Introduction
Reconstruction of the historical and palaeoenvironmental occurrence of sea ice is a key component of efforts to understand climate change (Polyak et al. Reference Polyak, Alley, Andrews, Brigham-Grette, Cronin, Darby, Dyke, Fitzpatrick, Funder, Holland, Jennings, Miller, O'Regan, Savelle, Serreze, St. John, White and Wolff2010). Since sea ice is obviously a transient indicator of climate, numerous sediment proxies of historical and palaeosea ice occurrence have been proposed and used (Armand & Leventer Reference Armand and Leventer2010, Polyak et al. Reference Polyak, Alley, Andrews, Brigham-Grette, Cronin, Darby, Dyke, Fitzpatrick, Funder, Holland, Jennings, Miller, O'Regan, Savelle, Serreze, St. John, White and Wolff2010). Amongst these proxies is a hydrocarbon designated IP25 (I, Fig. 1), which is a mono-unsaturated C25 highly branched isoprenoid (HBI monoene). Highly branched isoprenoids are secondary metabolites synthesized by a limited number of diatom species (e.g. Rowland et al. Reference Rowland, Belt, Wraige, Massé, Roussakis and Robert2001, Damsté et al. Reference Damsté, Muyzer, Abbas, Rampen, Massé, Allard, Belt, Robert, Rowland, Moldowan, Barbanti, Fago, Denisevich, Dahl, Trindade and Schouten2004). A large number of studies have described the widespread occurrence of di- to penta- (and occasionally mono-) unsaturated HBIs from freshwater, brackish or marine settings ranging from low to high latitudes. However, IP25 has to date only been reported in Arctic sea ice, sediments and sedimenting particles (Brown et al. Reference Brown, Belt, Philippe, Mundy, Massé, Poulin and Gosselin2010 and references therein). Belt et al. (Reference Belt, Massé, Rowland, Poulin, Michel and LeBlanc2007) described the structure of (I) isolated from both sea ice and sediments collected from the Canadian Arctic. The relative abundances of (I) in sections of a sediment core collected from the north Icelandic shelf showed a strong correlation with documented historical sea ice occurrences (Massé et al. Reference Massé, Rowland, Sicre, Jacob, Jansen and Belt2008). Belt et al. (Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008) measured the 13C composition of various HBI isomers (including IP25) isolated from sea ice, trap samples and sediments collected from a wide range of locations across the Canadian Arctic with IP25 values ranging between -23.2 and -16.3‰, consistent with an origin from sea ice derived organic matter (Schubert & Calvert Reference Schubert and Calvert2001). In contrast, the δ13C values of more unsaturated HBIs were between -35 and -42.2‰ (Belt et al. Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008) consistent with an origin from phytoplankton or freshwater plankton. Using the results of an examination of grain size, benthic foraminiferal composition, δ18O ratios, sediment mineral composition and magnetic properties, in conjunction with abundances of IP25 for sediment samples from a core collected off the north-westernmost coast of Iceland, Andrews et al. (Reference Andrews, Belt, Olafsdottir, Massé and Vare2009) documented sea ice and marine climate variability over the last 2000 years. Recently, Vare et al. (Reference Vare, Massé, Gregory, Smart and Belt2009) and Belt et al. (Reference Belt, Vare, Massé, Manners, Price, MacLachlan, Andrews and Schmidt2010) reconstructed the Holocene (0–10 kyr bp) sea ice history of the north-west passage in the Canadian Arctic Archipelago using measurements of IP25 in sections of dated sediments, whilst Müller et al. (Reference Müller, Massé, Stein and Belt2009) demonstrated that the use of IP25 could be extended to longer timescales ( > 30 ky bp) and examined sections of a sediment core collected from northern Fram Strait. They determined that in the early part of the sedimentary record of their core, sea ice was permanently present in the area, whilst during the Holocene, the sea ice regime over the coring site was seasonal and similar to present-day conditions. These studies confirm the usefulness of IP25 as a proxy for Arctic sea ice in addition to other proxies which are already well used and accepted (e.g. Armand & Leventer Reference Armand and Leventer2010, Polyak et al. Reference Polyak, Alley, Andrews, Brigham-Grette, Cronin, Darby, Dyke, Fitzpatrick, Funder, Holland, Jennings, Miller, O'Regan, Savelle, Serreze, St. John, White and Wolff2010).
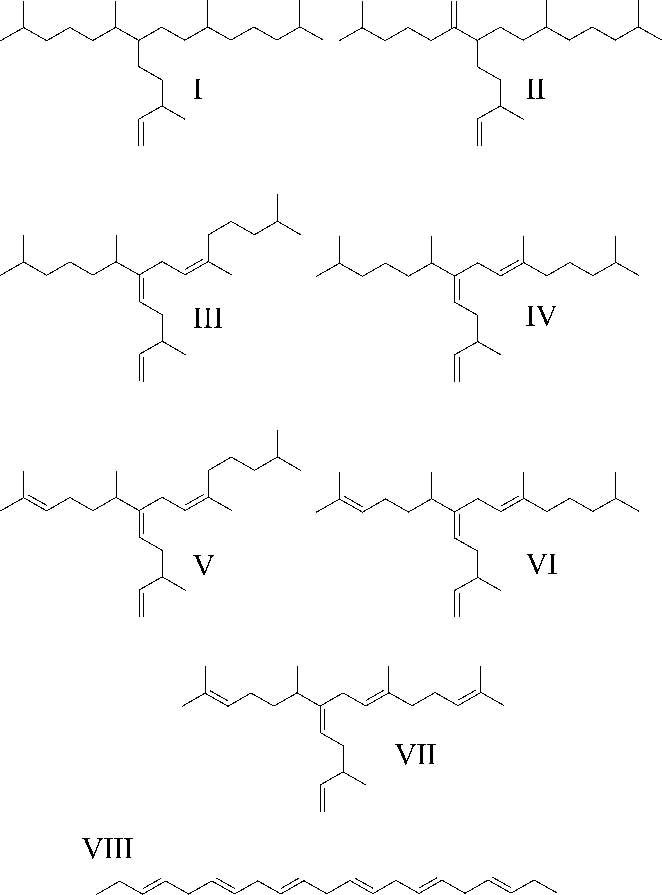
Fig. 1 Structures of the HBI isomers described in the current study. I, IP25; II, di-unsaturated HBI; III-IV, tri-unsaturated HBIs; V-VI, tetra-unsaturated HBIs; VII, penta-unsaturated HBI; VIII, n-C21:6 (henicosahexaene).
