The amygdala and medial prefrontal cortex (mPFC) are brain regions important for emotion regulation. The amygdala is involved in emotional learning and may facilitate attention to salient cues (Phelps & LeDoux, Reference Phelps and LeDoux2005), whereas the mPFC has been implicated in higher order emotional functioning (Forbes & Grafman, Reference Forbes and Grafman2010; Miller, Reference Miller2002). As the mPFC is suggested to provide top-down regulation of amygdala reactivity, functional coupling of the amygdala and mPFC may be particularly important for regulating emotions (Hariri, Mattay, Tessitore, Fera, & Weinberger, Reference Hariri, Mattay, Tessitore, Fera and Weinberger2003; Ochsner, Bunge, Gross, & Gabrieli, Reference Ochsner, Bunge, Gross and Gabrieli2002; Phan et al., Reference Phan, Fitzgerald, Nathan, Moore, Uhde and Tancer2005). In children, the amygdala–mPFC circuit is still developing. While the amygdala–mPFC circuit is immature, parents may play an important role in child emotion regulation (Tottenham, Reference Tottenham2015). As a consequence, adverse caregiving may affect amygdala and mPFC development. Several studies have suggested that extreme caregiving experiences, such as institutionalized care, are associated with amygdala and mPFC structure and function (Banihashemi, Sheu, Midei, & Gianaros, Reference Banihashemi, Sheu, Midei and Gianaros2015; Dannlowski et al., Reference Dannlowski, Kugel, Huber, Stuhrmann, Redlich, Grotegerd and Suslow2013; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Casey2010; Whittle et al., Reference Whittle, Yap, Yucel, Sheeber, Simmons, Pantelis and Allen2009), as well as amygdala–mPFC circuit development (Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013). However, results obtained in high-risk samples may not be generalizable to the general population. Here, we examined whether the development of the amygdala–mPFC circuit is modulated by typical variation in parental care.
Evidence suggests that the amygdala develops earlier than the mPFC, which may not show mature structure until late adolescence or early adulthood (Giedd et al., Reference Giedd, Vaituzis, Hamburger, Lange, Rajapakse, Kaysen and Rapoport1996; Shaw et al., Reference Shaw, Kabani, Lerch, Eckstrand, Lenroot, Gogtay and Wise2008). This would imply that during childhood, the immature mPFC may not be capable of fully regulating amygdala (re)activity. Amygdala reactivity to fearful stimuli decreases with age (Gee, Humphreys, et al., Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013; Guyer et al., Reference Guyer, Monk, McClure-Tone, Nelson, Roberson-Nay, Adler and Ernst2008). Moreover, Gee, Humphreys, et al. (Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) showed that this decrease in amygdala activity with age is accompanied by a switch from positive to negative coupling of the amygdala–mPFC circuit at approximately 10 years of age. Further evidence for the continuing development of the amygdala–mPFC circuit during childhood and adolescence comes from studies showing that task-based connectivity of the amygdala–mPFC circuit increases with age (Decety, Michalska, & Kinzler, Reference Decety, Michalska and Kinzler2012; Perlman & Pelphrey, Reference Perlman and Pelphrey2011). For children younger than 10.5 years, no evidence was found for functional coupling between the amygdala and the mPFC in the brain at rest, whereas for children from 10.5 years onward, there was a positive association between amygdala–mPFC connectivity and age (Gabard-Durnam et al., Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014).
In the absence of a mature amygdala–mPFC circuit, the child's emotionality may be externally regulated by parental presence (Tottenham, Reference Tottenham2015). For example, children show greater approach-related behavior, greater exploration, and lower levels of stress hormones in the presence of their parent than in the presence of a stranger or left alone (Ainsworth & Bell, Reference Ainsworth and Bell1970; Hostinar, Johnson, & Gunnar, Reference Hostinar, Johnson and Gunnar2015). In rodent pups, maternal presence reduced amygdala activity during odor-shock conditioning (Moriceau & Sullivan, Reference Moriceau and Sullivan2006), while in human participants, parental presence normalized anxious youth’ mPFC activation in response to stressful stimuli (Conner et al., Reference Conner, Siegle, McFarland, Silk, Ladouceur, Dahl, Coan and Ryan2012).
