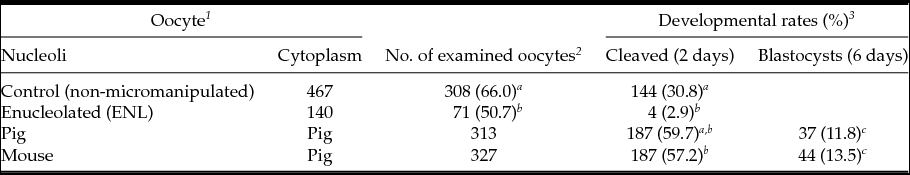Introduction
The nucleoli of growing mammalian oocytes are composed of three well defined compartments: the fibrillar centre (FC), dense fibrillar component (DFC) and granular component (GC). The active oocyte nucleolus is the site for transcription of ribosomal DNA, processing of rRNA transcripts and assembling of pre-ribosomal particles (Lefevre, Reference Lefevre2008). At the end of the oocyte growth phase the gradual downregulation of nucleolar synthetic activity occurs and this change is accompanied by the transformation of these nucleoli into unique structures – nucleolus-like bodies (NLBs or NPBs – nucleolus precursor bodies; to avoid some confusion the term ‘nucleolus like bodies – NLBs’ will be used throughout this article) (Shishova et al., Reference Shishova, Lavrentyeva, Dobrucki and Zatsepina2015). Typically, single large and morphologically distinguishable NLB is seen in various species oocytes including mouse, rat, pig, cattle, and human (Tesarik et al., Reference Tesarik, Travnik, Kopecny and Kristek1983; Antoine et al., Reference Antoine, Lepoint, Baeckeland and Goessens1987; Kopecny et al., Reference Kopecny, Biggiogera, Laurincik, Pivko, Grafenau, Martin, Fu and Fakan1996; Hyttel et al., Reference Hyttel, Viuff, Fair, Laurincik, Thomsen, Callesen, Vos, Hendriksen, Dieleman, Schellander, Besenfelder and Greve2001; Ogushi et al., Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008). During the germinal vesicle breakdown (GVBD), NLBs are disassembled and dissolved in the oocyte cytoplasm but after meiotic division and fertilization, NLBs appear again in male and female pronuclei (Flechon & Kopecny, Reference Flechon and Kopecny1998). It has been believed that in developing embryos, NLBs gradually change their morphology again, and embryos at more advanced stages of development regain typical differentiated and transcriptionally active nucleoli. The very recent results, however, demonstrate that the oocyte NLB material rather persists in zygotes and early embryo developmental stages and is only necessary during a very short time period post fertilization. Functional embryonic nucleoli are then rather formed de novo. (Fulka & Aoki, Reference Fulka and Aoki2016). This formation of functional fibrillogranular nucleoli is accompanied with the activation of ribosomal RNA genes and de novo ribosomal synthesis as a part of major transcriptional activation (MTA; Bjerregaard et al., Reference Bjerregaard, Wrenzycki, Strejcek, Laurincik, Holm, Ochs, Rosenkranz, Callesen, Rath, Niemann and Maddox-Hyttel2004), occurring at different developmental embryonic stages depending on mammalian species (e.g., 2-cell stage in mice, 4-cell stage in pig and at the 8-cell to 16-cell stage in human and cow) (Fulka & Fulka Jr, Reference Fulka and Fulka2010).
Ogushi et al. (Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008) reported that mouse and pig oocytes from which NLBs were micro-surgically removed (enucleolated – ENL) from germinal vesicles are capable to resume meiosis in culture and extrude the first polar bodies. After their fertilization or parthenogenetic activation, pronuclei lacking NLBs were, however, formed. These zygotes cleave up to 2-cell or 4-cell stage but they never reached the blastocyst stage (Lefevre, Reference Lefevre2008). Interestingly, when NLBs from germinal vesicle stage oocyte were re-injected into mature eggs that were previously enucleolated, normal NLBs were formed in zygotes (pronuclei) after fertilization and these zygotes developed to the blastocysts stage (Ogushi & Saitou, Reference Ogushi and Saitou2010). These nucleolus re-injection experiments clearly indicate that NLBs from mature oocytes are necessary for further embryonic development. Later on, it has been demonstrated that NLBs are essential for correct chromatin remodeling, especially during very early post fertilization stages (Fulka & Langerova, Reference Fulka and Langerova2014; Kyogoku et al., Reference Kyogoku, Kitajima and Miyano2014).
So far it has not been reported that NLBs originated from the oocyte of one mammalian species could support rRNA transcription, nucleologenesis and embryonic development of another mammalian species. In other words, it has not been reported whether development of enucleolated oocytes of one species can be rescued by the re-injection of NLBs from another species. The aim of our current experiments was to test this in experiments in which mouse NLBs were re-injected into previously enucleolated porcine oocytes.
Materials and Methods
Chemicals and media
Unless otherwise indicated, all chemicals were purchased from Sigma Chemical Co. (Prague, Czech Republic).
Collection of porcine germinal vesicle (GV) stage oocytes and their in vitro maturation
Porcine ovaries were obtained at a local slaughterhouse and transported to the laboratory in a Dulbecco's phosphate-buffered saline supplemented with 0.01% polyvinyl-alcohol (PBS-PVA) at 30–35°C. After three times washing with PBS-PVA, the GV stage oocytes were aspirated from large antral follicles (3–5 mm, in diameter) as previously reported (Barnetova et al., Reference Barnetova, Morovic, Strejcek, Østrup, Hyttel, Niemann, Laurincik, Fulka and Fulka2012). The oocytes surrounded by several layers of compact cumulus cells (cumulus–oocyte complexes – COCs) were selected and washed three times in M2 medium. COCs were in vitro matured in bicarbonate-buffered medium 199 supplemented with 4 mg/ml of bovine serum growth proteins (GPBos; Sevapharma, Prague, Czech Republic), 0.5 μg/ml sheep FSH (Sigma, F8174), 0.5 μg/ml sheep LH (Sigma, L5269), 40 μg/ml sodium pyruvate, 70 μg/ml l-cysteine and 50 μg/ml gentamicin (GIBCO Invitrogen, Prague, Czech Republic) (IVM) for 30 min at 38.5°C and 5% CO2 in humidified atmosphere. Subsequently, the cumulus cells were completely removed by a narrow-bore pipette and the oocytes were enucleolated, i.e. NLBs were micro-surgically removed from them (Fulka et al., Reference Fulka, Moor, Loi and Fulka2003). The enucleolated oocytes (ENL) were cultured for approximately 40 h and then freshly isolated NLBs, either from porcine or mouse immature oocytes, were injected into them. The nucleoli (NLBs) re-injected oocytes were then parthenogenetically activated (see below). Non-micromanipulated (intact) porcine oocytes were used as controls; i.e. matured in vitro and then parthenogenetically activated.
Collection of mouse GV stage oocytes and their cultivation in vitro
Mouse ovaries were isolated from 6- to 10-week-old ICR female mice (Anlab, Prague, Czech Republic). To obtain GV stage oocytes, females were injected with 5 IU equine chorionic gonadotropin (eCG; Intervet International, Boxmeer, Holland) and after about 44 h, the GV oocytes were collected from large antral follicles. COCs were briefly pre-cultured in αMEM supplemented with BSA (4 mg/ml), gentamicin (50 µg/ml), Na-pyruvate (20 mM) and dbcAMP (150 µg/ml) at 37°C under an atmosphere of 5% CO2 in air. The oocytes were then freed from follicular cells and used for enucleolation.