Although IP25 has a widespread occurrence in Arctic sea ice (Brown et al. Reference Brown, Belt, Philippe, Mundy, Massé, Poulin and Gosselin2010 and references therein), it has never been reported in Antarctic sea ice and sediments. However, a number of studies have reported the presence of a di-unsaturated HBI in samples from the Antarctic. Nichols et al. (Reference Nichols, Volkman, Palmisano, Smith and White1988) reported a HBI diene in mixed sea ice diatom samples, and Venkatesan (Reference Venkatesan1988) showed that this diene was present in sediment horizons dated as over 2.5 ky old. Later Johns et al. (Reference Johns, Wraige, Belt, Lewis, Massé, Robert and Rowland1999) identified the diene as (II) (Fig. 1) by comparing the mass spectrum and gas chromatographic retention index of the diene with samples authenticated by nuclear magnetic resonance spectroscopy after isolation from cultures of the temperate diatom Haslea ostrearia (Gaillon) Simonsen. A HBI diene other than (II) and unidentified HBI trienes were reported from water column particulate material from the Bellinghausen Sea, as well as from a coastal site close to Signy Island (South Orkney Islands) (Cripps Reference Cripps1995, Cripps & Clarke Reference Cripps and Clarke1998). In the samples collected from Signy Island, Cripps & Clarke (Reference Cripps and Clarke1998) reported the presence of unidentified di- and tri-unsaturated HBIs in the material trapped during the summer (January and February). Cripps (Reference Cripps1995) also reported the presence of a tri-unsaturated HBI isomer in particulate matter and sediments collected during spring under the pack ice in the Bellinghausen Sea area. Matsumoto et al. (Reference Matsumoto, Matsumoto, Sasaki and Watanuki1992) identified a HBI diene in sections of sediment cores from Lützow-Holm Bay and suggested that it originated from sea ice diatoms. More recently, Damsté et al. (Reference Damsté, Rijpstra, Coolen, Schouten and Volkman2007) reported the presence of (II) in anoxic sediments from Ellis Fjord in East Antarctica. This fjord is a small meromictic basin where the water stratification favours the development of anoxic conditions. These authors interpreted the co-occurrence of (II) and a structurally-related thiane as evidence for the rapid diagenetic incorporation of sulphur into (II). Damsté et al. (Reference Damsté, Rijpstra, Coolen, Schouten and Volkman2007) also determined the 13C isotopic composition of (II) and suggested that δ13C values of -9.1 to -9.4‰ were consistent with a sea ice diatom origin for the alkene.
In the present study, we extend reports of numerous HBI alkenes in Antarctic sediment samples, and notably we also report the presence of (II) in sea ice and other HBIs in phytoplankton together with the δ13C composition of individual HBIs in both sea ice and sediments. Differences in the structures and isotopic compositions allowed us to assign the origins of HBI isomers present in recent sediments collected from the Adélie Land area in the East Antarctic continental shelf area, representing 13 months of sedimentation. We propose the presence of isotopically 13C enriched HBI diene (II) as a proxy indicator for contributions of sea ice diatom organic matter to recent Antarctic sediments. In addition, subject to limitations placed by diagenetic effects, a ratio of the concentrations of diene/triene concentrations might reflect the relative contributions of sea ice organic matter and phytoplankton derived organic matter to Antarctic sediments. Two recent studies, comparing diene/triene ratios to diatom counts, have suggested that such a ratio is applicable to Holocene sediments, in which diagenetic effects have not been extensive (Barbara et al. Reference Barbara, Crosta, Massé and Ther2010, Denis et al. Reference Denis, Crosta, Barbara, Massé, Renssen, Ther and Giraudeau2010).
Methods
Sea ice and phytoplankton samples
Sea ice sampling was performed in 2006 during the biogeochemical sea ice sampling of the Winter Weddell Outflow Study (WWOS) with the RV Polarstern (September–October 2006; Fig. 2, area 1) and at Casey research station (November 2006; Fig. 2, area 2). Samples were collected during the spring–summer period when sea ice algal growth is at a maximum. The biology-rich bottom ice horizons (5 cm) were used from the Weddell Sea ice and chlorophyll a concentrations ranged from 19 to > 2000 μg l-1. The ice samples from the Weddell Sea were collected using a Kovacs Mark II ice corer from predominantly first year ice. Ice cores were sectioned immediately into 10 cm sections in order to avoid brine drainage. Sectioned cores were then melted in the dark at 4°C and the particulate material collected onto GF/F filters by vacuum filtration. The samples were kept frozen until later analyses. For area 2 samples, the bottom ice flora was scraped by divers. In the laboratory, these were allowed to melt in the dark at 4°C in 0.2 μm filtered seawater. After complete melting, algal cells were concentrated via centrifugation and freeze-dried prior to analysis. Phytoplankton samples were obtained during the MD130-Image X CADO cruise (January 2003, area 3) using a 150 μm phytoplankton net. No identification of species composition was made. All samples were kept frozen prior to their analysis. A sample of the diatom Navicula glaciei Van Heurck (strain 2743) was obtained from the Provasoli-Guillard collection (National Center for Culture of Marine Phytoplankton, ME, USA).
Fig. 2 Map of Antarctica showing approximate sample locations.
Sediment samples
Surface sediments and core MD130-MC02 (66°24.74′S, 140°25.26′E, 982 m water depth) were retrieved during the MD130-Images X-CADO cruise in 2003 on board the RV Marion Dufresne II (Denis et al. Reference Denis, Crosta, Schmidt, Carson, Ganeshram, Renssen, Crespin, Ther, Billy and Giraudeau2009).
Hydrocarbon analyses, compound specific isotope analysis
Lipids were extracted from samples using a CH2Cl2/MeOH (2/1) mixture and purified using open column chromatography (after Belt et al. Reference Belt, Massé, Rowland, Poulin, Michel and LeBlanc2007). Hydrocarbon fractions were analysed using a Hewlett-Packard 5890 Series II gas chromatograph (GC) fitted with a 30 m fused silica HP-1 column (0.25 mm i.d., 0.25 μm film) and coupled to a 5970 Series mass selective detector (MSD). Spectra (m/z 40–550) were collected using Hewlett-Packard MS-Chemstation software. Individual HBIs were identified on the basis of comparison between their GC retention indices and mass spectra with those of previously authenticated HBIs (e.g. Johns et al. Reference Johns, Wraige, Belt, Lewis, Massé, Robert and Rowland1999).
Determination of the relative 13C isotopic composition of individual HBIs was performed by gas chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS) at the NERC Life Science Mass Spectrometry Facility (University of Bristol, UK, application LSMSFBRISTOL007; Belt et al. Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008).
