Introduction
Polychlorinated biphenyls (PCBs) are among the major persistent organic pollutants (POPs). In the past, commercial mixtures of PCBs were widely used in numerous industrial applications, including the production of dielectric fluid for electrical transformers, flame retardants, plasticizers, resins, waxes, paints, copy paper, and sealants for wood and cement surfaces (FAO/UNEP 1992). PCB congeners are stable, persistent, highly lipophilic, and show particle reactivity and low vapour pressure. As a result, these toxic compounds are ubiquitously distributed throughout both aquatic and terrestrial environments and tend to bioaccumulate through the food chain, thus threatening ecosystems and human health.
PCBs were first reported in Antarctica in the 1960s and 1970s (Risebrough et al. Reference Risebrough, Rieche, Peakall, Herman and Kirven1968, Reference Risebrough, Walker, Schmidt, De Lappe and Connors1976). Their presence in this relatively isolated region is thought to be the result of long-range transport in the atmosphere, precipitation, and cold condensation (Wania & Mackay Reference Wania and Mackay1993, Loganathan & Kannan Reference Loganathan and Kannan1994). On the other hand, several studies pointed out the pollution from local sources of POPs around research stations (Risebrough et al. Reference Risebrough, De Lappe and Younghans-Haug1990, Larsson et al. Reference Larsson, Järnmark and Södergren1992, Borghini et al. Reference Borghini, Grimalt, Sanchez-Hernandez and Bargagli2005, Klanova et al. Reference Klanova, Matykiewiczova, Macka, Prosek, Laska and Klan2008). Therefore, increasing PCB levels in various environmental matrices in Antarctica are a matter of growing concern. Several reports have documented PCB levels in Antarctic soil, seawater, and sediment (Fuoco et al. Reference Fuoco, Colombini, Ceccarini and Abete1996, Montone et al. Reference Montone, Taniguchi and Weber2001b, Negoita et al. Reference Negoita, Covaci, Gheorghe and Schepens2003), as well as in various organisms from various trophic levels (Reinhardt & Van Vleet Reference Reinhardt and Van Vleet1986, Focardi et al. Reference Focardi, Bargagli and Corsolini1995, Montone et al. Reference Montone, Taniguchi, Sericano, Weber and Lara2001a, Weber & Goerke Reference Weber and Goerke2003).
The accumulation of PCBs occurs mainly in soil, which plays an important role in their global fate, and this process occurs via mass flow in the atmosphere. In addition, vegetation is the primary site of bioaccumulation via the uptake of lipophilic organic compounds from the air into plant tissues. In Antarctica, the distribution of terrestrial vegetation is limited to ice free areas, mainly coastal regions, and biodiversity is restricted to mostly lichens and mosses. Lichens are slow-growing organisms with large surface areas that facilitate the direct absorption of pollutants from the atmosphere. Therefore, lichens have been widely used for biomonitoring air pollution. However, there have been few studies on PCBs in Antarctic lichens (Focardi et al. Reference Focardi, Gaggi, Chemello and Bacci1991, Negoita et al. Reference Negoita, Covaci, Gheorghe and Schepens2003).
This study was performed to determine the distribution and concentration profiles of PCBs in the air, soil, and lichen collected in the maritime Antarctic. To evaluate the potential risk to the Antarctic ecosystem, the concentrations of 12 dioxin-like PCB congeners listed by the World Health Organization (WHO) were measured in soil and in the lichen Usnea aurantiaco-atra (Jacquinot) Bory, which has a circumpolar distribution with the greatest abundance along the Antarctic Peninsula.