Enucleolation of porcine and mouse GV oocytes
The porcine and mouse GV stage oocytes were enucleolated (ENL) essentially as described previously (Fulka et al., Reference Fulka, Moor, Loi and Fulka2003; Ogushi et al., Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008). Briefly, GV stage oocytes were manipulated with a micromanipulator equipped with a Piezo injector (PrimeTech, Tsukuba, Japan) in M2 medium supplemented with 7.5 μg/ml cytochalasin D (CD), 0.1 μg/ml demecolcine (DM) and 0.1% PVA (Enu-M2). Immediately before the enucleolation, the porcine oocytes were centrifuged for 5 min at 1350 g in Enu-M2 to visualize GV NLBs. Following the centrifugation, the oocytes were transferred into 10 μl droplets of Enu-M2 covered with paraffin oil their zonae pellucidae (ZP) were opened by Piezo pulses and the enucleolation pipette (inner diameter; 10 μm) inserted into the vicinity of NLBs. Then a mild suction was applied and NLBs were removed first from GVs and subsequently from the oocyte. The mouse oocytes were enucleated similarly.
Nucleolus re-injection and parthenogenetic activation
Freshly isolated porcine or mouse NLBs were re-injected into the matured ENL porcine eggs (P + P and P + M, respectively), as described previously (Ogushi et al., Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008). The NLB re-injected and intact (parthenogenetic control) porcine MII oocytes were cultured in porcine zygote medium-3 (PZM3) supplemented with 3 mg/ml BSA (PZM3-BSA) for 4 h at 38.5°C, 5% CO2 in air and then parthenogenetically activated. The oocytes were transferred into 5.5% mannitol solution and activated by a single DC pulse of 1500 V/cm for 100 μs (CF-150; BLS, Budapest, Hungary). The activated oocytes were incubated in PZM3-BSA medium supplemented with 5 µg/ml cytochalasin B (CB) for 6 h to inhibit the second polar body extrusion. Then activated oocytes were cultured in CB-free PZM3-BSA medium for 6 days.
Immunodetection of nucleolar proteins
The following primary antibodies against key nucleolar proteins were used: mouse monoclonal anti-nucleolin (C23; 1:1000) (Santa Cruz, sc-8031) and mouse monoclonal anti-upstream binding factor (UBF; 1:50) (Abnova, Taiwan, H00007343-M01).
The embryos of all groups were collected at the 2-cell stage (22 to 24 hpa; h post activation), 4-cell stage (46 to 48 hpa), 8-cell stage (70 to 72 hpa) and blastocyst stage (140 to 144 hpa). The embryos were fixed in 4% paraformaldehyde (PFA) for 1 h at room temperature (RT), permeabilized in 0.2% Triton X-100 in PBS for 1 hour at RT, blocked in 5% goat serum in PBS for 2 h and incubated overnight with the appropriate primary antibody at 4°C. Redundant primary antibodies were removed by washing three times in PBS with bovine serum albumin (3 mg/ml; PBS-BSA) prior to incubation with Alexa fluor 488 goat anti-mouse (C23) and Alexa 594 goat anti-mouse (UBF) secondary antibodies for 2 h at RT. Finally, the embryos were mounted on glass in Vectashield mounting medium (Vector Laboratories, Peterborough, UK) containing 4,6-diamidino-2-phenylindole (DAPI) and observed under the fluorescence microscope (Olympus IX 71; Olympus Optical Co., Tokyo, Japan).
Autoradiography
Embryos harvested at above defined time points were labeled by 3H-uridine (sp. act. 962 GBq/mmol; Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany) at a final concentration of 4 MBq/mmol (Laurincik et al., Reference Laurincik, Thomsen, Hay-Schmidt, Avery, Greve, Ochs and Hyttel2000) for 20 min in gas-equilibrated culture medium. After incubation with the radioactive precursor, the specimens were repeatedly washed in 3H-uridine free culture medium and fixed as described below.
Processing for light microscopic autoradiography and transmission electron microscopy
Three to five embryos per group were evaluated for nuclear and nucleolar ultrastructure. Embryos were fixed in 3% glutaraldehyde in 0.1 M Na phosphate buffer (pH 7.2), washed in 0.1M Na phosphate buffer, post-fixed in 1% OsO4 in 0.1M Na phosphate buffer, embedded in Epon and serially sectioned into semithin sections (2 µm). Sections were stained with basic toluidine blue and evaluated by bright-field light microscopy. Selected semithin sections were re-embedded (Hyttel & Madsen, Reference Hyttel and Madsen1987) and processed for ultrathin sectioning (70 nm). The ultrathin sections were contrasted with uranyl acetate and lead citrate and examined on a Philips CM100 transmission electron microscope.
Statistical analysis
All experiments were replicated more than eight times. The maturation and developmental rates of each group were analyzed by Fisher's Exact test and the chi-squared test. A value of P < 0.05 was determined to be statistically significant.
Ethics
Handling of the farm animals was conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Mice were housed and handled according to institutional guidelines, and experimental procedures are approved by the IAS board, with full respect to the EU Directive 2010/63/EU for animal experimentation.
Results
In vitro embryonic development of nucleolus re-injected oocytes
First, we compared embryonic development of ENL and intact porcine oocytes after parthenogenetic activation. As shown in Table 1, the cleavage rate of ENL oocytes was lower (50.7%, 71/140) than that of control oocytes (intact, 66.0%, 308/467). Moreover, the blastocyst development rate of the ENL group (2.9%, 4/140) was significantly lower when compared with controls (30,8%: 144/467) and to both experimental groups (11.8 to 13.5%, Table 1); i.e. when mouse NLBs were re-injected into the porcine enucleolated oocytes, 13.5%, of them were developed to the blastocyst stage (13.5%, 44/327) or when porcine NLBs were re-injected into the porcine enucleolated oocytes (11.8% blastocysts, 37/313) (Table 1).
Table 1 In vitro development of NLB re-injected porcine oocyte after parthenogenetic activation
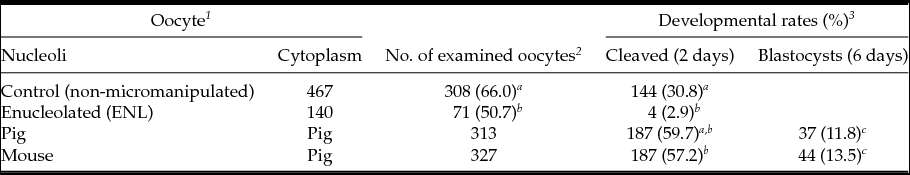
a,b,c Values with different superscripts are significantly different (P < 0.05).
1 Oocytes were cultured with TCM-199 medium supplemented with l-cysteine, pyruvate and GPBOS for 40 h (re-injection groups) or 44 h (control groups).