Diatom analysis
Sediments were prepared as outlined in Crosta et al. (Reference Crosta, Romero, Armand and Pichon2005). Diatom identification was performed to species level and the relative abundance of each species was expressed as a percentage of the total diatom abundance.
Sediment geochemistry
Carbon isotopic composition (δ13C, 0.1‰ reproducibility) of the sediments from multi-core MD130-MC02 retrieved at CADO site 2 was determined using a Carlo Erba 2500 elemental analyser in line with a VG Isoprime IR-MS (Crosta et al. Reference Crosta, Romero, Armand and Pichon2005). Biogenic silica (BSi) was determined (2% reproducibility, Crosta et al. Reference Crosta, Romero, Armand and Pichon2005).
Results and discussion
Sea ice
In studies of numerous sediment samples from the Arctic (e.g. Massé et al. Reference Massé, Rowland, Sicre, Jacob, Jansen and Belt2008, Andrews et al. Reference Andrews, Belt, Olafsdottir, Massé and Vare2009, Belt et al. Reference Belt, Vare, Massé, Manners, Price, MacLachlan, Andrews and Schmidt2010, Vare et al. Reference Vare, Massé and Belt2010), we noted the co-occurrence of HBI monoene IP25 and HBI diene (II). Here we show the correlation between the relative concentrations of IP25 and (II) in 214 Holocene sediment samples collected in the north Icelandic shelf (Fig. 3). A linear algorithm describes 94% of the variance of this correlation. Despite the absence of IP25, the distributions of HBI (II) in Antarctic sediments might reflect the contributions of sea ice diatom derived organic matter, as suggested previously for (II) and an unidentified HBI diene based on their identification in a sample of mixed sea ice diatoms ((II); Nichols et al. Reference Nichols, Volkman, Palmisano, Smith and White1988, Johns et al. Reference Johns, Wraige, Belt, Lewis, Massé, Robert and Rowland1999) and sediments from Ellis Fjord and Lützow-Holm Bay (Matsumoto et al. Reference Matsumoto, Matsumoto, Sasaki and Watanuki1992 (unidentified HBI diene), Damsté et al. Reference Damsté, Rijpstra, Coolen, Schouten and Volkman2007 (II)). In order to test this hypothesis further, we obtained nine sea ice samples from two well separated regions of the Antarctic (Fig. 2) and examined the hydrocarbon fractions of extracts using GC–MS (gas chromatography-mass spectrometry) (e.g. Fig. 4a). These revealed the occurrence of C25 HBI diene (II) together with n-C21:6 (henicosahexaene) in all of the samples (Table I). The diene was identified by comparison of its retention index and mass spectrum with those reported previously for (II) identified in Antarctic sea ice diatoms and sediments (Nichols et al. Reference Nichols, Volkman, Palmisano, Smith and White1988, Johns et al. Reference Johns, Wraige, Belt, Lewis, Massé, Robert and Rowland1999) and in Arctic sea ice (Belt et al. Reference Belt, Massé, Rowland, Poulin, Michel and LeBlanc2007, Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008). Diene (II) was also reported previously in cultures of the diatom Haslea ostrearia a benthic diatom commonly observed in temperate areas, when cultured in the laboratory at 5°C (Rowland et al. Reference Rowland, Belt, Wraige, Massé, Roussakis and Robert2001). Although H. ostrearia has never been reported in polar environments, some Haslea spp. are commonly reported in the sympagic flora both in the Arctic and the Antarctic (Belt et al. Reference Belt, Massé, Rowland, Poulin, Michel and LeBlanc2007 and references therein). To date, every attempt to grow these sympagic species in our laboratories under the exacting conditions prevailing in sea ice has failed. However, every Haslea species investigated to date, as well as numerous members of the related genera Navicula, Pleurosigma and Gryosigma have been shown to biosynthesize HBIs (Damsté et al. Reference Damsté, Muyzer, Abbas, Rampen, Massé, Allard, Belt, Robert, Rowland, Moldowan, Barbanti, Fago, Denisevich, Dahl, Trindade and Schouten2004). These observations further support the suggestion (Damsté et al. Reference Damsté, Rijpstra, Coolen, Schouten and Volkman2007, Matsumoto et al. Reference Matsumoto, Matsumoto, Sasaki and Watanuki1992) that the source of (II) in Antarctic sea ice could be one or more of these diatom genera. Coolen et al. (Reference Coolen, Muyzer, Schouten, Volkman and Damsté2006), using specific primers to amplify fossil DNA from recent Antarctic sediments, restricted the list of potential sources for this sea ice derived isomer to members of closely related Navicula /Haslea genera. Examination of an extract of a single sample of the diatom Navicula glaciei (Strain CCMP2743) from the Provasoli-Guillard culture collection herein, however, failed to reveal any HBIs.
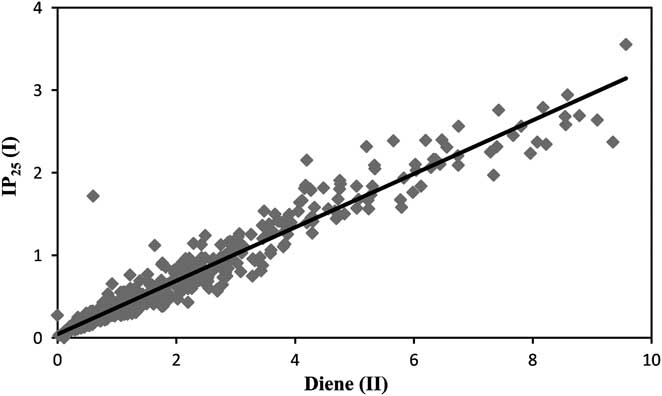
Fig. 3 Concentrations of IP25 HBI monoene vs concentrations of HBI diene (II) in 214 sediments from the north Icelandic shelf (Massé et al. Reference Massé, Rowland, Sicre, Jacob, Jansen and Belt2008). Concentrations are given in ng g-1 of dry sediments.