Early caregiving adversity affects brain development. In rats, maternal deprivation has been associated with accelerated development of fear-based learning (Callaghan & Richardson, Reference Callaghan and Richardson2011), which has been ascribed to the amygdala–mPFC circuit (Akirav & Maroun, Reference Akirav and Maroun2007). Moreover, institutionalized care has been associated with structural abnormalities in the amygdala (Mehta et al., Reference Mehta, Golembo, Nosarti, Colvert, Mota, Williams and Sonuga-Barke2009; Tottenham et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse, Gilhooly and Casey2010). Adults who report a history of early maltreatment exhibit exaggerated amygdala reactivity in response to emotional stimuli (Dannlowski et al., Reference Dannlowski, Kugel, Huber, Stuhrmann, Redlich, Grotegerd and Suslow2013), and previously institutionalized children have been shown to exhibit reduced amygdala discrimination between mothers versus strangers, which is associated with indiscriminate friendliness (Olsavsky et al., Reference Olsavsky, Telzer, Shapiro, Humphreys, Flannery, Goff and Tottenham2013). Comparable to fear-based learning, maturation of the amygdala–mPFC circuit may be accelerated in the absence of supportive parental care. Although family-reared children do not show negative coupling between the amygdala and the mPFC until the age of 10 years (while watching fearful faces; Gee, Humphreys, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013), previously institutionalized youth have been shown to demonstrate negative coupling earlier in development (Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013).
The finding of accelerated maturation in response to caregiving adversity is in line with the Belsky, Steinberg, and Draper (Reference Belsky, Steinberg and Draper1991) evolutionary theory of reproductive development. They theorized that children adaptively adjust their development to match local conditions. Parental care and investment provide children with information about availability and predictability of resources and relationships. Low levels of parental care or investment suggest scarcity of resources and low quality of interpersonal relationships. According to this theory, children respond to low levels of parental care by speeding up development, as this may ultimately increase their reproductive success. Building on this theory, Callaghan and Tottenham (Reference Callaghan and Tottenham2016b) suggest that early life stress may prematurely activate the development of neural structures important for emotion regulation. Although a long childhood is critical for the development and practice of highly competent behavior and long-term well-being, it may be adaptive in the short run to self-regulate instead of rely on a parent for emotion regulation when quality of parental care is low, even at the cost of long-term health or well-being (Belsky, Ruttle, Boyce, Armstrong, & Essex, Reference Belsky, Ruttle, Boyce, Armstrong and Essex2015; Hochberg & Belsky, Reference Hochberg and Belsky2013).
Due to the relatively extreme caregiving experiences, results obtained in high-risk samples such as previously institutionalized children may not be generalizable to the general population. More important, the influence of unmeasured confounders and third variables (such as nutritional deficiencies, congenital diseases, and lack of cognitive stimulation) in the mostly somewhat “messy” designs that are inevitably characteristic of deprivation-related studies of child development in high-risk samples makes converging evidence from relatively normal, typical variations in parenting and the effects on brain development even more crucial. Ultimately, developmental pathways can only be understood when both typical and atypical processes are considered (Cicchetti, Reference Cicchetti2016; Sroufe, Reference Sroufe1990). Although there is compelling evidence that normal variation in early caregiving has a long-term impact on child behavior, emotional regulation, and executive functioning (Bernier, Carlson, Deschenes, & Matte-Gagne, Reference Bernier, Carlson, Deschenes and Matte-Gagne2012; Kok, Linting, et al., Reference Kok, Linting, Bakermans-Kranenburg, van IJzendoorn, Jaddoe, Hofman, Verhulst and Tiemeier2013; Lucassen et al., Reference Lucassen, Kok, Bakermans-Kranenburg, van IJzendoorn, Jaddoe, Hofman and Tiemeier2015; Newton, Laible, Carlo, Steele, & McGinley, Reference Newton, Laible, Carlo, Steele and McGinley2014; Rijlaarsdam et al., Reference Rijlaarsdam, Stevens, van der Ende, Hofman, Jaddoe, Mackenbach and Tiemeier2013), research on normal variation in parental care and brain development is scarce. Sensitive parental care is characterized by prompt and adequate responses to the child's signals and needs (Ainsworth, Bell, & Stayton, Reference Ainsworth, Bell, Stayton and Richards1974). Maternal sensitivity has been shown to predict reduced growth in the amygdala and greater thinning of the orbitofrontal cortex 4 years later (Whittle et al., Reference Whittle, Simmons, Dennison, Vijayakumar, Schwartz, Yap and Allen2014). Moreover, insecure attachment during infancy may predict larger amygdala volume in adulthood (Moutsiana et al., Reference Moutsiana, Johnstone, Murray, Fearon, Cooper, Pliatsikas and Halligan2015), and lower levels of early parental sensitivity in a typically developing, nonclinical sample was associated with smaller total brain volume and gray matter volume (controlled for infant head size) later in childhood (Kok et al., Reference Kok, Thijssen, Bakermans-Kranenburg, Jaddoe, Verhulst, White and Tiemeier2015). However, this study did not find an association between early parental insensitivity and amygdala volume at 8 years.