2 The survived and matured oocytes were used for experiments.
3 Cleavage and blastocysts rates were examined 2 and 6 days after activation, respectively.
Immunofluorescent analysis of nucleolar proteins
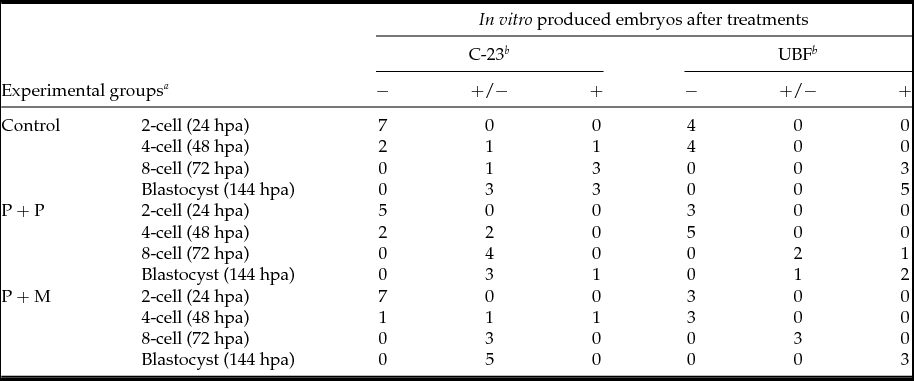
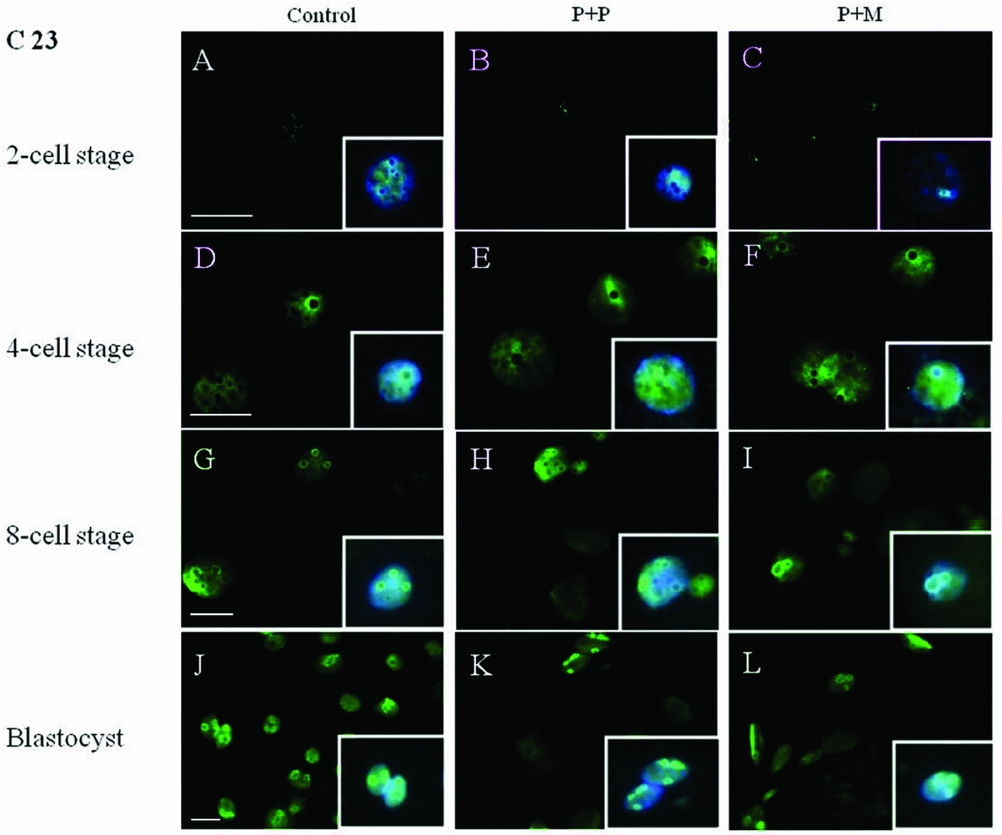
The localization of proteins involved in the rRNA processing (nucleolin, C23) and transcription (upstream binding factor, UBF) was determined by immunocytochemistry. The labeling of C23 was first observed on the periphery of the nucleoli in a form of non-focused clusters at the 4-cell stage embryos in all experimental groups (Table 2 and Fig. 1 D–F). At the 8-cell and blastocyst stage, the labeling of C23 was much stronger and localized as a shell like areas surrounding nucleoli (Fig. 1 G–L). However, as shown in Table 2, the number of embryos in P + P (n = 1) and P + M (n = 1) groups labeled with C23 in all blastomeres was lower than in control groups (n = 7).
Table 2 Number of embryos displaying the localization of C-23 and UBF
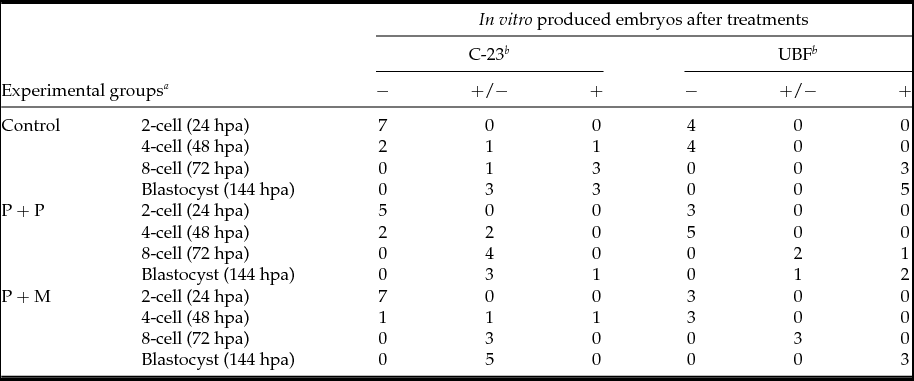
Control: porcine non-micromanipulated parthenogenetic control, P + P: porcine cytoplasm + porcine nucleolus, P + M: porcine cytoplasm + mouse nucleolus.
a hpa: h post activation.
b −: no blastomeres; +/−: some blastomeres; +: all blastomeres.