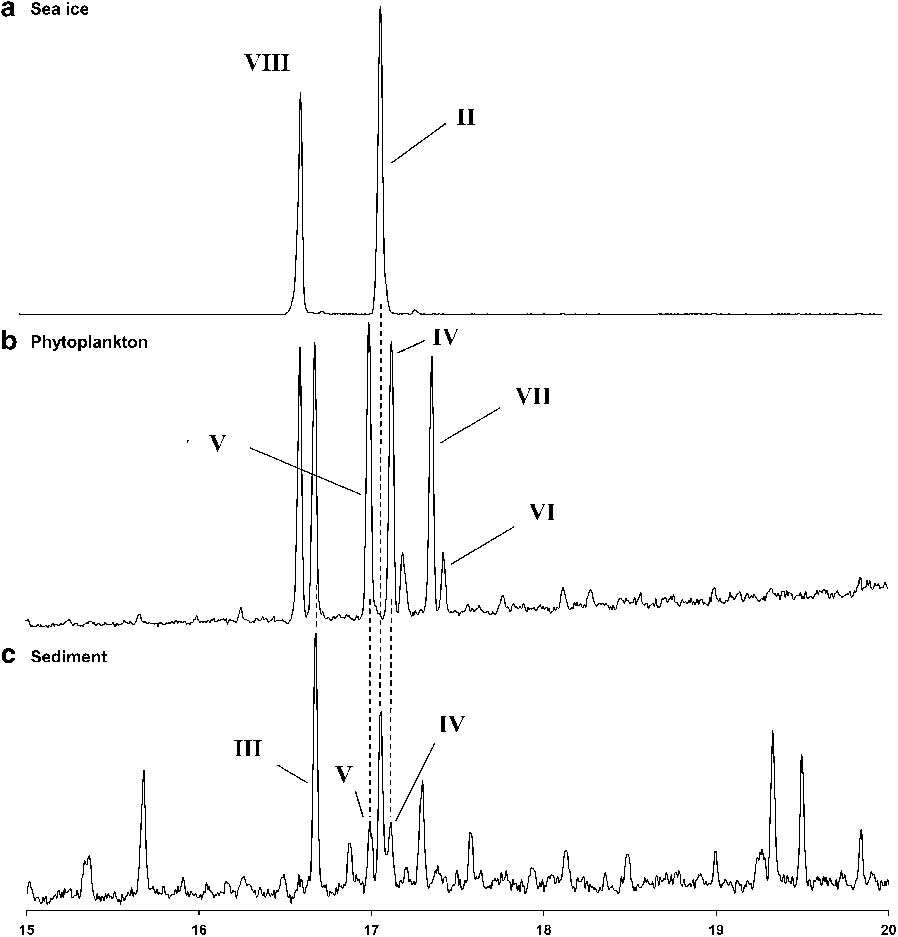
Fig. 4 Gas chromatography-mass spectrometry (GC-MS) total ion current chromatograms of hydrocarbon extracts of samples from the Antarctic. a. Sea ice (O'Brien Bay 1), b. mixed phytoplankton (MD130-PN07), c. sediment (MD130-MC02 0–0.5 cm).
Determination of the stable carbon isotopic compositions (δ13C) of HBI II in the sea ice produced average values of -8.5 and -5.7‰ for the samples collected in the Weddell Sea and Stevenson Cove in East Antarctica (Table II). These values are similar to those reported by Damsté et al. (Reference Damsté, Rijpstra, Coolen, Schouten and Volkman2007) for (II) in sediments from Ellis Fjord, Antarctica (-9.1 and -9.4‰) and are entirely consistent with an origin from diatoms growing within the sea ice where CO2 concentrations are sometimes depleted, depending on the physical condition of the ice (Gibson et al. Reference Gibson, Trull, Nichols, Summons and McMinn1999, Kennedy et al. Reference Kennedy, Thomas, Kattner, Haas and Dieckmann2002, Belt et al. Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008).
Phytoplankton
Highly branched isoprenoid (II) was not detected in the hydrocarbon fractions obtained from phytoplankton samples collected in Antarctic open waters during a summer algal bloom (Fig. 4b) but GC-MS analysis did reveal a suite of tri-, tetra- and penta-unsaturated HBI isomers (III–VII, Fig. 1). Comparison of their mass spectra and retention times with authenticated standards revealed that these isomers were identical to those previously observed in some temperate Pleurosigma spp. (Belt et al. Reference Belt, Allard, Massé, Robert and Rowland2000).
The δ13C composition of the HBIs in the phytoplankton samples ranged from -38 to -41‰ (Table II). Belt et al. (Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008) measured δ13C values from -35 to -40‰ for HBI (III) associated with a suite of HBI isomers not detected in our phytoplankton samples, in marine sediments collected in the Canadian Arctic Archipelago, although they were unable to conclude on the specific origin of the latter HBIs. Given the values obtained for our phytoplankton derived HBIs, it seems probable that the polyunsaturated HBI isomers observed in Arctic sediments were also of phytoplankton origin.
Surface sediments
We next carried out analyses of hydrocarbon fractions of extracts from core tops of sediment cores from six locations in the Adélie Land area of Antarctica. Briefly, the surface sediment samples (CADO 2-4, 6-8), were obtained from an area between 138°33′–144°46′E and 59°50′–66°25′S (Figs 2 & 5, Table I). Except for the northernmost station (CADO 8), all the sites experience sea ice cover during at least one to two months per year (Fig. 5). For the more coastal stations (e.g. CADO 2, 3 & 4), a landfast ice buttress extending between 135° and 142°E is present over the sites during seven to ten months per year (Giles et al. Reference Giles, Laxon and Ridout2008, Fraser et al. Reference Fraser, Massom and Michael2010). Although landfast ice is a recurrent feature in the area, it does not usually reach latitudes lower than 65°S, so stations 6 and 7 are usually covered by the drifting pack ice which is usually observed up to 60°S (Fraser et al. Reference Fraser, Massom and Michael2010). This pack ice results from the interception by fast ice and icebergs grounded on the ‘d'Urville bank’ of the sea ice formed in the Mertz Glacier polynya and swept westward by both katabatic and synoptic winds. When examined by GC–MS, nearly all the hydrocarbon fractions from the CADO surface sediments were found to contain (II), co-occurring with the HBIs (III–VII) which were those observed in the phytoplankton samples (Figs 4c & 6). The only sediment samples that did not contain (II) were those collected at the CADO 8 site, where only trace amounts of the tri-unsaturated HBI isomer (III) were observed. Satellite observations indicate that sea ice does not occur at this site, even during winter (Fig. 5). At the CADO 7 site, tri-unsaturated isomers dominated the HBI distribution, with tetra-unsaturated isomers (V & VI) also present, whilst only trace amounts of the sea ice derived isomer (II) could be detected. The low abundance of (II), in particular, probably reflects the ice conditions in the area, which only occurs for one to two months per year. In contrast, a relatively high abundance of the tri-unsaturated isomers reflects the high primary productivity and enhanced export efficiency associated with the marginal ice zone (Buesseler et al. Reference Buesseler, Barber, Dickson, Hiscock, Moore and Sambrotto2003). Gas chromatography-mass spectrometry analysis of the hydrocarbons extracted from the surface sediments obtained from the more coastal sites (CADO 2, 3 & 4) revealed the presence of di-, tri-, tetra- and penta-unsaturated HBI isomers. For CADO 3 and 4, although the sea ice derived isomer (II) was more abundant, the tri- and tetra-unsaturated HBIs (III–VI) remained the dominant isomers with (III) approximately 50 and 5 times more abundant than (II) in CADO 3 and CADO 4 samples, respectively. In contrast, for CADO 2 sediments, although the same HBI isomers were present, diene (II) dominated the HBI distributions, being approximately ten times more abundant than triene (III).