The present cross-sectional magnetic resonance imaging (MRI) study examined whether the association between age and amygdala–mPFC connectivity in 6- to 10-year-old children is correlated with normal variation in parental care. Gabard-Durnam et al. (Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014) found that resting-state functional coupling between the amygdala and mPFC increases from age 10.5 years onward. We hypothesized that children from less sensitive parents show accelerated development of the amygdala–mPFC circuit, with amygdala–mPFC coupling already increasing over age in the period from 6 to 10 years. In children from more sensitive parents, amygdala–mPFC coupling would not yet show an increase with age in the current sample. Moreover, as several studies provide evidence that parents may have differential effects on the neurobiology of male and female offspring (Ellis, Schlomer, Tilley, & Butler, Reference Ellis, Schlomer, Tilley and Butler2012; Wu et al., Reference Wu, Song, Wang, Shui, Tai, Qiao and He2014; Yu et al., Reference Yu, An, Tai, Zhang, He, Wang and Wu2012), we explored Sensitivity × Age × Sex interaction effects.
Methods
Participants
The study was embedded within the Generation R Study, a prospective cohort investigating growth, development, and health from fetal life onward in Rotterdam, The Netherlands (Jaddoe et al., Reference Jaddoe, van Duijn, Franco, van der Heijden, van IJzendoorn, de Jongste and Hofman2012). Detailed measurements, including parental sensitivity, were obtained in a subgroup of children of Dutch national origin (the children, their parents, and their grandparents were all born in The Netherlands) to reduce confounding and effect modification (Tiemeier et al., Reference Tiemeier, Velders, Szekely, Roza, Dieleman, Jaddoe and Verhulst2012). The study was approved by the Medical Ethics Committee of the Erasmus Medical Centre. Written informed consent was obtained from all adult participants.
Resting-state functional MRI (rs-fMRI) and parental sensitivity scores were available for 147 children: for 134 children data on maternal sensitivity was available, while for 147 children paternal sensitivity scores were available. We excluded 23 children (16%) due to poor quality rs-fMRI data. Analyses regarding maternal sensitivity were performed on 112 children (57 girls), whereas analyses regarding paternal sensitivity were performed on 124 children (64 girls).
Measures
Sensitivity
Maternal and paternal sensitivity was observed when the children 4 years of age. Parents and children were observed during four 3- to 4-min tasks that were too difficult for the child: building a tower and an Etch-a-Sketch task. Sessions were coded using the revised Erickson 7-point rating scales for supportive presence and intrusiveness (Egeland, Erickson, Clemenhagen-Moon, Hiester, & Korfmacher, Reference Egeland, Erickson, Clemenhagen-Moon, Hiester and Korfmacher1990). Overall sensitivity scores were created by aggregating the standardized subscale scores of both subscales in both tasks (reversing the intrusiveness scores). The intraclass correlation was 0.84 (Kok, van IJzendoorn, et al., Reference Kok, van IJzendoorn, Linting, Bakermans-Kranenburg, Tharner, Luijk and Tiemeier2013). The correlation between fathers and mothers was 0.18 (p = .06). A composite parental sensitivity score was created by averaging the standardized maternal and paternal sensitivity scores, as was done in Kok et al. (Reference Kok, Thijssen, Bakermans-Kranenburg, Jaddoe, Verhulst, White and Tiemeier2015), but maternal and paternal sensitivity were also analyzed separately. When results are stronger for the composite score than for the separate maternal and paternal sensitivity scores, this may suggest that the overall level of sensitivity experienced by the child is of greater importance than the sex of the parent providing care.