Materials and methods
Sample collection
King George Island, the largest island in the South Shetland Islands, is located c. 120 km north of the Antarctic Peninsula (Fig. 1). Most of the island is covered with glaciers, and outcrops are exposed only along the shorelines in restricted areas. The island has a cold oceanic climate and with an average annual temperature of -1.8°C to -1.6°C, relative humidity of 89%, precipitation of 437.6 mm, and a mean wind velocity of 7.9 m s-1, predominantly from the north-west and south-west, as recorded at the Korean Antarctic Research Station (King Sejong Station, 62°13′S, 58°47′W) on Barton Peninsula (Lee et al. Reference Lee, Yoon, Chae and Choi2007). Most of the ice free area is covered by relatively abundant vegetation, dominated by cryptogamic species. The flora includes two flowering plants, 33 bryophytes, and 62 lichens (Lee Reference Lee1992, Kim et al. Reference Kim, Ahn, Hong, Andreev, Lim, Oh, Koh and Hur2006). The Chottaebawi area on the southern coast of Barton Peninsula is one of the most important penguin colonies in the maritime Antarctic region (Fig. 1). In addition, the cape area near the King Sejong Station is populated by seabirds, mainly south polar skua (Catharacta maccormicki Saunders) and penguins (Pygoscelis papua Forster and Pygoscelis antarctica Forster etc.). In total, 14 soil samples were collected near the King Sejong Station. Five soil samples for the analysis of all PCB congeners were collected in January 2006, and nine additional soil samples and eight lichen samples were collected for the analysis of 12 dioxin-like PCB congeners in January 2008 in the ice free area (Fig. 1). After collection, all samples were stored at -20°C until extraction. Locations and detailed descriptions of sampling sites are shown in Table I and Fig. 1.

Fig. 1 Locations of the study area and sampling sites. For details refer to Table I.
Table I Description of samples and sampling locations.

alichen: Usnea aurantiaco-atra.
Analytical procedure
Samples were treated, extracted, and analysed according to the US Environmental Protection Agency method 1668A (US EPA 1999). Samples of approximately 50 g of soil were spiked with 13C-labelled PCB internal standards (1, 3, 4, 15, 19, 37, 54, 77, 81, 104, 105, 114, 118, 123, 126, 155, 156, 157, 167, 169, 188, 189, 202, 205, 206, 207, and 209), and extracted for 16 h using a hot Soxhlet manifold with 300 ml of toluene. The extract was collected and concentrated to c. 2 ml using a rotary evaporator, and further purified using a column filled with silica gel. The column consisted of (from bottom to top) quartz glass wool, 0.9 g of silica gel, 3 g of 2% KOH silica gel, 0.9 g of silica gel, 4.5 g of 44% H2SO4 silica gel, 6 g of 22% H2SO4 silica gel, 0.9 g of silica gel, 3 g of 10% AgNO3 silica gel, and 6 g of sodium sulphate. The column was washed with 50 ml of hexane immediately prior to use. The extract was passed through the column and eluted with 120 ml of hexane. The eluate was concentrated to 5 ml using a rotary evaporator and then reduced to 0.5 ml under a gentle stream of nitrogen gas. Finally, 1.2 ng of 13C12-labelled injection standards (9 l, 52 l, 101 l, 138 l and 194 l) were added as internal standards prior to analysis. Dioxin-like PCB congeners were identified and quantified by high-resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) on a DFS mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) using EPA method 1668A (US EPA 1999) for PCB congeners. The mass spectrometer was operated in electron impact mode (36 eV) with helium gas as a carrier. The GC column was a DB-5MS fused silica column (60 m × 0.32 mm i.d., 0.20 μm film thickness). The column oven temperature was programmed to increase at a rate of 20°C min-1 from an initial temperature of 120°C (3 min hold) to a temperature of 220°C (5 min hold), then at 4°C min-1 to 260°C (17 min hold). The injector, transfer line, and ion source were all at a temperature of 260°C. The identification of dioxin-like PCBs was performed based on the retention times of the 13C-labelled standard and isotope ratios M/(M + 2) or (M + 2)/(M + 4). The concentrations of native analytes with corresponding 13C-labelled surrogate standards were calculated using the isotope dilution method of quantification based on EPA method 1668A (US EPA 1999). The average recovery of internal standards was 89 ± 27%. The limits of detection for selected major PCBs found in samples were 0.001–0.041 pg g-1 dry weight.
