000). The result of such costs is the impairment of the reproductive performance of the resistant individuals, due to resource reallocation from a basic physiological process to the protection against insecticides, favoring their survival at the expense of their reproduction (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000; Foster et al., Reference Foster, Denholm and Devonshire2000; Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006).
Adaptative costs associated with insecticide resistance were reported in some populations of the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) (Fragoso et al., Reference Fragoso, Guedes and Peternelli2005; Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Ribeiro et al., Reference Ribeiro, Guedes, Corrêa and Santos2007; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). This insect species is a key pest of cereal grains, whose infestation starts in the field before harvest and extends throughout the storage period (USDA, 1980; Rees, Reference Rees, Subramanyam and Hagstrum1996). The main control method used against maize weevil infestations in warm climates is the use of insecticides, particularly pyrethroids, due to the lack of suitable control alternatives (White & Leesch, Reference White, Leesch, Subramanyam and Hagstrum1996; Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003). The over-reliance on insecticides for maize weevil control has made insecticide resistance a major concern in this species (Guedes et al., Reference Guedes, Lima, Santos and Cruz1995; Subramanyam & Hagstrum, Reference Subramanyam, Hagstrum, Subramanyam and Hagstrum1996; Perez-Mendoza, Reference Perez-Mendoza1999; Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003). Recent demographic and competition studies of pyrethroid-resistant and -susceptible populations of the maize weevil showed that some resistant populations exhibit fitness disadvantages while others do not (Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Corrêa, Damasceno and Santos2005, Reference Oliveira, Guedes, Tótola and De Marco2007). The underlying costs of insecticide resistance and their mitigation mechanisms have not been determined.
O2 consumption or CO2 production are proportional to metabolism and may represent energetic costs (Clarke, Reference Clarke1993; Marais & Chown, Reference Marais and Chown2003). Variations in respiration rate may, therefore, assist in detecting stress and stress response with potential for detecting costs associated with insecticide resistance, while modifications in fat body morphology indicate the availability and mobilization of energy reserves for the individual's maintenance, leading to its survival when exposed to toxic compounds (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006). These patterns and tools of investigation were developed in studies with populations of maize weevil resistant to pyrethroids (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006), where the resistant population, showing mitigation of fitness costs associated with insecticide resistance, exhibited higher respiration rate and body mass than the susceptible and another resistant population. Higher respiration rates may be correlated with larger size, which may help to promote greater energy storage and mitigate costs of insecticide resistance, allowing the maintenance of the resistance mechanism without compromising reproductive performance.
The objective of the present study was to determine whether costs of insecticide resistance are correlated with changes in the activity of specific pathways of intermediary metabolism. These data may eventually lead to studies that determine the mechanisms that reduce the costs of insecticide resistance in populations of the maize weevil. Assays were, therefore, carried out for enzymes involved in sugar and lipid digestion (amylase and lipase) and energy metabolism (glycogen phosphorylase, glycosidae, trehalase and lipase). Lipases are responsible for the mobilization of triglycerides, the main lipid form in insects, while glycogen phosphorylases mobilize glycogen, increasing trehalase levels in the insect hemolymph (Steele, Reference Steele1982; Candy et al., Reference Candy, Becker and Wegener1997; Thompson et al., Reference Thompson, Borchardt and Wang2003; Arrese et al., Reference Arrese, Patel and Soulages2006; Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006). Lipases also catalyze food breakdown in the insect gut to release stored energy, allowing its eventual accumulation (Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006). In addition, lipases are also important in lipid mobilization from the insect fat body (Arrese et al., Reference Arrese, Patel and Soulages2006).