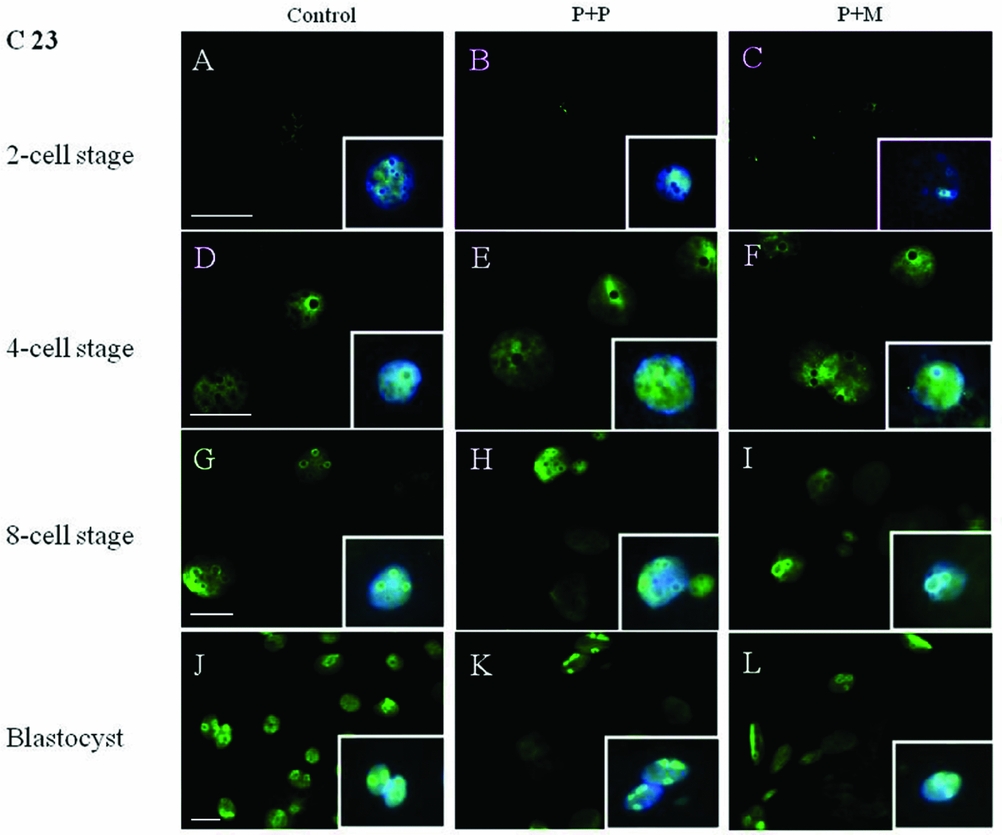
Figure 1 (A–L) Localization of DNA (Hoechst staining – blue) and C23 (green) in NLBs and nucleoli of control and re-injected (P + P or P + M) groups. Scale bars: 10 µm (main panels). Insets: Details of nuclei.
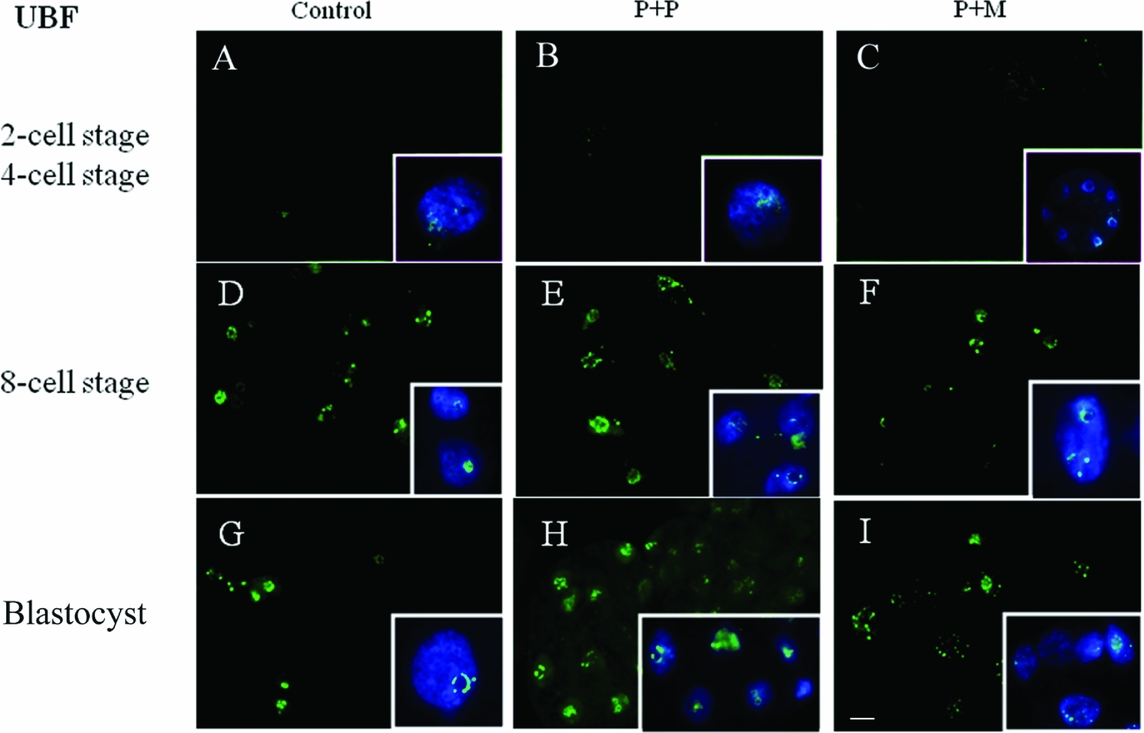
The localization of UBF was first detected in 8-cell stage embryos in the form of small foci surrounding or penetrating nucleoli in all analyzed groups (Table 2 and Fig. 2 D–F). Interestingly, we found the differences in UBF localization at the blastocyst stage. While the UBF labeling in the control and P + P group typically surrounded or penetrated nucleoli as small foci (Fig. 2 G, H), in P + M blastocysts the labeling of UBF was scattered also within the nucleoplasm (Fig. 2 I). Furthermore, the proportion of embryos displaying labeled foci in all blastomeres increased with embryonic developmental stages (control: from 3 to 5; P + P: from 1 to 2; P + M: from 0 to 3) (Table 2).
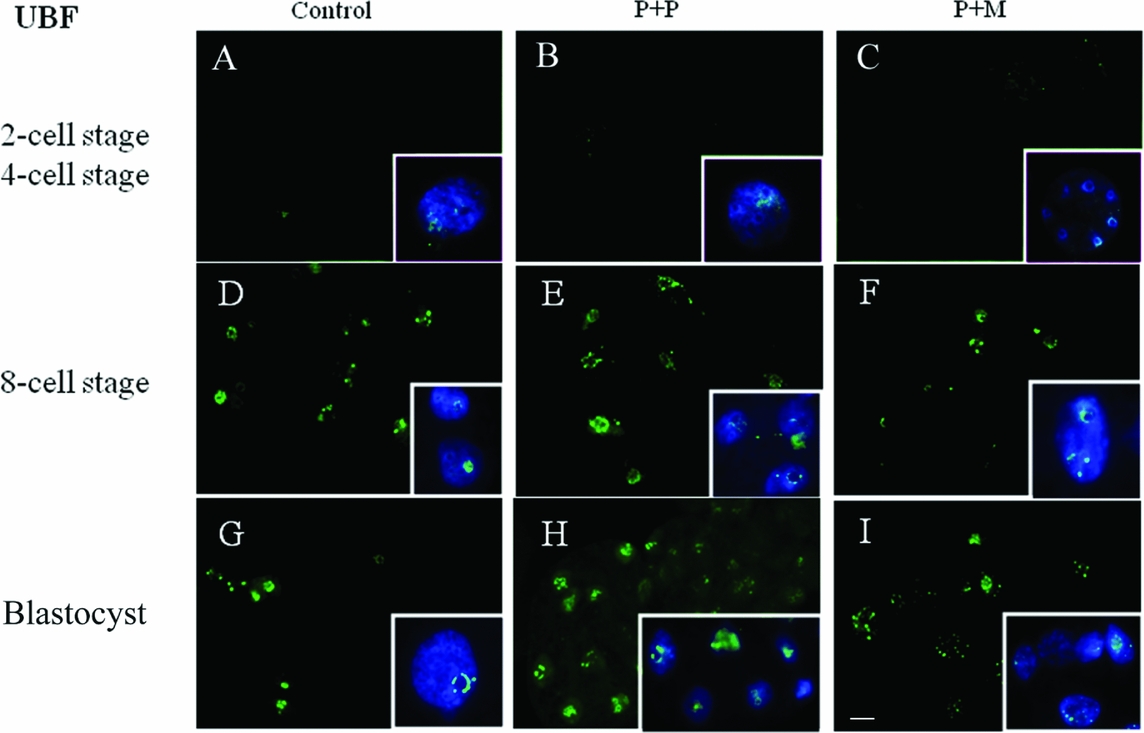
Figure 2 (A–I) Localization of DNA (Hoechst staining – blue) and UBF (green) in the nucleus of control and re-injected (P + P or P + M) embryos. Scale bar: 10 µm (main panels). Insets: Details of nuclei.
