Introduction
The expandable ear wick is routinely used in the treatment of otitis externa and as a dressing in the ear canal following middle-ear surgery.Reference Badran and Jani1–Reference Taylor9 In both cases, the compressed, desiccated wick is inserted into the ear canal under direct vision and expands with hydration following the application of ear drops.
The expanded wick confers a number of benefits in the management of otitis externa. It provides a reservoir of the therapeutic preparation, thus prolonging contact of the medication with the inflamed canal skin. It also exerts gentle pressure on the oedematous meatal wall, as well as ensuring distribution of medication to the medial aspects of the meatus. Depending on the clinical situation, various ear drop preparations can be instilled onto the wick on a regular basis; these include antibiotic, anti-fungal, steroid and astringent preparations.Reference Rosenfeld, Singer, Wasserman and Stinnett10 The expanded wick remains in situ until canal swelling reduces sufficiently for it to fall out or to be removed on a subsequent visit.
The relationship between different preparations of ear drops and the use of the expandable ear wick has never been documented. It has been noted that the degree of expansion of the wick differs according to the ear drop preparation used. The effectiveness of the wick as a useful adjunct in the management of otitis externa lies in its ability to absorb and retain medication and to remain in situ until the canal becomes more patent. For this reason, it is important that the wick expands sufficiently to remain in the canal and carry out its function.
This study aimed to examine the effect of different ear drop preparations on a standard expandable otowick in vitro, in order to identify which preparations are likely to be most effective in clinical practice.
Materials and methods
Eight of the most frequently used ear drops were tested (Gentisone HC, Sofradex, Otomize, Betnesol, Exocin, Canesten, Locorten-Vioform and EarCalm; see Table I for manufacturer's details), along with water as a positive control. Ichthammol glycerin was also tested as its use is well documented in the treatment of otitis externa.Reference Nilssen, Wormald and Oliver12, Reference Ahmed, Roberts and Mannion13 Each preparation's base type – aqueous or oil-based – was noted. An expandable otowick (Merocel Pope Oto-Wick, Medtronic Xomed, Jacksonville, Florida, USA) was used.
Outcomes
The following measurements were taken: weight to within 1 mg (Mettler AC 100 precision balance; Mettler-Toledo, Columbus, Ohio, USA), and width and length to within 0.1 mm (Vernier calliper; Draper, Chandlers Ford, UK). The wick was then placed in a petri-dish containing at least 30 drops of the respective ear drop preparation (double the maximum absorptive dose determined by a pilot study). The length, width and weight of each wick were recorded at 30, 60, 120, 300 and 600 seconds. Five wicks were tested in each of the 10 preparations.
Statistical analysis
Data were analysed using the Excel 2003 (Microsoft Corporation, Redmond, Washington, USA) and Statistical Package for the Social Sciences version 11.0 (SPSS Inc, Chicago, Illinois, USA) software packages.
Results
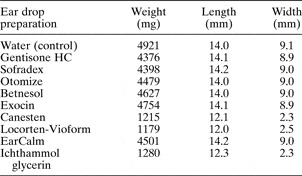
The rate of expansion and absorption of the otowick differed between drops. The results for change in weight, length and width over 10 minutes are tabulated in Table II. Figures 1 and 2 demonstrate graphically these weight and width data.
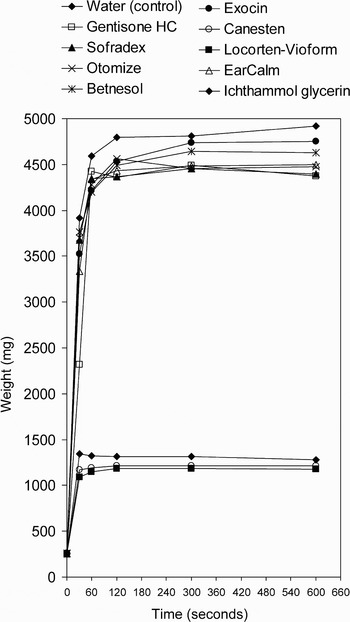
Fig. 1 Mean weight of otowick following application of ear drops.