Amylase catalyzes starch breakdown in the insect gut and is a particularly important hydrolytic enzyme in grain beetles, such as the maize weevil, which feed on cereal grains rich in starch (Baker, Reference Baker1986, Reference Baker1988; Baker & Woo, Reference Baker and Woo1992; Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006). In addition, amylase polymorphism has already been reported in populations of the maize weevil, but its significance is not known (Baker, Reference Baker1987). Glycosidases are also important in carbohydrate digestion in the maize weevil (Baker, Reference Baker1991), and the carbohydrate-metabolizing enzyme trehalase is particularly important in energy mobilization because the disaccharide trehalose is the main carbohydrate in the insect hemolymph (Friedman, Reference Friedman, Kerkut and Gilbert1985; Suarez et al., Reference Suarez, Darveau, Welch, O'Brien, Roubik and Hochachka2005; Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006).
The levels of activity of lipid- and carbohydrate-metabolizing enzymes obtained in the present study were compared with results of concentration-mortality bioassays and respiration rates in populations of maize weevil. Higher activity levels of digestive enzymes were expected in the insecticide-resistant population without fitness disadvantage (higher body mass), unlike in the insecticide-susceptible population and the insecticide-resistant population with fitness disadvantage. In contrast, activity levels of nutrient mobilization enzymes were surveyed to document differences in intermediate metabolism among insecticide-susceptible and -resistant populations (with and without associated fitness costs). Higher intermediate metabolism and, consequently, higher levels of some nutrient mobilization enzymes were expected in the insecticide-resistant populations because they are likely to require higher energy mobilization to maintain their resistance mechanisms in addition to their basic physiological processes. The study reported here focused on three insect populations and should be considered a preliminary investigation of how these enzyme levels may explain the observed variation in resistance levels and associated fitness costs.
Material and methods
Insects and chemicals
Three populations of S. zeamais were used in the present investigation. These populations are termed here as ‘susceptible’, ‘resistant cost’ and ‘resistant no-cost’. The susceptible population was collected by mid-1980 in Sete Lagoas County (state of Minas Gerais, Brazil). The resistant cost population was collected in Juiz de Fora County (state of Minas Gerais, Brazil) in 1999. It is resistant to pyrethroids but has reduced fitness in the absence of pyrethroid exposure (Fragoso et al., Reference Fragoso, Guedes and Rezende2003, Reference Fragoso, Guedes and Peternelli2005). The resistant no-cost population was collected in Jacarezinho County (state of Paraná, Brazil) in the late 1980s (Guedes et al., Reference Guedes, Lima, Santos and Cruz1994, Reference Guedes, Lima, Santos and Cruz1995). It is also resistant to pyrethroids but does not demonstrate reduced fitness in the absence of pyrethroid exposure (Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). The two resistant populations share the same major insecticide resistance mechanism (Guedes et al., Reference Guedes, Lima, Santos and Cruz1995; Fragoso et al., Reference Fragoso, Guedes and Rezende2003, Reference Fragoso, Guedes and Oliveira2007).
The three populations were maintained in whole maize grains free of insecticides under controlled temperature (25±2°C), relative humidity (70±5%) and photoperiod (LD 12:12 h). All reagents were purchased from Sigma-Aldrich Química Brasil (São Paulo, Brazil) except acetone, which was obtained from Cromato Prod. Quim (Diadema, São Paulo, Brazil), and technical grade permethrin, which was provided by Syngenta (São Paulo, Brazil).
Insecticide and respirometry bioassays
Insecticide bioassays were carried out as described elsewhere using 20 ml glass scintillation vials (Fragoso et al., Reference Fragoso, Guedes and Rezende2003; Ribeiro et al., Reference Ribeiro, Guedes, Oliveira and Santos2003). Three flasks containing 20 insects each were used in respirometry determinations for each population in a completely closed system (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). Production of CO2 was measured in a CO2 Analiser (TR2, Sable Systems International, Las Vegas, NV, USA) using methods described by Guedes et al. (Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006). The measurements were obtained by injecting CO2-free air into the flasks, which directed the CO2 produced within the flask to an infrared reader connected to the system. Respiration values were presented as μmol CO2 produced per hour. Body mass was determined for insects of each population, after their removal from the respirometer flasks, using an analytical balance (Sartorius BP 210D, Germany).