The total carbon (TC) content of the nine soil samples collected in 2008 were analysed using an element analyser (FlashEA 1112; Thermo Fisher Scientific) by measuring CO2 generated by combustion at 950°C. The total inorganic carbon (TIC) content was analysed using a CO2 coulometer (UIC Inc, Joliet, IL, USA) by measuring the CO2 gas generated by reaction of c. 50 mg of powdered bulk samples with 42.5% phosphoric acid at 80°C for 10 min. The total organic carbon (TOC) content was determined from the difference between the TC and TIC content.
Results and discussion
PCB congener distribution and concentrations in soil
At five sampling location (Fig. 1, Sites A–E), the distribution and the concentration of each PCB congener in soil were analysed. Results of detected PCBs (ΣPCB) in five soil samples collected in 2006 are shown in Table II. Thirty-two PCB congeners (IUPAC numbers 1, 2, 3, 4, 15, 18, 19, 28, 37, 52, 77, 81, 101, 105, 111, 114, 118, 126, 128, 153, 155, 156, 157, 167, 169, 178, 180, 189, 202, 205, 206, and 209) were identified in soil samples taken from Sites A–E. The mean concentration of ΣPCB in these five samples was 22.0 pg g-1 dry weight (range, 8.0–33.8 pg g-1 dry weight; Table II). The soil samples taken from Sites B and D showed the highest and lowest ΣPCB concentrations, respectively. The PCB levels of surface soils from Barton Peninsula were comparable to those in soils from other areas in Antarctica, but much lower than those in soils from James Ross Island, West Antarctica (Klanova et al. Reference Klanova, Matykiewiczova, Macka, Prosek, Laska and Klan2008), and slightly lower than those in soils from East Antarctic coastal regions (Negoita et al. Reference Negoita, Covaci, Gheorghe and Schepens2003). The presence of PCBs in Antarctica is thought to be the result of global contamination through long-range transport, as well as local contamination by human activity at the research stations. Recently, Choi et al. (Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008) reported that the atmospheric levels of 31 PCB congeners detected in this area using passive air samplers were 2.1–8.07 pg m-3, and they observed that PCB levels in atmospheric samples decreased with increasing distance from the main building of King Sejong Station. The study area is located downwind from not only ten other research stations operating on King George Island, but also from a Chilean air force base, thus, it is probable that atmospheric PCB contamination also occurred through local emissions. But PCB concentration in soil samples of this study did not show any significant correlation with distance from the research station. The most abundant classes of PCB isomers were of low molecular weight. All soil samples (Sites A–E) had similar homologue patterns, with high fractions of di-, tri-, and penta-CBs accounting for more than 75% of the total homologue concentrations (Fig. 2). This PCB profile might be suggestive of long range transport from distant sources to the polar region. In contrast, two soil samples collected near the research station (Sites A and E) showed elevated highly chlorinated homologues (penta-CBs and higher) among five samples, implying the potential influence of local pollution. In addition, the significant levels of highly persistent congeners, such as PCBs 118, 153, and 180, may suggest local contamination of soil caused by wildlife, such as seabird excrement and carcasses (Table II).
Table II Concentrations of PCB congeners (pg/g dry weight) in five soil samples from Barton PeninsulaFootnote a.

a ND = Not detected.
b Coplanar (dioxin-like) congeners are shown in bold.

Fig. 2 PCB homologue patterns in Antarctic soil.