Covariates
Information on sex and date of birth was obtained from midwives and hospital registries. IQ was estimated from the mosaics and categories subtest of the Snijders–Oomen Nonverbal Intelligence Test—Revised when the children were on average 6 years old (M = 108.42, SD = 13.08; Tellegen, Winkel, Wijnberg-Williams, & Laros, Reference Tellegen, Winkel, Wijnberg-Williams and Laros2005). IQ data was missing for 11 children. For these children, the score was imputed. Parental education was assessed via questionnaire when the children were on average 6 years of age. Parental education level was defined as the highest completed education and was categorized as secondary (lower and intermediate vocational training, n = 28 [23%] and n = 26 [21%] for maternal and paternal education, respectively) and higher (higher vocational education and university, n = 96 [73%] and n = 98 [71%] for maternal and paternal education, respectively) education.
fMRI acquisition and processing
Rs-fMRI acquisition
The neuroimaging component of the Generation R Study has been presented in White et al. (Reference White, El Marroun, Nijs, Schmidt, van der Lugt, Wielopolki and Verhulst2013). In brief, children were 6 to 10 years of age at the time of the MRI assessment (M = 8.04, SD = 0.96). Prior to the MRI, the children were first familiarized with a mock scanning session. MRI scanning was performed on a GE Discovery MR 750 3 T scanner (General Electric, Milwaukee, MI, USA) using an 8-channel head coil. Rs-fMRI utilized a gradient-echo blood oxygen level dependent echo planar imaging sequence with repetition time = 2000 ms, echo time = 30 ms, flip angle = 85°, matrix 64 × 64, and voxel resolution of 3.6 × 3.6 × 4.0 mm3. The total duration of the rs-fMRI session is 5 min 20 s. The children were asked to keep their eyes closed during the rs-fMRI sequence and to think about nothing in particular.
MR data preprocessing
Data were preprocessed using the Functional MRI of the Brain Software Library (v5.0.5, FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith2012). FSL's FMRI Expert Analysis Tool was used for preprocessing the rs-fMRI data, which consisted of exclusion of the first four volumes, motion correction, high-pass temporal filtering (σ = 50 s), brain extraction, prewhitening, and spatial filtering (full width at half maximum = 8 mm). Registration of the rs-fMRI data to an in-house generated age-appropriate, standard space T1-weighted template was achieved using a two-step process. The rs-fMRI data were registered to the T1-weighted anatomical image with the FSL-Linear Registration Tool (FLIRT), using 6 degrees of freedom and the boundary based registration cost function. In the second stage, the T1-weighted image was registered to the age-appropriate, standard space T1-weighted template image using FLIRT with 12 degrees of freedom. The two resulting transformation matrices were concatenated and applied to the preprocessed rs-fMRI data.
Given the age of the sample, it was important to use an age-appropriate template for registration of the functional data to standard space (Muetzel et al., Reference Muetzel, Blanken, Thijssen, van der Lugt, Jaddoe, Verhulst and White2016). One hundred thirty T1-weighted images from children without behavioral problems, also rated as having excellent quality, were used to construct the structural template for registration. An iterative approach using both linear and nonlinear algorithms was used (Sanchez, Richards, & Almli, Reference Sanchez, Richards and Almli2012), and is represented graphically in Figure 1. Briefly, T1-weighted images from each of the 130 subjects were first aligned to the MNI-152 1-mm brain using a linear, 6 degree of freedom approach (FLIRT). All registered images were then averaged and used as the template brain for the subsequent step, which was a nonlinear registration (FNIRT). Once again, the result from the nonlinear registration was averaged and used as the template for the subsequent iteration. This routine continued for a total of five nonlinear iterations, where it has been shown the template image stabilizes considerably (Sanchez et al., Reference Sanchez, Richards and Almli2012). The result of the fifth and final nonlinear registration was averaged, resampled to 2 mm isotropic resolution, and then used as the standard-space template for all rs-fMRI data sets.