Preparation of enzyme extracts
Three batches of 300 unsexed adult insects of each population were used as the enzyme source for the determination of glycosidase and trehalase activity after their immersion in 1.5% KCl and subsequent homogenization in 6.0 ml 0.1 M Tris-HCl buffer (pH 8.0). The crude homogenate was filtered through glass-wool and centrifuged at 10,000 g max for 15 min. The pellet was discarded and aliquots of the supernatant were taken for determination of protein content and enzyme activity. Three batches of 100 adult insects (unsexed) were used for the determination of glycogen phosphorylase activity using the same amount of buffer, while batches of 20 insects were used for amylase and lipase determinations in homogenates with 5 ml buffer.
Protein determination and enzyme assays
Protein concentration was determined following Warburg & Christian (Reference Warburg and Christian1941). Glycosidase activity was determined as described by Hill & Orchard (Reference Hill and Orchard2005) and complemented by the method of the reducing sugar using 3,5-dinitrosalicylic acid (DNS) developed by Miller (Reference Miller1959). The substrate was prepared from 1.33% sacharose in 50 mM sodium acetate buffer (pH 5.4). The reaction was started by the addition of 1 ml enzyme extract to 1 ml substrate, which was incubated at 40°C for 20 min. The reaction was stopped with the addition of 1 ml of 0.044 M DNS, which reduced the glycose released in the reaction. The absorbance readings were carried out at 540 nm.
Trehalase activity was determined following Dahlqvist (Reference Dahlqvist1968) and using trehalose as the substrate (50 mM trehalose), also complemented by the reducing sugar method (Miller, Reference Miller1959). Glycogen phosphorylase was determined by the method of Tolman & Steele (Reference Tolman and Steele1980). The inorganic phosphate was determined at 725 nm, following Fiske & Subbarow (Reference Fiske and Subbarow1925), after release from glycose-1-phosphate in the presence of glycogen, which sparked the reaction. Amylase activity was determined with the K003 enzymatic kit from BIOCLIN (QUIBASA – Química Básica Ltda, Belo Horizonte, Minas Gerais, Brazil) by incubating the samples with starch, following the method modified by Caraway (Reference Caraway1959). The soluble starch shows a blue color in the presence of iodine, and the starch hydrolysis by amylase progressively eliminates the blue color. The absorbance is read at 660 nm. Lipase activity was determined using the K025 enzymatic kit, also from BIOCLIN, following methods adapted from Cherry & Crandall (Reference Cherry and Crandall1932). This method is based on the activity of lipases over a glycerol ester, releasing a chromogenic compound quantified at 410 nm. Activity values for amylase and lipase were expressed as amylase units (AU dL−1) and international units (IU), respectively. Amylase unit (AU dL−1) refers to the amount of amylase that hydrolyzes 10 mg starch in 30 min at 37°C, while international unit of lipase activity (IU) refers to the amount of lipase that releases 1 μmol of fatty acid per minute. The kinetic parameters were determined using increasing substrate concentrations and fitting the results into a non-linear regression (Michaelis-Menten equation).
Statistical analyses
Concentration-response bioassays with deltamethrin were analyzed using probit analysis (PROC PROBIT: SAS Institute, 2002). Body mass and respiration rates for the insect population were analyzed using analysis of variance and Fisher's LSD test (p<0.05) (PROG GLM: SAS Institute, 2002). The levels of enzyme activity were analyzed using analysis of variance and Fisher's LSD test (p<0.05), if appropriate. Non-linear regression (Michaelis-Menten equation) was used to estimate the kinetic parameters (K m and V max) using the curve-fitting procedure of SigmaPlot (SPSS, 2000).