Autoradiography and ultrastructure
To determine the timing of major transcription activation and the formation of functional nucleoli, the light microscopical autoradiography and TEM were used. None of the evaluated embryos (from 1-cell to 4-cell stage of all groups) displayed autoradiographic staining assuming the lack of major transcriptional activity at these stages.
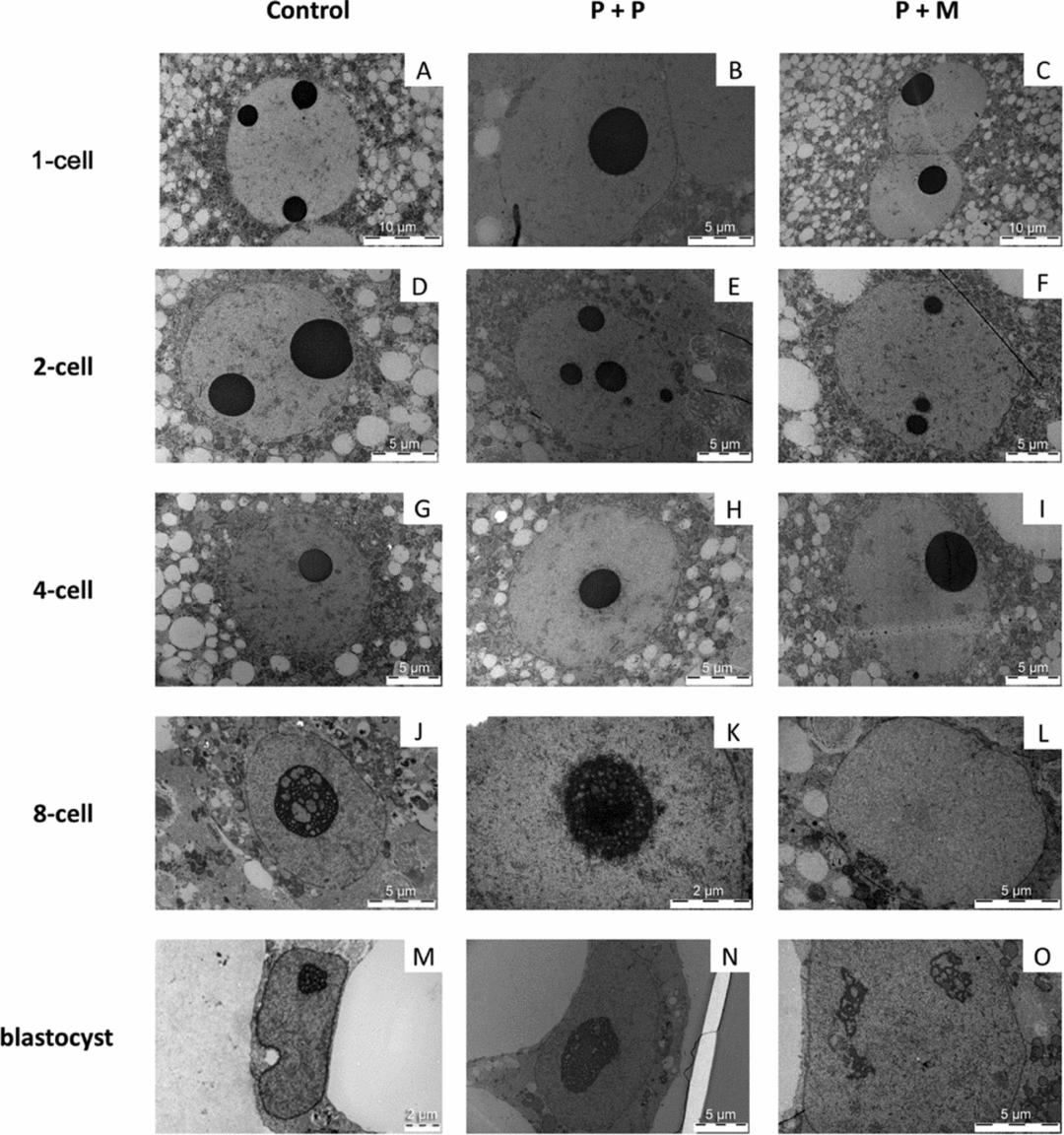
One-cell stage
The three evaluated 1-cell embryos of control group displayed one pronucleus (n = 2) or two centrally apposed pronuclei (n = 1). Chromatin was organized mainly in the form of euchromatin with spare heterochromatin equally distributed throughout the nucleoplasm (Fig. 3 A). Nuclei of zygotes have one to three electron-dense NLBs (Fig. 4 A).

Figure 3 (A–O) TEM analysis of nuclei in control and interspecies (P + P and P + M) groups.