Figure 1. Study-specific, age-appropriate template for registration. Reprinted from “Resting-State Networks in 6-to-10 Year Old Children,” by R. L. Muetzel, 2016, Human Brain Mapping, 37, supplemental materials. Copyright 2016 by Wiley. Reprinted with permission.
Subject-level independent component analysis (ICA)-based artifact removal
In addition to standard fMRI preprocessing, each data set was cleaned to remove potential biases resulting from subject motion, cardiac/respiratory physiology, and scanner noise using the FMRIB ICA-based Xnoiseifier (FIX v1.06, Griffanti et al., Reference Griffanti, Salimi-Khorshidi, Beckmann, Auerbach, Douaud, Sexton and Smith2014; Salimi-Khorshidi et al., Reference Salimi-Khorshidi, Douaud, Beckmann, Glasser, Griffanti and Smith2014). With the aid of a training set, FIX automatically classifies subject-level ICA components as signal or noise, and subsequently “denoises” the rs-fMRI data by regressing out time series classified as noise. A thorough and sophisticated cleaning procedure, such as FIX, is especially relevant in the context of pediatric rs-fMRI, given recent reports on the impact of motion on functional connectivity (Power, Barnes, Snyder, Schlaggar, & Petersen, Reference Power, Barnes, Snyder, Schlaggar and Petersen2012; Satterthwaite et al., Reference Satterthwaite, Wolf, Loughead, Ruparel, Elliott, Hakonarson, Gur and Gur2012). The FIX classifier was trained using manually labeled, subject-level ICA data from a random sample of 50 subjects (mean leave one out classifier performance results: 93.4% true-positive rate, 85.8% true-negative rate). All rs-fMRI data sets underwent a single-session ICA using the MELODIC (v5.0.5) tool from FSL, followed by the artifact removal with FIX, including the removal of motion confounds (Griffanti et al., Reference Griffanti, Salimi-Khorshidi, Beckmann, Auerbach, Douaud, Sexton and Smith2014).
Data quality
Data quality was assessed in two steps. First, as the subject-level ICA denoising of the data is not sufficient in data sets severely corrupted by motion, a mean root mean squared relative motion greater than 0.5 mm was used as a cutoff to exclude data of poor quality (n = 19, 13%). Second, all standard space registrations were examined using the middle functional volume from the time series, and poorly registered data sets were excluded (n = 4, 3%).
Statistical analyses
The left and right amygdala masks from the Harvard-Oxford-subcortical atlas were used for analyses (Frazier et al., Reference Frazier, Chiu, Breeze, Makris, Lange, Kennedy and Biederman2005). The mPFC mask was created by combining the medial frontal gyrus, medial orbitofrontal cortex, and anterior cingulate cortex using Wake Forest University PickAtlas (Maldjian, Laurienti, Kraft, & Burdette, Reference Maldjian, Laurienti, Kraft and Burdette2003). The regions of interest were spatially normalized to the in-house template space by applying an inverse transform matrix from MNI template space to the in-house child template space. Amygdala functional connectivity was assessed at the single-subject level using the general linear model within FSL's FMRI Expert Analysis Tool with the time series of the amygdalae (obtained using fslmeants) as the design matrix.