Table I Summary of sea ice, phytoplankton and sediment samples.
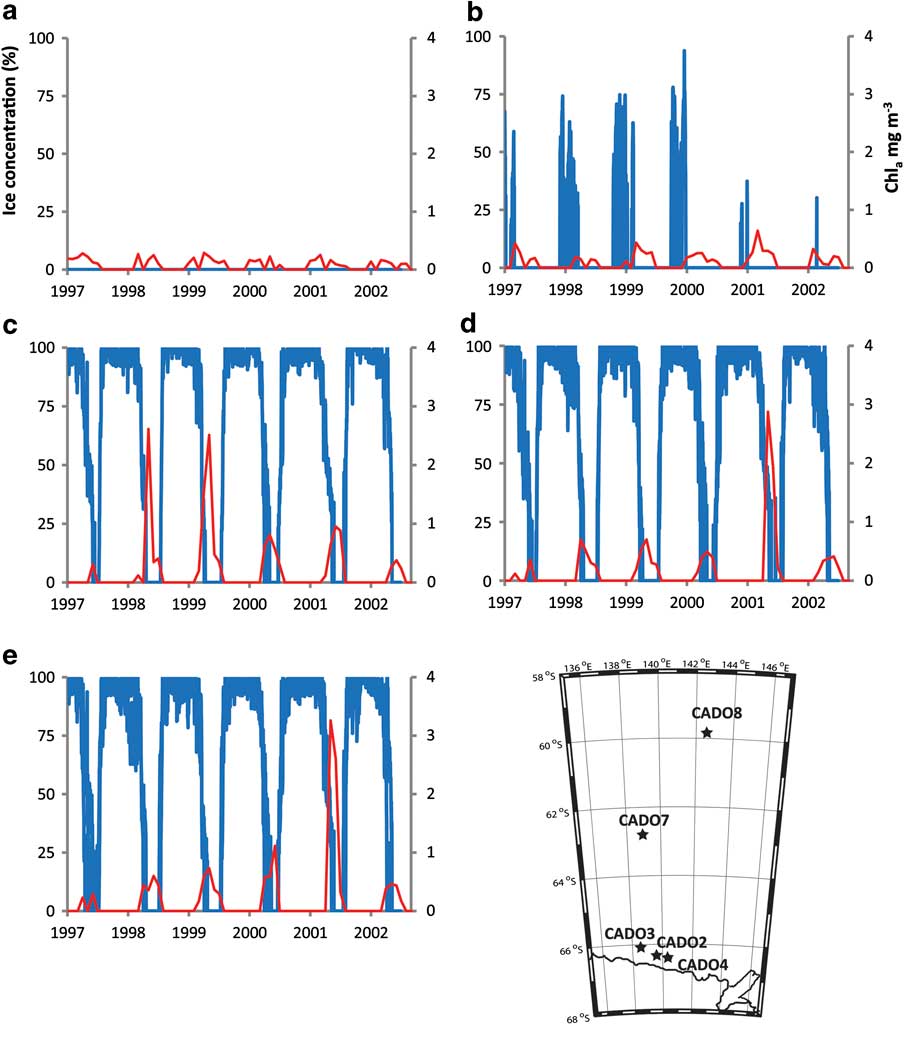
Fig. 5 September 1997–September 2002 satellite derived sea ice (blue line, SSMI, 25 km resolution) and chlorophyll a (red line, SEAWIFS, 9 km) concentrations. a. CADO 8, b. CADO 7, c. CADO 4, d. CADO 3, and e. CADO 2.
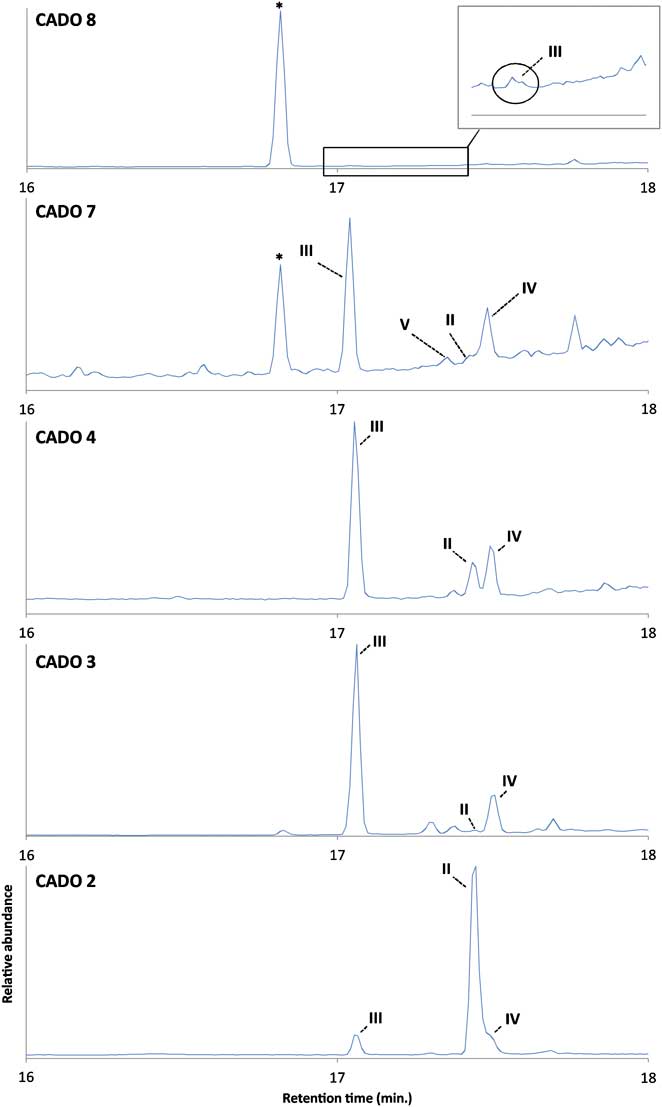
Fig. 6 Gas chromatography-mass spectrometry (GC-MS) total ion current chromatograms of hydrocarbon extracts of surface sediments collected in CADO 2, 3, 4, 7 and 8 sites. * = unidentified hydrocarbon.