Results
Insecticide resistance and respiration rate
The results of the χ2 tests (χ2 and p values) used to measure how well the data of each concentration-mortality curve fit the assumption of the probit model indicate that the model was suitable for the data (low χ2-values and p>0.05; table 1). The resistant cost and no-cost populations showed resistance ratios of 286-fold and 121-fold compared with the susceptible population at the LC50 (table 1). The insect respiration rates also differed among the populations (F2,6=23.05, p=0.003) with the resistant no-cost population respiring at a greater rate than either the susceptible or the resistant cost population (fig. 1a). However, because there were differences in body mass among the insects from these populations (F2,6=82.30, p<0.0001; fig. 1b), which followed the same trend as the respiration rates, we calculated normalized respiration rates (i.e. divided by body mass), which were similar for all three populations (0.022±0.001 μmol CO2/h/mg; F2,6=0.57, p=0.60).

Fig. 1. (a) Respiration rate (μmol CO2 produced/h/insect) (±SEM) and (b) body mass (±SEM) of adult insects from a susceptible and two pyrethroid-resistant populations (cost and no-cost) of the maize weevil, Sitophilus zeamais. Histogram bars with the same letter are not significantly different by Fisher's LSD test (p<0.05).
Table 1. Toxicity of the pyrethroid insecticide permethrin to a susceptible and two pyrethroid-resistant (cost and no-cost) populations of the maize weevil, Sitophilus zeamais.

Enzyme activity
Among the enzymes involved in mobilization of carbohydrate (glycogen phosphorylase and trehalase) and sugar hydrolysis from metabolism (glycosidase), only trehalase activity differed among the populations (F2,6=72.39, p<0.0001), with the insects from the resistant cost population showing higher specific activity than the remaining populations, which showed similar activity (table 2). Activity of glycogen phosphorylase (F2,6=0.25, p=0.79) and glycosidase (F2,6=1.43, p=0.31) were similar in all three populations. In contrast, activity levels of amylase and lipase differed among populations (F2,6=20.19, p=0.002 for amylase; and F2,6=22.80, p=0.002 for lipase), with the resistant no-cost population exhibiting the highest levels of amylase activity and the resistant cost exhibiting the highest levels of lipase activity (table 2).
Table 2. Specific activity of carbohydrate- and lipid-metabolizing enzymes in a susceptible and two pyrethroid-resistant (cost and no-cost) populations of the maize weevil, Sitophilus zeamais.
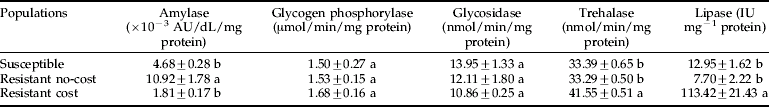
Means (±SEM) followed by the same letter in a column are not significantly different by Fisher's LSD test (p<0.05).
Kinetic trends
Maize weevil trehalase activity follows the Michaelis-Menten kinetics within the range of substrate concentrations used (fig. 2). Amylase and lipase activities from the populations also follow the Michaelis-Menten kinetics (figs 3 and 4) and the kinetic parameters K m and V max were, therefore, estimated. Although the susceptible population showed higher substrate affinity (lower K m) for amylase, lipase and trehalase, it showed lower levels of catalytic activity (represented by lower V max values) than the resistant populations, except for amylase, which was higher than for the resistant cost population, but much lower than for the resistant no-cost population (table 3). The resistant no-cost population showed much higher amylase catalytic activity than the other populations (fig. 3, table 3), while the resistant cost population showed higher trehalase activity and particularly higher lipase activity (fig. 4, table 3), following the trend observed in the preliminary enzyme assays for amylase and lipase (table 2).

Fig. 2. Michaelis-Menten plots of trehalase activity from a susceptible and two pyrethroid-resistant populations (cost and no-cost) of the maize weevil, Sitophilus zeamais (p<0.001; R2>0.90). Insert: Lineweaver-Burk plot (double reciprocal) (p<0.001; R2>0.90). (○, Susceptible; ▿, Resistant cost; □, Resistant no-cost).