Kok et al. (Reference Kok, Thijssen, Bakermans-Kranenburg, Jaddoe, Verhulst, White and Tiemeier2015) showed that maternal and paternal sensitivity were similarly related to child brain structure. Therefore, we first examined the interaction effect of sensitivity and age using the combined parental sensitivity score. In case of a significant interaction effect, analyses were repeated for maternal and paternal sensitivity scores separately in order to assess whether both parents contributed to the effect. Higher level analyses of the Sensitivity × Age interaction effect were performed using FSL's FLAME I (FMRI's local analysis of mixed effects) module, controlling for sensitivity, age, sex, Sensitivity × Sex, and Sex × Age for the left and the right amygdala separately. In exploratory analyses, we assessed Sensitivity × Age × Sex interaction effects (controlling for sensitivity, age, sex, Sensitivity × Age, Sensitivity × Sex, and Sex × Age). All variables were mean-centered. Higher level analyses were restricted to the voxels in the mPFC mask. Statistical maps were thresholded using clusters determined by Z > 2.3 and a cluster corrected significance threshold of p < .05. In order to assure that results are specific to amygdala–mPFC connectivity, we repeated the higher level analyses using a lateral PFC mask (superior frontal gyrus, middle frontal gyrus, and inferior frontal gyrus from the Wake Forest University Pick Atlas).
In order to interpret significant interaction effects, we extracted mean Z values of all significant voxels for all participants. The Z values were then imported into SPSS. Post hoc analyses on interaction effects were conducted using the PROCESS SPSS custom dialog, using IQ and parental education as covariates.
Results
Combined parental sensitivity
Figure 2 and Table 1 show the clusters for which amygdala connectivity was significantly predicted by a Parental Sensitivity × Age interaction effect. Post hoc analyses (Tables 2 and 3 for full models and Table 4 for Sensitivity × Age interaction effects) showed that in children with lower combined parental sensitivity, amygdala–mPFC connectivity was related to age (b = 0.11, p = .004 and b = 0.06, p = .06, right and left amygdala, respectively). In children with highly sensitive parents, the association between right amygdala–mPFC connectivity and age was not significant (b = –0.07, p = .12 and b = –0.06, p = .12, right and left amygdala, respectively).
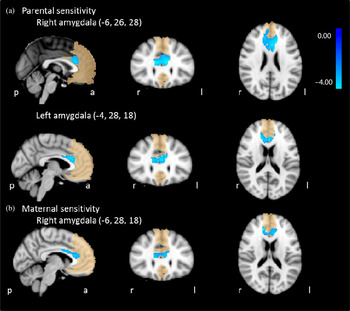
Figure 2. (Color online) Sensitivity × Age interaction effect on amygdala–medial prefrontal cortex functional connectivity. (a) Parental sensitivity and (b) maternal sensitivity. Medial prefrontal cortex mask shown in background.
Table 1. Sensitivity × Age Effects on amygdala–medial prefrontal cortex functional connectivity

Note: The results of the general linear model of the Sensitivity × Age interaction effects on amygdala–medial prefrontal cortex functional connectivity. The table displays the six most significant voxels per contrast and is not a conclusive list of significant regions. R, Right; WM, white matter; ACC, anterior cingulate cortex; L, left.
Table 2. Full model of Parental Sensitivity × Age Interaction effect on right amygdala–medial prefrontal cortex connectivity

Note: The results of the PROCESS regression analysis examining the Parental Sensitivity × Age interaction effect on right amygdala–medial prefrontal cortex connectivity. The top row of the table describes the model characteristics, and the lower rows describe characteristics of the dependent variables. LLCI, Lower limit confidence interval; ULCI, upper limit confidence interval; rs-fMRI, resting state functional magnetic resonance imaging.
Table 3. Full model of Parental Sensitivity × Age interaction effect on left amygdala–medial prefrontal cortex connectivity

Note: The results of the PROCESS regression analysis examining the Parental Sensitivity × Age interaction effect on left amygdala–medial prefrontal cortex connectivity. The top row of the table describes the model characteristics, and the lower rows describe characteristics of the dependent variables. LLCI, Lower limit confidence interval; ULCI, upper limit confidence interval; rs-fMRI, resting state functional magnetic resonance imaging.
Table 4. Post hoc analyses of Sensitivity × Age interaction effects

Note: The table describes the regression coefficients of age on amygdala–medial prefrontal cortex connectivity at ±1 SD of sensitivity.