The hydrocarbons of sediment sections of an additional 20 cm long core collected at the CADO 2 site (core MD130-MC02), were also analysed (Fig. 4c) and the 13C isotopic compositions of HBIs in the uppermost 0–0.5 cm interval were determined (Table II). Thus, δ13C values were obtained for the diene (II), trienes (III, IV) and one of the tetraenes (VI). The δ13C value of diene (II) was -17.8±1.0‰. This is somewhat different to that of (II) in the sea ice samples (-8.5 and -5.7‰, Table II), similar to that of IP25 in Arctic sea ice and sediments (-22.3±0.4‰ (sea ice), -19.6±1.1‰ (sediment traps) and -19.3±2.3‰ (sediments); Belt et al. Reference Belt, Massé, Vare, Rowland, Poulin, Sicre, Sampei and Fortier2008) and to both IP25 and (II) in Arctic macrofauna (-17 to -18‰, Brown Reference Brown2011). Further, this value was different to those found for the polyunsaturated HBIs (III–VII) in phytoplankton (-38 to -41‰, Table II). We interpret these findings to indicate that (II) in the Antarctic sediments originated from sea ice diatoms, and that the sedimentary isotopic signature (c. -18‰) reflects inputs of (II) biosynthesized both during periods of ice melt (when the ice is more permeable to CO2 and nutrient replenishment is possible, Kennedy et al. Reference Kennedy, Thomas, Kattner, Haas and Dieckmann2002 and references therein) and during earlier phases, when maximum algal growth occurs and CO2 supply becomes limited (Gibson et al. Reference Gibson, Trull, Nichols, Summons and McMinn1999). In contrast to (II), HBIs (III, IV and V) were significantly depleted in 13C with δ13C values of -41.6‰, -36.1‰ and -39.1‰, respectively (Table II), in excellent agreement with the corresponding values obtained from the phytoplankton samples.
Table II 13C isotopic data for selected HBI alkenes in samples of Antarctic sea ice, phytoplankton and sediment.

*only one replicate
We next examined the concentrations of HBIs in further, deeper, sections of the interface core MD130-MC02 collected in February 2003 (Fig. 7). Activities of 210Pb excess presents a mean value of about 265 mBq g-1 from 0–21 cm, revealing virtually no decrease down core (Table III). This indicates that either the sediments were heavily bioturbated, or that the sedimentation rate was extremely high. Exceptionally high sedimentation rates with an average of 2.3 cm yr-1 over the entire Holocene have been measured previously at this site (Costa et al. Reference Costa, Dunbar, Kryc, Mucciarone, Brachfeld, Roark, Manley, Murray and Leventer2007). Visual examination of the sediments revealed a succession of multi-centimetre dark and light seasonal laminations, indicating that the sediments were not bioturbated. It is probable, therefore, that the sediments from this 20 cm long interface core represent only a few months of sedimentation, although in the absence of a confirmed age model, interpretations of temporal changes can only be speculative. Since the core was collected in February 2003 during the summer, we suggest that sediments from the 0–2.5 cm interval correspond mainly to particles that were sedimenting during December 2002–January 2003 when ice was largely absent. Gas chromatography-mass spectrometry analysis of the hydrocarbon fractions revealed low concentrations of (II) and (III) in this period (although sufficient amounts were present for δ13C determinations), suggesting that most of the organic matter produced during the spring bloom of phytoplankton had not reached the seafloor at the time of collection of the core (explaining also the low excess of the short-lived 234Th at the interface of MD130-MC02). In the 2.5–8 cm interval, (II) was two to three times more abundant than in the other sections. We suggest that these elevated relative concentrations of (II) reflect the inputs of organic matter from the melting sea ice in September–December 2002. The proposed increased contribution of the ice derived organic matter (OM) is also reflected in the slightly higher δ13C values of the total OM during this period (Fig. 7).
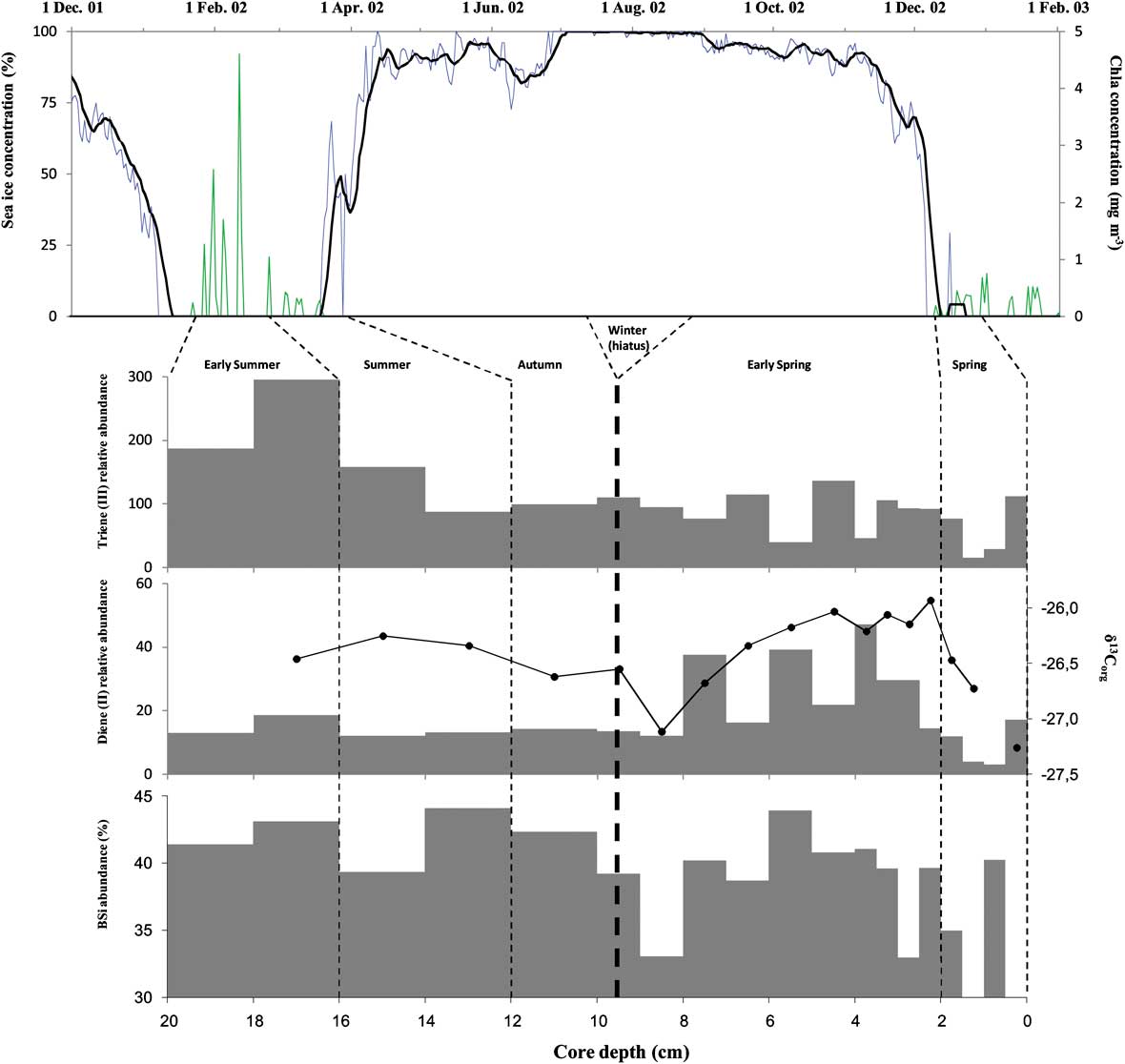
Fig. 7 2001–03 satellite derived sea ice (SSMI, 25 km resolution) and chlorophyll a (SEAWIFS, 9 km) concentrations at CADO 2 coring site. Highly branched isoprenoid, δ13C and BSi concentration along core MD130-MC02.