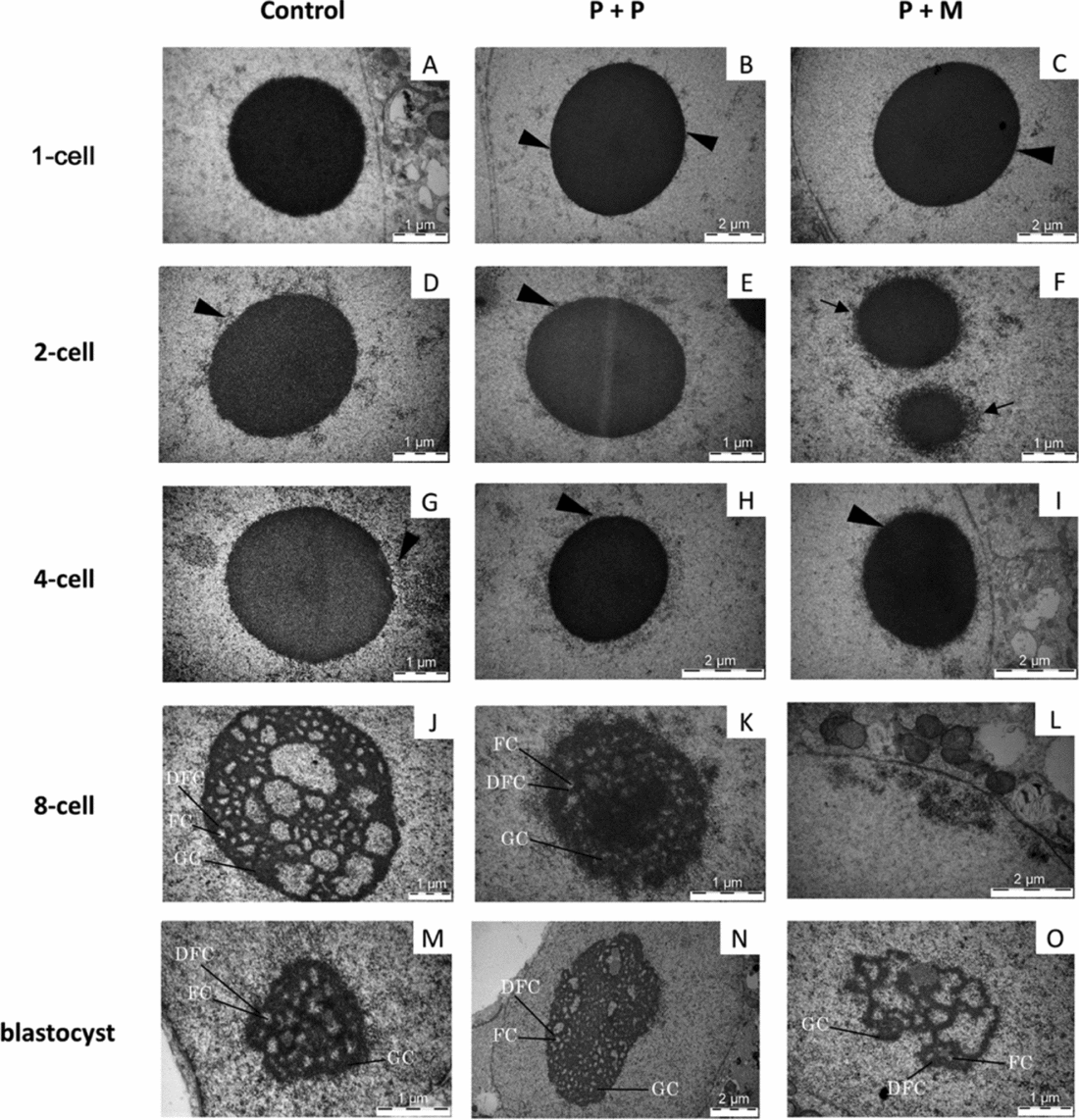
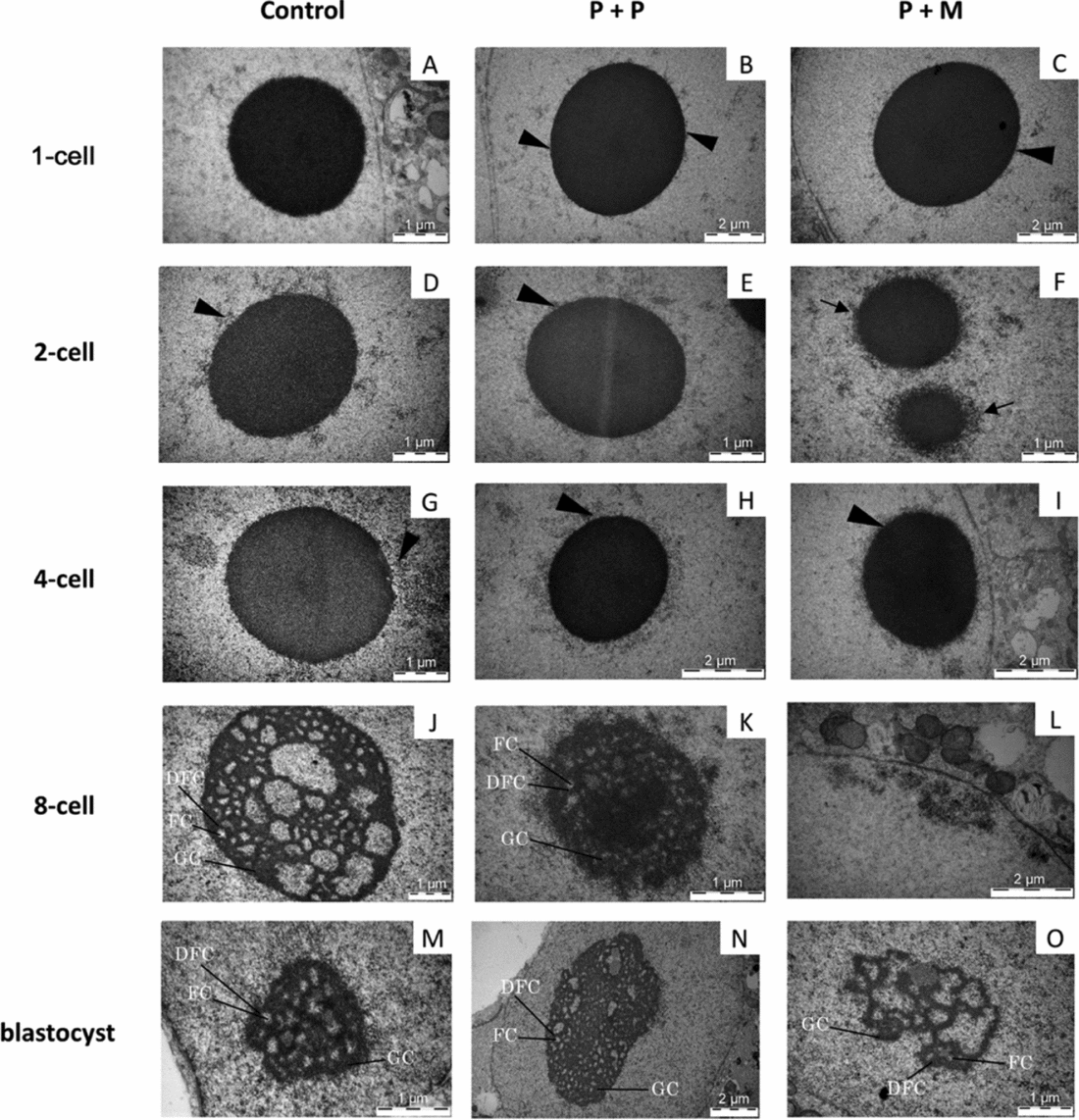
Figure 4 (A–O) TEM analysis of NLBs/nucleoli in control and interspecies (P + P and P + M) groups. Arrowheads indicate heterochromatin clusters attached to the NLBs. Arrows indicate the electron-dense fibre structure attached to NLBs. Detail showing the nucleolus presenting fibrillar centres (FC), dense fibrillar component (DFC), and granular component (GC).
In the P + P group, all evaluated embryos had only one pronucleus localized either centrally (2/3) or at the cytoplasmic periphery (1/3). The pronuclei contained mainly euchromatin with rare heterochromatin clusters localized in the nucleoplasm (Fig. 3 B). All 1-cell embryos had only one NLB with attached heterochromatin (Fig. 4 B).
Two out of three evaluated P + M embryos at 1-cell stage had two apposed pronuclei and one embryo contained two migrating pronuclei. The pronuclei contained euchromatin with heterochromatin clusters localized at the sites of apposition or at the periphery of pronuclei (Fig. 3 C). All 1-cell embryos displayed only one NLB with prominent heterochromatin clusters (Fig. 4 C).