Fig. 3. Michaelis-Menten plots of amylase activity from a susceptible and two pyrethroid-resistant populations (cost and no-cost) of the maize weevil, Sitophilus zeamais (p<0.001; R2>0.95). Insert: Lineweaver-Burk plot (double reciprocal) (p<0.001; R2>0.90). (○, Susceptible; ▿, Resistantant cost; □, Resistant no-cost).

Fig. 4. Michaelis-Menten plots of lipase activity from a susceptible and two pyrethroid-resistant populations (cost and no-cost) of the maize weevil, Sitophilus zeamais (p<0.001; R2>0.90). Insert: Lineweaver-Burk plot (double reciprocal) (p<0.001; R2>0.80). (○, Susceptible; ▿, Resistant cost; □, Resistant no-cost).
Tabela 3. Kinetic parameters of amylase, lipase and trehalase from an insecticide-susceptible and two insecticide-resistant (cost and no-cost) populations of the maize weevil, S. zeamais.

Discussion
The levels of permethrin resistance, body mass and the respiration rates observed in the present study reinforce those obtained by Guedes et al. (Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006), who hypothesized that the higher respiration rate, body mass and energy reserves from an insecticide-resistant population may mitigate the fitness cost usually associated with insecticide resistance. Such mitigation allows the maintenance of insecticide resistance mechanisms without impairing other physiological processes, such as reproduction. The study reported here aimed to further test this hypothesis and to provide insights on the potential mechanisms underlying the mitigation of insecticide resistance costs.
As previously reported by Guedes et al. (Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006), the resistant no-cost population respired at a greater rate as a consequence of its greater body mass than the other populations. In contrast, the body mass and respiration rate of the resistant cost population was similar to those of the susceptible population. This may be a consequence of the need for higher energy mobilization in the resistant cost insects compared with those from the resistant no-cost population to maintain the higher levels (>two-fold) of insecticide resistance observed in the resistant cost population.
Differences in activity among enzymes from intermediary metabolism were expected between susceptible and resistant populations and also between resistant populations with and without fitness costs associated with insecticide resistance. Indeed, some differences were observed in the present study. Activity of trehalase, amylase and lipase were always higher in one of the resistant populations. The resistant cost population exhibited higher activity of trehalase and lipase than the resistant no-cost population, which exhibited higher amylase activity. The differences were particularly high for amylase and lipase.
Amylase cleaves starch and related polysaccharides, allowing their eventual storage and use as an energy source, which becomes the substrate of activity of another group of carbohydrases (e.g. glycosidase and trehalase) that hydrolyze oligosaccharides and disaccharides (Baker, Reference Baker1991; Chown & Nicolson, Reference Chown and Nicolson2004). Trehalase, which is widespread in insects, hydrolyses trehalose into glucose (Friedman, Reference Friedman, Kerkut and Gilbert1985; Suarez et al., Reference Suarez, Darveau, Welch, O'Brien, Roubik and Hochachka2005; Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006); lipases are involved in both lipid digestion and lipid mobilization, but we were unable to distinguish both classes in our in vitro bioassays (Arrese et al., Reference Arrese, Patel and Soulages2006; Kunieda et al., Reference Kunieda, Fujiyuki, Kucharski, Foret, Ament, Toth, Ohashi, Takeuchi, Kamikouchi, Kage, Morioka, Beye, Kubo, Robinson and Maleszka2006). Lipid hydrolysis was more efficient in the resistant cost population, while starch digestion was more efficient in the resistant no-cost population. The higher activity of amylases in the resistant no-cost population is suggestive of its greater efficiency of energy extraction from the food and greater consequent storage, resulting in a higher body mass, confirming previous results by Guedes et al. (Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006). The higher trehalase activity in the resistant cost population is probably indicative of greater energy mobilization, minimizing its potential accumulation and consequent increase in body mass. The importance of higher lipase activity in the resistant cost population is difficult to assess because the enzyme source was the whole insect body and the lipase classes (involved in either lipid digestion or lipid mobilization) were not distinguished. However, considering the higher trehalase activity and the clear presence of resistance costs in the resistant cost population (Fragoso et al., Reference Fragoso, Guedes and Peternelli2005; Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007), the greater lipase activity observed in this population seems more likely an indicator of greater lipid mobilization.