Table III 210Pbexcess data for core MD130-MC02.

Using sediment traps deployed in shallow waters near Davis Station (East Antarctica), Gibson et al. (Reference Gibson, Trull, Nichols, Summons and McMinn1999) also observed a similar increase in the 13C content of OM sedimenting during springtime and attributed this to the same phenomenon. They observed a 5‰ difference between the OM collected in traps during the spring months (October–December) compared to that of OM collected from April–September. The OM from core MD130-MC02 sediments exhibited much more depleted 13C values (-26.4 ± 0.4‰) than those observed in the surface sediments examined by Gibson et al. (Reference Gibson, Trull, Nichols, Summons and McMinn1999; -19.4 ± 0.6‰) but these large differences can probably be explained by a more pronounced influence of sea ice OM in the shallow coastal area sampled by Gibson et al. (Reference Gibson, Trull, Nichols, Summons and McMinn1999). These authors also observed a pronounced depletion of 13C in the trapped OM (September) concomitant with near zero sedimentation during the preceding winter months (August–September). We suggest that the depletion of the δ13Corg in the 8–9 cm interval in the MD130-MC02 core also reflects limited winter sedimentation. Significantly lower biogenic silica is observed during this interval, probably as a consequence of reduced biological productivity during winter when the ice coverage was at a maximum. The highest concentrations of biogenic silica were observed in the 14–20 cm core interval along with a corresponding increase of the concentration of triene (III). We suggest that this interval corresponds to a period where the particles from the 2002 phytoplankton bloom reached the seafloor.
The distributions and 13C isotopic compositions of HBI alkenes in the Antarctic sediments we have examined here, suggest that in the uppermost sediments at least, they reflect the relative inputs of sea ice diatoms (II) and water column phytoplankton (III–VII), respectively. Thus, a ratio of (II)/(III), for example, might reflect these relative contributions in Antarctic sediments and be a useful additional proxy for the duration of certain sea ice conditions. Whether such a signature is generally preserved during diagenesis is yet to be more fully established.
Clearly, unlike IP25 which, to date at least, has not been reported in environments other than the Arctic, diene (II) cannot be claimed to be unique to the Antarctic, especially as it has been reported from a wide range of environments (e.g. Johns et al. Reference Johns, Wraige, Belt, Lewis, Massé, Robert and Rowland1999 and references therein). However, in the Polar Regions the presence of 13C isotopically enriched (II) can be taken as more characteristic of a sea ice origin (cf. Brown Reference Brown2011).
Our results, therefore, suggest that HBIs have the potential to become useful proxies for the relative contributions of organic matter derived from sea ice diatoms (II) and phytoplankton (III). Although there are already well-established proxy methods for sea ice or productivity reconstruction (Armand & Leventer Reference Armand and Leventer2010), HBIs can be detected in extremely small sediment samples (< 1 g) and therefore permit high-resolution studies to be achieved routinely. Our analysis of the laminated sediments from core MD130-MC02 provides evidence for the potential of HBIs to provide information about the oceanic conditions (in particular sea ice) at an unprecedented sub-seasonal resolution.
Based on suggestions we made earlier (Massé et al. Reference Massé, Belt, Rowland, Sicre and Crosta2007), Barbara et al. (Reference Barbara, Crosta, Massé and Ther2010) and Denis et al. (Reference Denis, Crosta, Barbara, Massé, Renssen, Ther and Giraudeau2010) examined variations in the ratio (II)/(III), in sections of dated sediment cores from the Prydz Bay and Adélie Land areas and observed a strong correlation with the relative abundances of the diatom species Fragilariopsis curta (Van Heurck) and Fragilariopsis kerguelensis (O'Meara) Hustedt. They proposed the use of the ratio of (II)/(III) as a complementary proxy to diatom counts. Although F. curta occurs in open waters, it is commonly considered as representative of the sea ice environment (Armand et al. Reference Armand, Crosta, Romero and Pichon2005), while F. kerguelensis blooms around Antarctica during summer in open waters (Crosta et al. Reference Crosta, Romero, Armand and Pichon2005). In order to test this hypothesis further, we performed diatom counts on sediments from core MD130-MC02. The diatom population was found to be relatively diverse with over 50 species identified in the samples. Amongst the major groups, Fragilariopsis species were found to dominate the assemblage, representing on average over 55% of the total diatom population. Fragilariopsis curta was found to be most abundant, representing between 26 and 51% of the total diatom population, while F. kerguelensis represented between 2 and 10%. Chaetoceros species (vegetative cells and spores) were also abundant, accounting for between 6 and 32% of the total diatom population. Figure 8 shows the (II)/(III) and F. curta/F. kerguelensis ratios