Two-cell stage
Control embryos showed one centrally localized nucleus with equally distributed heterochromatin (Fig. 3 D). Intact NLBs were surrounded with varying amounts of heterochromatin (Fig. 4 D). Two-cell stage embryos in both experimental groups displayed centrally positioned nucleus. In P + P embryos, the nuclei contained mainly euchromatin with spare heterochromatin aggregates (Fig. 3 E) and NLBs were intact, with or without attached heterochromatin (Fig. 4 E). Conversely, nuclei of interspecies P + M embryos exhibited more heterogeneous chromatin in amounts comparable with control embryos (Fig. 3 F). Intact NLBs were surrounded by a heterochromatin halo, in one embryo largely permeating the electron-dense fibre structure (Fig. 4 F).
Four-cell stage
Control embryos at 4-cell stage displayed one nucleus. Heterochromatin was equally distributed in the nucleoplasm (n = 1) or localized at the nuclear and nucleolar (NLB) periphery (n = 2) (Fig. 3 G). NLBs were intact with no signs of transformation into active nucleoli (Fig. 4 G).
Experimental P + P and P + M embryos at the 4-cell stage displayed identical nuclear morphology with large euchromatin and heterochromatin mainly localized at nuclear envelope and NLBs periphery (Fig. 3 H, I). All NLBs were intact (Fig. 4 H, I).
Eight-cell stage
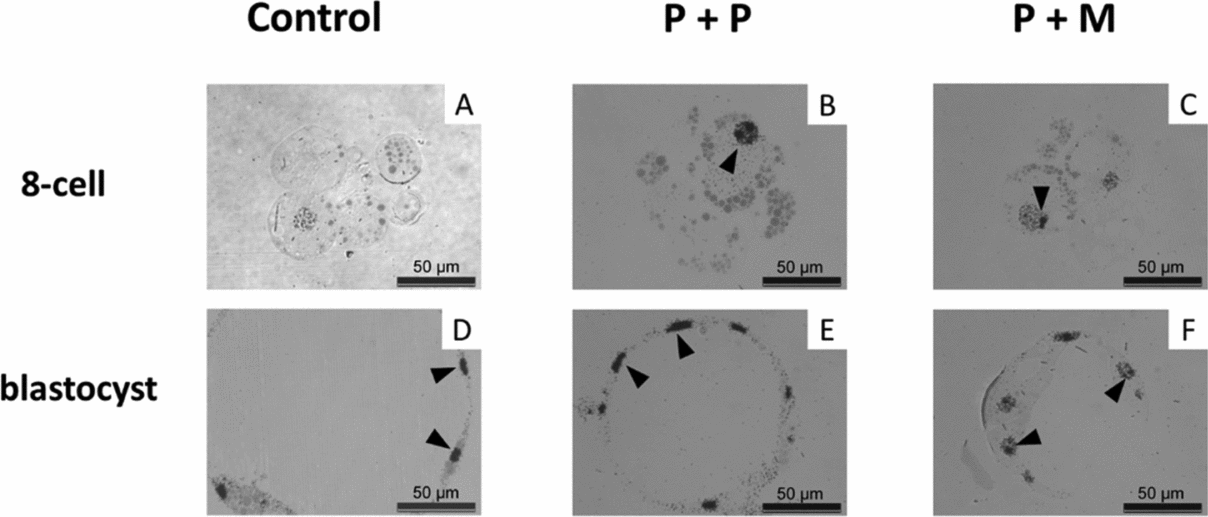
The nuclei of control embryos exhibited increased amount of heterochromatin that was homogenously distributed throughout the nucleoplasm and the fibrillogranular nucleoli (Fig. 3 J). Occasionally, developing nucleoli and NLBs were observed along with fibrillogranular nucleoli (Fig. 4 J). Two from three tentative 8-cell embryos displayed autoradiographic labeling over the nucleoplasm (Fig. 5 A).

Figure 5 (A–F) Light microscopical autoradiogram of control and interspecies (P + P and P + M) groups of embryos showing major genome activation (MGA). Note the autoradiographic labeling over the nucleoplasm and the nucleoli (arrowheads). Scale bars: 50 µm.
Blastomeres of P + P embryos displayed one nucleus with heterochromatin mainly attached to nucleolar periphery (Fig. 3 K). NLBs were intact (n = 2) or in the different levels of transformation into fibrillo-granular nucleolus (Fig. 4 K). All four tentative 8-cell embryos displayed intensive autoradiographic labeling over both the nucleoplasm and nucleoli in all nuclei (Fig. 5 B).
Eight-cell P + M embryos had blastomeres with one nucleus mainly containing euchromatin with spare heterochromatin localized at nuclear periphery (Fig. 3 L). No NLBs were observed. Instead, the structures formed by granules at different size (resembling GC), and/or by fibres and granules (resembling granular and DFC) were observed (Fig. 4 L). Two tentative 8-cell embryos presented the same autoradiographic and ultrastructural findings as the previous group (Fig. 5 C).
Blastocyst stage
In all groups of blastocyst stages, pronounced autoradiographic labeling over nucleoplasm and nucleoli was observed (Fig. 5 D–F).
All blastocysts in control group contained only very few presumptive inner cell mass (ICM) cells, whereas the trophectoderm (TE) was morphologically well defined and developed. Both ICM and TE cells were large and in three out of five blastocysts, binucleated cells were observed. The nuclei contained higher amounts of heterochromatin (Fig. 3 M). Interestingly, both NLBs (not shown) and developing fibrillogranular nucleoli within the nuclei were observed (Fig. 4 M).
The cells in blastocysts of both experimental groups were structurally very similar and contained one, transcriptionally active nucleolus with well defined nucleoli. The nuclei in presumptive ICM exhibited mainly euchromatin. The nuclei in the presumptive TE contained also heterochromatin distinctly localized at nuclear periphery (Fig. 3 N, O). On some occasions nucleolar structures formed by granular mass were observed in blastomeres of P + M group (Fig. 4 O).
Discussion
Major genome activation is an essential event in embryonic development and is related to the formation of the functional nucleolus (Lagutina et al., Reference Lagutina, Zakhartchenko, Fulka, Colleoni, Wolf, Fulka, Lazzari and Galli2011). It has been reported that the low efficiency of somatic cell nuclear transfer (SCNT) and interspecies somatic cell nuclear transfer (iSCNT) embryo production is accompanied with abnormal embryonic nucleologenesis, insufficient rRNA transcription and irregular expression of nucleolus-related proteins (Song et al., Reference Song, Lee, Kim, Kim, Park, Kim, Chang, Han, Lee, Lee and Koo2009; Lagutina et al. Reference Lagutina, Fulka, Brevini, Antonini, Brunetti, Colleoni, Gandolfi, Lazzari, Fulka and Galli2010, Reference Lagutina, Zakhartchenko, Fulka, Colleoni, Wolf, Fulka, Lazzari and Galli2011; Østrup et al., Reference Østrup, Strejcek, Petrovicova, Lucas-Hahn, Morovic, Lemme, Petersen, Laurincikova, Niemann, Laurincik and Hyttel2011). These reports strongly indicate that the quality of maternal nucleolus plays a key role in these developmental processes. The contribution of maternal nucleolus to embryonic development has been characterized further in detail especially after the invention of a new method for microsurgical removal of NLBs (enucleolation) (Fulka Jr et al., Reference Fulka, Moor, Loi and Fulka2003). Subsequent NLBs manipulation studies, mainly in the mouse, showed that the enucleolated MII oocytes develop into the 1-cell embryos without NLBs in their pronuclei after fertilization or parthenogenetic activation in vitro. The development of these embryos was generally arrested at the 2-cell stage and only exceptionally some of these reached the blastocyst stage (5%). However, very interestingly, when mouse NLBs (isolated from GV stage oocytes) were re-injected into the enucleolated mouse MII oocytes, 50% of these were developed to the blastocyst stage after fertilization (Ogushi & Saitou, Reference Ogushi and Saitou2010). These results strongly indicated that NLBs from the oocyte (maternal NLBs) are essential for embryonic development.