Fig. 2 Mean width of otowick following application of drops.
Table II Mean Weight, Length and Width of Otowicks After 10 Minutes
Subgroup analysis
There appeared to be two distinct subgroups, confirmed by post hoc Scheffe's test. Changes in weight and size over time were similar for water, Gentisone HC, Sofradex, Otomize, Betnesol, Exocin and EarCalm. However, Canesten, Loctoen-Vioform and ichthammol glycerin showed poorer rates of absorption and no significant otowick expansion. This difference corresponded to the oil or water base of the ear drop preparation.
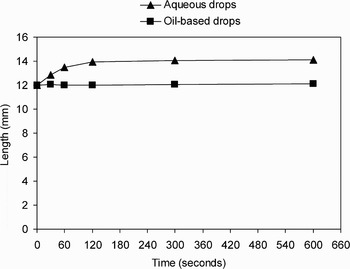
Data were then pooled according to the composition of the drops (i.e. oil-based or aqueous) and analysed (Figures 3 to 5). There was a statistically significant difference in the weight, length and width of the otowick following application of all aqueous drops (i.e. water, Gentisone HC, Sofradex, Otomize, Betnesol, Exocin and EarCalm), compared with application of oil-based drops (i.e. Canesten, Locorten-Vioform and ichthammol glycerin), at all time intervals after immersion (independent samples t-test, p < 0.001).

Fig. 3 Mean weight of otowick following application of drops.

Fig. 4 Mean length of otowick following application of drops.

Fig. 5 Mean width of otowick following application of drops.
Discussion
When oil-based drops were used in isolation with otowicks, they were absorbed poorly and failed to produce otowick expansion. In comparison, all aqueous drops were equally well absorbed, and all expanded the otowick.
Implications for clinical practice
Whilst there is a range of topical agents available for the treatment of otitis externa, no single preparation has been shown to be superior.Reference Rosenfeld, Brown, Cannon, Dolor, Ganiats and Hannley2, Reference Rosenfeld, Singer, Wasserman and Stinnett10 The choice of topical preparation rests with the attending clinician. The author recommends that only aqueous drops should be prescribed when using expandable otowicks.
• An in vitro study examined the weight, length and width of an expandable ear wick when commonly prescribed ear drops were applied
• There was a marked difference in otowick absorption and expansion when different drops were used
• This corresponded to the aqueous or oil base of the preparation; all aqueous drops (i.e. Gentisone HC, Sofradex, Otomize, Betnesol, Exocin and EarCalm) produced similar rates of absorption into, and expansion of, the otowick, whereas oil-based drops (i.e. Canesten, Loctoen-Vioform and ichthammol glycerin) failed to expand the wick and showed poor rates of absorption into the wick
• The author concludes that expandable ear wicks should only be used in conjunction with aqueous ear drops; when such wicks are used in treating fungal otits externa, acetic acid drops should be prescribed, as all other anti-fungal drops are oil-based
Otomycosis
Of the prescription ear drops tested, only Locorten-Vioform and Canesten are licenced for treatment of fungal otitis externa; both of these drops are oil-based. Acetic acid has been shown to have anti-fungal properties and can be used in isolation (EarCalm) or in combination with steroids or antibiotics (Otomize) to treat otomycosis.Reference Rosenfeld, Brown, Cannon, Dolor, Ganiats and Hannley2, Reference Rosenfeld, Singer, Wasserman and Stinnett10 Combination preparations are superior to acetic acid alone in the treatment of acute otitis externa.Reference van Balen, Smit, Zuithoff and Verheij14 However, this benefit has not been proven for otitis externa of fungal origin.
Conclusions
Expandable ear wicks should only be used in conjunction with aqueous ear drops. When using an expandable otowick as an adjunct to treating fungal otits externa, drops containing acetic acid should be considered, as other anti-fungal drops are oil-based.
Acknowledgements
The author wishes to thank Mr C E Hall for assistance in the production of this manuscript, and Mr D D Pothier for statistical analysis.