Dioxin-like PCBs in soil
The dioxin-like PCB congeners (IUPAC numbers 77, 81, 105, 114, 118, 123, 126, 156, 157, 167, 169, and 189) listed by the WHO were measured in nine soil samples collected in 2008 (Table III). The sum of all 12 dioxin-like PCB congeners (Σ12PCB) showed a mean concentration of 3.666 pg g-1 dry weight (range 0.639−13.477 pg g-1 dry weight) among the nine samples collected. As shown in Table III, clear site-to-site variation and a wide range in values were observed. In particular, the soil sample from Site I showed the highest level of Σ12PCB (13.477 pg g-1 dry weight). The PCB concentrations in soil samples appeared to be unaffected by geological characteristics, but did appear to be affected by the presence of animal life. The soil sample from Site I was collected on the beach with nearby colonies of seabirds (penguins and skuas) and marine animals (fur seals and elephant seals), and seabird droppings and penguin carcasses were commonly found in this area. These observations suggested that such high levels of PCBs were influenced by animal-derived pollutants. Several recent papers have suggested that the bioaccumulation of POPs can result in heavy contamination on a local scale (Negoita et al. Reference Negoita, Covaci, Gheorghe and Schepens2003, Goerke et al. Reference Goerke, Weber, Bornemann, Ramdohr and Plotz2004, Yogui & Sericano Reference Yogui and Sericano2008). The TOC contents of soil samples from Sites F−N ranged from 0.15−2.40 wt.%. In general, ΣPCB content is known to correlate well with the soil organic carbon content (e.g. Cousins et al. Reference Cousins, Gevao and Jones1999). Figure 3 shows Σ12PCB concentration and normalized Σ12PCB concentration to soil TOC content. Correlation analysis showed a high correlation coefficient between Σ12PCB concentration and TOC content in soil samples (r = 0.768, P ≤ 0.05). However, two soil samples collected from sites F and N show high Σ12PCB/TOC values, although their Σ12PCB concentrations were lower than mean Σ12PCB concentration. The soil sample from Site F (near the station) shows the lowest TOC content (0.15 wt.%) but highest Σ12PCB/TOC ratio, suggestive of local contamination caused by human activities near the station. The soil sample collected from site N also shows relatively high Σ12PCB/TOC value compared with its low TOC content (0.26 wt.%). It can be explained by the fact that no vegetation was present at site N because it is newly exposed due to recent glacial retreat.
Table III Concentrations of dioxin-like PCB congeners in soil and lichen samples collected from Barton Peninsula.

a pg g-1, b Choi et al. (Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008), c pg m-3, d wt.%, ND: not detected.

Fig. 3 Concentrations of Σ12PCB congeners, and Σ12PCB/TOC in soil samples from Barton Peninsula. Insert: Σ12PCB concentrations and TOC contents in soil samples.
Dioxin-like PCBs in lichen
Lichen is the dominant type of terrestrial vegetation in Antarctica, and U. aurantiaco-atra is commonly found not only in and around the coastal region of the ice free area, but also in adjacent island areas. The concentrations of pollutants measured in lichens reflect absorption from the atmosphere, which is integrated over a long period and should be correlated with the mean atmospheric concentration of pollutants because of the lack of root-like structures in lichen (Villeneuve et al. Reference Villeneuve, Fogelqvist and Cattini1988). However, there have been only a few reports regarding the presence of POPs in lichens (Villeneuve et al. Reference Villeneuve, Fogelqvist and Cattini1988, Negoita et al. Reference Negoita, Covaci, Gheorghe and Schepens2003, Yogui & Sericano Reference Yogui and Sericano2008). In this respect, the concentration of dioxin-like PCB congeners in U. aurantiaco-atra collected on Barton Peninsula were measured (Table III). The mean concentration of Σ12PCB was 17.133 pg g-1 dry weight (range 5.49–43.765 pg g-1 dry weight). In general, the PCB concentrations in lichen samples decreased with increasing distance from the research station, except at Sites H and I (Table III). These observations were in good agreement with a previous atmospheric analysis from the King Sejong Station (Choi et al. Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008). However, the lichen samples from Sites H and I showed very high levels of PCBs (43.8 pg g-1 and 34.4 pg g-1, respectively), suggesting the influence of local pollution. As these two sites are located near a penguin rookery and skua nests, respectively, the high PCB levels in these samples were thought to be influenced by wildlife, such as seabird droppings and carcasses. The dominant PCB congeners in the lichens were PCB 118 and PCB 105. Interestingly, the PCB 114 congener was detected in over 30% of lichen samples from Sites I and J, but was detected at markedly lower levels in soil samples from the same sites. PCB 118 was also detected at higher levels than other congeners in soil and atmospheric samples (Choi et al. Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008). Focardi et al. (Reference Focardi, Bargagli and Corsolini1995) reported that the most abundant congeners were penta-CB, hexa-CB, and hepta-CB in Antarctic fish, skua, penguins, and Weddell seals, because lower chlorinated biphenyl homologues are the most biodegradable PCB congeners. Comparison of the compositions of PCB congeners in soil and lichen samples showed some important differences (Fig. 4). The soil samples showed markedly higher concentrations of hexa-CBs, but the lichen samples contained large amounts of penta-CBs because of the lack of PCB 169.