in the sediment core. The HBI ratio is highest in the 2.5–8 cm interval, corresponding to spring, when the ice derived OM was reaching the seafloor. In contrast, the (II)/(III) ratio is at a minimum in the 14–20 cm interval when the ice cover in the area was minimal and phytoplankton were blooming. A somewhat similar trend is observed in the F. curta vs F. kerguelensis ratio, with the highest relative abundance of F. curta in the 2.5–10 cm interval. However, the maximum in F. curta cell numbers occurred somewhat earlier than the maximum in the (II)/(III) ratio. These differences in the timing of the maxima in the two ratios are probably attributable to differences in the ecology of the Fragilariopsis spp. compared to HBI producing diatom species. Although, so far as we are aware, neither F. curta or F. kerguelensis have been examined for HBI hydrocarbons, investigation of Fragilariopsis cylindrus (Grunow) Krieger (strain CCMP11022), which is a sea ice diatom species, as well as other species from the Bacillariales, failed to reveal any HBI producers (Damsté et al. Reference Damsté, Muyzer, Abbas, Rampen, Massé, Allard, Belt, Robert, Rowland, Moldowan, Barbanti, Fago, Denisevich, Dahl, Trindade and Schouten2004). It is probable, therefore, that F. curta and F. kerguelensis are not the diatom species responsible for the presence of HBIs in the Antarctic environment. It has been observed previously, in temperate environments, that maxima in HBI concentrations do not correlate exactly with those of other diatom biomarkers. Thus, in sediments of the Tamar Estuary, UK, seasonal maxima in the ubiquitous diatom biomarkers fucoxanthin and henicosahexaene, slightly post dated maxima in HBI concentrations over two year periods (Hird & Rowland Reference Hird and Rowland1995, Cooke et al. Reference Cooke, Barlow, Green, Belt and Rowland1998). Although F. kerguelensis blooms around Antarctica during summer in open waters, maximum abundances of F. curta were observed in both fast and pack ice of regions experiencing nine to eleven months of sea ice per year but also in waters near the ice edge (Armand et al. Reference Armand, Crosta, Romero and Pichon2005). In our MD130-MC02 sediments, relative abundances of F. kerguelensis are at a maximum in the sediments corresponding to the summer–autumn interval (10–18 cm). This species is then rapidly replaced by more cryophilic species (e.g. F. curta) during the winter–early spring interval (2.5–10 cm). Relatively low abundances of Chaetoceros spp. are also observed in the 12–18 cm and 2.5–10 cm intervals while a sharp increase in both vegetative cells and spores are observed in the 10–12 and 0–2.5 cm intervals (Fig. 8). Chaetoceros (CRS) blooms usually result from enhanced water stratification following the ice melt (e.g. spring) or reflect the degradation of the growth conditions when sea ice starts to form in autumn (Armand et al. 2005). In our MD130-MC02 sediments, the high CRS abundances in the 10–12 cm interval reflect the late autumn export with the formation of sea ice in the area promoting spore formation and settling. In contrast, the large abundance of CRS in the early spring interval (0–2.5 cm) reflects the enhanced water stratification induced by the ice melt.
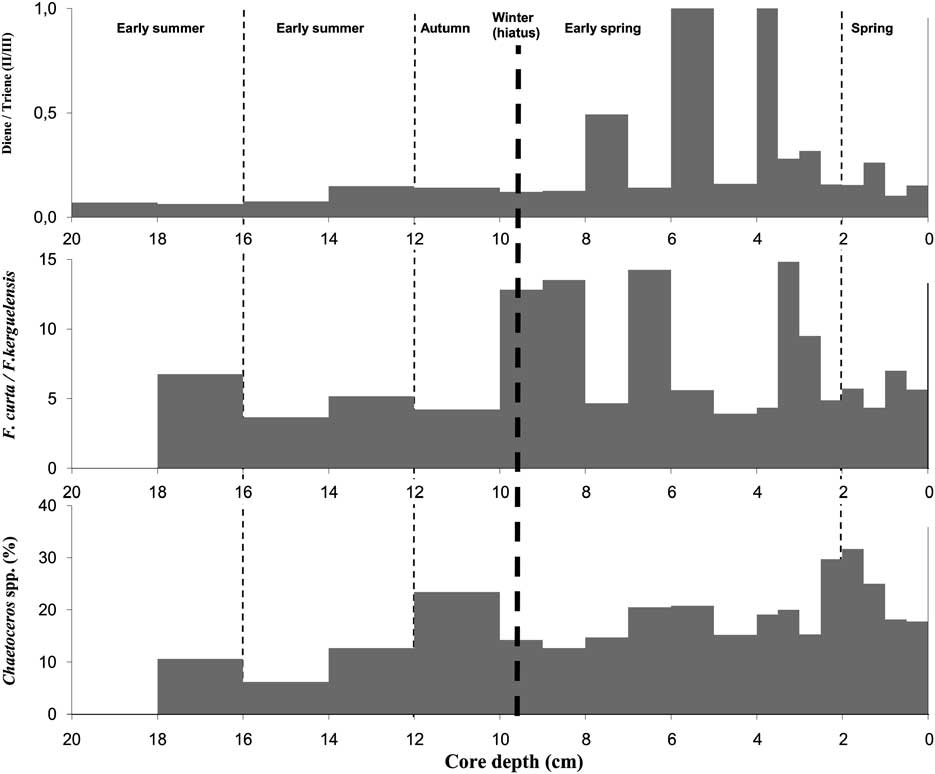
Fig. 8 Relative concentrations of Diene (II) vs Triene (III), Fragilariopsis curta vs Fragilariopsis kerguelensis and relative abundances of Chaetoceros spp. along core MD130-MC02.
Although there are some differences in the profiles of the two ratios (F. curta/F. kerguelensis and HBI ((II)/(III)), there appears to be a general agreement between the two. The small temporal differences might be induced by differences in the ecology of the two Fragilariopsis spp. and sea ice (II) and phytoplanktonic (III) HBI producing diatoms.
Conclusions
We have extended previous reports of HBI alkenes in sediment samples from the Antarctic and report an HBI diene (II) in sea ice and other HBIs in phytoplankton. We determined the δ13C composition of individual HBIs in both sea ice and sediments and used the differences in the structures and isotopic compositions to assign HBI isomers present in sediments from the East Antarctic continental shelf to specific origins. We propose that the presence of isotopically 13C enriched HBI diene (II) might be a useful proxy for contributions of organic matter derived from sea ice diatoms to recent Antarctic sediments. Although limitations due to diagenetic effects need to be considered, a ratio of the concentrations of (II)/(III) might reflect the relative contributions of sea ice organic matter and phytoplankton derived organic matter to Antarctic sediments.
Acknowledgements
This work was supported by the (UK) Natural Environment Research Council (NERC, NE/D000068/1 & NER/A/S/2003/00340) and the European Research Council (Grant number: 203441). DT is grateful to the master and crew of the RV Polarstern as well as S. Papadimitriou, L. Norman and G.S. Dieckmann for help in the field. We are very grateful to Dr I.D. Bull at the Life Sciences Mass Spectrometry Facility at the University of Bristol (Application LSMSFBRISTOL007) for conducting the carbon isotopic measurements.