We observed similar results with porcine oocytes (Table 1). Almost a half of enucleolated porcine oocytes (50.7%, 71/140) cleaved within 2 days after activation. However, similar to previous reports with mouse oocytes, further embryonic development to the blastocyst stage dramatically decreased (2.9%, 4/140; Table 1).
In our study, only 11.8% (37/313) of porcine oocytes re-injected with porcine NLBs developed to the blastocyst. When enucleolated porcine MII oocytes were re-injected with NLBs from mouse oocytes, 57.2% of these interspecies NLBs transferred embryos regained their cleavage potential and 13.5% developed to blastocyst. To our knowledge, this report is the first to show that the mouse NLB can support the embryonic development after its re-injection into the enucleolated porcine oocyte.
The formation of a functional nucleolus during MTA is tightly related with activation of ribosomal genes (rDNA) transcription and de novo ribosomal synthesis (Tomanek et al., Reference Tomanek, Kopecny and Kanka1989; King et al., Reference King, Niar, Chartrain, Betteridge and Guay1988). Activation of rDNA transcription requires the formation of the transcription initiation complex consisting of an upstream-bindig factor (UBF), SL1 factors containing the TATA-binding protein (TBP), and RNA polymerase I (Chen & Huang, Reference Chen and Huang2001).
Based on the localization of these key nucleolar proteins, the rDNA transcription occurs between the FC and the DFC (Maddox-Hyttel et al., Reference Maddox-Hyttel, Svarcova and Laurincik2007). Newly synthesized rRNA is further processed resulting in 18S, 5.8S and 28S rRNA production.
The final step of rRNA processing is the assembling of mature rRNAs with ribosomal proteins into pre-ribosomal particles maintained by nucleolin (C23) and nucleophosmin (B23) located in the peripheral regions of the DFC and GC (Hyttel et al., Reference Hyttel, Laurincik, Rosenkranz, Rath, Niemann, Ochs and Schellander2000). To evaluate the initial and terminal phases of ribosome synthesis, we performed the immunocytochemical staining of nucleolar proteins involved in rDNA transcription (UBF) and rRNA processing C23 at different stages of embryonic development as described previously in in vivo and in vitro produced embryos (Hyttel et al., Reference Hyttel, Laurincik, Rosenkranz, Rath, Niemann, Ochs and Schellander2000; Laurincik et al., Reference Laurincik, Bjerregaard, Strejcek, Rath, Niemann, Rosenkranz, Ochs and Maddox-Hyttel2004). In the present study the labeling of C23 was first observed at the 4-cell stage in control and experimental (P + P, P + M) groups. C23 was localized on the periphery of nucleoli in the form of non-compact rings. Further developmental stages were characterized by formation of C23 into shell-like bodies enclosing the NLBs or fibrillogranular nucleoli. The UBF was detected first at 8-cell stage in all analyzed groups as small foci surrounding the NLBs. The UBF labeling at blastocyst stage in the control and P + P groups typically surrounded or penetrated the nucleoli as small foci, whereas the UBF labeling in P + M blastocyst was scattered within the nucleoplasm. The timing and formation of C23 and UBF into nuclear entities resembled to the labeling observed in porcine embryos developed in vitro (Laurincik et al., Reference Laurincik, Bjerregaard, Strejcek, Rath, Niemann, Rosenkranz, Ochs and Maddox-Hyttel2004) except for UBF localization at blastocyst stage in P + M group. UBF labels evenly distributed in entire nucleoplasm may relate with more relaxed form of nucleoli observed in P + M blastocysts.
NLBs are also involved in the regulation of chromatin reorganization during early embryonic development (Fulka & Langerova, Reference Fulka and Langerova2014). The surface of the NLBs plays an important role in the creating of chromosomal compartments like α-satellite DNA repeats and centromeric and pericentric silent heterochromatin (Fulka & Langerova, Reference Fulka and Langerova2014; Gavrilova et al., Reference Gavrilova, Kuznetsova, Enukashvily, Noniashvili, Dyban and Podgornaya2009; Ogushi et al., Reference Ogushi, Palmieri, Fulka, Saitou, Miyano and Fulka2008) contributing to the formation of chromocentres with regulatory potential for transcriptional silencing (Ahmed et al., Reference Ahmed, Dehghani, Rugg-Gunn, Fussner, Rossant and Bazett-Jones2010; Gavrilova et al., Reference Gavrilova, Kuznetsova, Enukashvily, Noniashvili, Dyban and Podgornaya2009; Martin et al., Reference Martin, Beaujean, Brochard, Audouard, Zink and Debey2006; Pichugin et al., Reference Pichugin, Le Bourhis, Adenot, Lehmann, Audouard, Renard, Vignon and Beaujean2010). In our study, the chromatin reorganization and MTA was analyzed by TEM and autoradiography in intact and NLBs re-injected embryos. One-cell embryos of control group contained two centrally apposed pronuclei containing abundant euchromatin with spare heterochromatin evenly distributed in the nucleoplasm. In 1-cell stage embryos originating from nucleolus re-injected oocytes the heterochromatin clusters were localized mainly towards the nuclear envelope and also surrounding the NLBs. Interestingly, the heterochromatin of NLBs re-injected embryos was more dynamic with floating repositioning within the nucleoplasm and weak indication of decondensation at 4-cell stage (both groups). The transient appearance of heterochromatin domains, which are typically observed in 2-cell stage in vivo produced porcine embryos (Østrup et al., Reference Østrup, Hall, Petkov, Wolf, Hyldig and Hyttel2009, Deshmukh et al., Reference Deshmukh, Østrup, Strejcek, Vejlsted, Lucas-Hahn, Petersen, Li, Callesen, Niemann and Hyttel2012), was observed lately at 4-cell stage with no signs of NLBs transformation into fibrillogranular nucleoli and negative autoradiographic labeling.
In conclusion, we showed that mouse NLBs can help to overcome the critical early cleavage embryo development period, when re-injected into porcine enucleolated oocytes which are subsequently activated. The re-injected P + M embryos showed moderate alterations in the nucleolar structures and different UBF localization at blastocyst stage compared with control and P + P groups. Finally, we believe that the results presented in this paper will form a solid basis for further studies aiming to elucidate the roles of NLBs and nucleoli in the process of regulation of mammalian oocyte maturation and early embryonic development.
Competing interests
The authors hereby declare that there are no conflicting interests.
Acknowledgements
This work was supported by Slovak Research and Development Agency under the contract No. APVV-14–0001, and also by the project ‘EXCELLENCE in molecular aspects of the early development of vertebrates’, CZ.02.1.01/0.0/0.0/15_003/0000460 from the Operational Programme Research, Development and Education and was co-funded by the projects VEGA 1/0022/15 and VEGA 1/0327/16. JF Jr and HF were supported from GACR 17–08605S. H.K. was supported by a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science.