Fig. 4 PCB homologue compositions (%) in soil and lichen samples from Barton Peninsula.
Comparison of dioxin-like PCBs in soil, lichen, and air
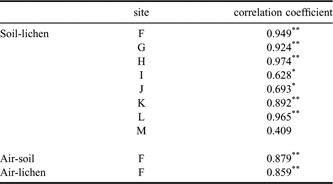
The greater proportion of PCB compounds found in Antarctica were probably transported through the atmosphere and deposited in the soil and lichen. Kallenborn et al. (Reference Kallenborn, Oehme, Wynn-Williams, Schlabach and Harris1998) reported the atmospheric long-range transport of POPs from South America to Signy Island in the South Orkney Islands. Furthermore, Montone et al. (Reference Montone, Taniguchi and Weber2003) reported a similar trend for PCBs on King George Island and Choi et al. (Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008) recently discussed the potential relationship between atmospheric PCB levels and long-range transport in the same region examined here. The sampling site F in this study is the same site that Choi et al. (Reference Choi, Baek, Chang, Wania, Ikonomou, Yoon, Park and Hong2008) collected air samples with passive air samplers. The relationship between the atmospheric and soil/lichen PCB profiles (Site F) was investigated by calculating Spearman’s correlation coefficients (Table IV). The PCB congener concentrations showed a strong correlation between air and soil (r = 0.879, P < 0.01), suggesting that these congeners accumulated in soil by partitioning from the atmosphere. A high correlation coefficient was also observed between air and lichen concentrations (r = 0.859, P < 0.01), which can be explained by the direct absorption of PCBs from the atmosphere due to the absence of root-like structures in lichen. The Σ12PCB level was about 5-fold higher in lichen than in soil. Soil and lichen samples collected from the same sites also showed significant correlations in PCB congener concentrations, except at Site M. Despite the limited number of sites these correlations suggest that atmospheric transport may be the main source of PCBs in the study area.
Table IV Correlations between PCB congener concentrations in air and soil samples, air and lichen samples, and soil and lichen samples collected at the same site.
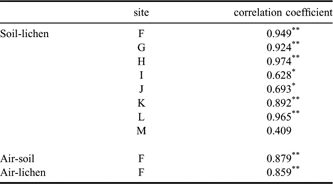
**P < 0.01, *P < 0.05.
In conclusion, the surface soil and lichen samples collected on King George Island showed very low levels of PCBs. Several pathways for PCB contamination in soil and lichen samples were suggested. Long-range atmospheric transport is thought to be the main source of PCBs detected on King George Island. However, PCB levels in soil and lichen samples also appeared to be influenced by local sources, such as the presence of wildlife. Our results suggested that, on a local scale, bioaccumulation and biologically mediated transport of PCBs could cause greater contamination than human impact from the research station. Therefore, further comprehensive studies on bioaccumulation are needed to clarify the pathways of PCB contamination in this area.
Acknowledgements
This study was supported by the Korea Polar Research Institute (grants PE09110, PE09040, and PE09010). We are grateful to Professor Yong Il Lee and Dr Jae Il Lee for their help with field work. The critical reviews of two anonymous reviewers were greatly appreciated.