Body mass is greater in the resistant no-cost population compared with the resistant cost population, which is a more resistant population and probably requires higher energy mobilization for the maintenance of its higher level of insecticide resistance (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). Higher amylase activity seems, therefore, a particularly efficient tactic to better exploit this insect food source (i.e. maize grains, which are a rich source of starch) leading to more efficient energy storage and greater body mass. Lipid digestion is poorly understood in insects (Arrese et al., Reference Arrese, Canavoso, Jouni, Pennington, Tsuchida and Wells2001) and may not be as important as starch digestion in maize weevil populations in relation to the mitigation of insecticide resistance costs because the resistant cost population, with high lipase activity, show a fitness disadvantage in the absence of insecticide (Fragoso et al., Reference Fragoso, Guedes and Peternelli2005; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007). Furthermore, part of the lipase activity detected in the present study is involved in energy mobilization, which is likely to be greater in the resistant cost population, partially preventing lipid accumulation and increase in body mass in this population, while allowing the maintenance of its higher levels of insecticide resistance.
Trehalase activity was greater in the resistant cost population, followed by the resistant no-cost population, suggesting that energy mobilization is also greater in the insecticide resistant populations. The K m values for trehalase from the resistant populations indicate lower affinity for the substrate (i.e. trehalase) than the enzyme from the susceptible population. The higher trehalase activity observed in the resistant populations is probably due to the higher levels of these enzymes in the resistant insects. Since trehalase activity was particularly higher in the resistant cost population, sugar mobilization is probably higher in insects from this population, preventing energy storage at the levels observed for the resistant no-cost population (Guedes et al., Reference Guedes, Oliveira, Guedes, Ribeiro and Serrão2006; Oliveira et al., Reference Oliveira, Guedes, Tótola and De Marco2007).
The other enzymes involved in energy mobilization and studied here (i.e. glycogen phosphorylases and glycosidase) were similar in all three populations. Therefore, trehalase and lipase are probably the main enzymes responsible for the higher energy mobilization in the resistant populations, which are required for maintaining active insecticide resistance mechanisms in the resistant insects. Extensive selection with insecticide may, however, favor selection of modifier genes that mitigate such cost. The mitigation of the cost of insecticide resistance may minimize the need for this high energy mobilization and favor energy storage instead (through a higher efficiency of starch digestion and consequent energy uptake), resulting in the maintenance of both insecticide resistance mechanisms and basic physiological processes without significant trade-offs between them, as observed in the resistant no-cost population, but not in the resistant cost population.
The high levels of either amylase or lipase activity in insecticide-resistant populations, showing or not showing associated fitness costs leading to fitness disadvantages in the absence of insecticides, may underlay the physiological basis of cost-mitigating mechanisms in pyrethroid-resistant populations. The purification and characterization of these enzymes in these populations should shed further light on this phenomenon, and future surveys of insecticide resistance in maize weevil associating insecticide resistance with fitness disadvantage and energy storage (and mobilization) will allow the testing of the suggested basis of physiological costs associated with insecticide resistance and its mitigation. In addition, our study focused on three insect populations, and it will be important to expand it to more populations of maize weevil (and even of other species) to determine the general physiological patterns of costs of insecticide resistance and their mitigation mechanisms.
Acknowledgements
The financial support, provided by the Minas Gerais State Agency for Research Aid (FAPEMIG) and the National Council of Scientific and Technological Development (CNPq), was greatly appreciated. The provision of technical grade permethrin by Syngenta was also greatly appreciated. The thoughtful comments and suggestions provided by the editor and two anonymous referees were appreciated, as well as the editing of the manuscript by Dr J.E. Throne.