Maternal sensitivity
For the right amygdala, we found a significant maternal Sensitivity × Age interaction effect on amygdala–anterior cingulate cortex connectivity (Table 1 and Figure 2). Post hoc analyses (Table 5 for full model, Table 4 for Maternal Sensitivity × Age interaction effects) showed that in children from less sensitive mothers, right amygdala–mPFC connectivity increased over age (b = 0.09, p = .01). In children from more sensitive mothers, the association between right amygdala–mPFC connectivity and age was not significant (b = –0.03, p = .52).
Table 5. Full model of Maternal Sensitivity × Age interaction effect on right amygdala–medial prefrontal cortex connectivity

Note: The results of the PROCESS regression analysis examining the Maternal Sensitivity × Age interaction effect on right amygdala–medial prefrontal cortex connectivity. The top row of the table describes the model characteristics, and the lower rows describe characteristics of the dependent variables. LLCI, Lower limit confidence interval; ULCI, upper limit confidence interval; rs-fMRI, resting state functional magnetic resonance imaging.
Paternal sensitivity
After correction for multiple testing, we did not find significant Paternal Sensitivity × Age interaction effects on amygdala–mPFC connectivity.
Exploratory Sensitivity × Age × Sex analyses
Combined parental sensitivity
Table 6 and Figure 3 show the clusters for which amygdala connectivity was significantly predicted by a Parental Sensitivity × Age × Sex interaction effect. Table 7 shows the results of the post hoc analyses of the Parental Sensitivity × Age × Sex interaction effect. In daughters from less sensitive parents, amygdala–mPFC connectivity was related to age (b = 0.26, p < .001 and b = 0.20, p < .001, right and left amygdala, respectively). In daughters from highly sensitive parents, amygdala–mPFC decreased with age (b = –0.12, p = .03 and b = –0.10, p = .08, right and left amygdala, respectively). In boys, no association between age and amygdala–mPFC connectivity was found (less sensitive parents b = –0.05, p = .34 and b = –0.04, p = .36, for right and left amygdala, respectively; highly sensitive parents b = –0.01, p = .94 and b = 0.07, p = .26, for right and left amygdala, respectively).
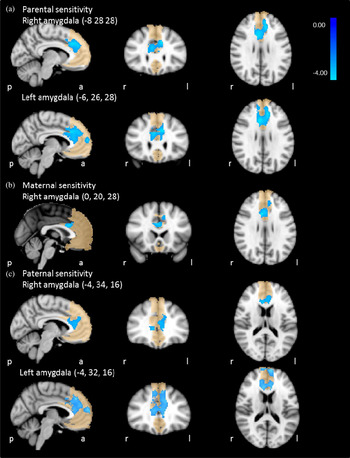
Figure 3. (Color online) Sensitivity × Age × Gender interaction effect on amygdala–medial prefrontal cortex functional connectivity. (a) Parental sensitivity, (b) maternal sensitivity, and (c) paternal sensitivity.
Table 6. Sensitivity × Age × Gender effects on amygdala–medial prefrontal cortex functional connectivity

Note: The results of the general linear model of the Sensitivity × Age × Gender interaction effects on amygdala–medial prefrontal cortex functional connectivity. The table displays the six most significant voxels per contrast and is not a conclusive list of significant regions. L, Left; R, right; ACC, anterior cingulate cortex; SFG, superior frontal gyrus; WM, white matter.
Table 7. Post hoc analyses of Sensitivity × Age × Gender interaction effects

Note: The table describes the regression coefficients of age on amygdala–medial prefrontal cortex connectivity at ±1 SD of sensitivity for boys and girls separately.
Maternal sensitivity
For the right amygdala, we found a significant Maternal Sensitivity × Age × Sex interaction effect on amygdala–mPFC functional connectivity. Specific information on the mPFC cluster in which this effect was found is presented in Table 6 and Figure 3. Post hoc analyses show that in daughters of less sensitive mothers amygdala–mPFC connectivity was related to age (b = 0.29, p < .001; Table 7). There was no association between age and amygdala–mPFC connectivity in daughters from highly sensitive mothers (b = –0.08, p = .17) or in sons (b = –0.04, p = .35 and b = –0.02, p = .80, with less or highly sensitive mothers, respectively).
Paternal sensitivity
Table 6 and Figure 3 show the clusters for which amygdala connectivity was significantly predicted by a Paternal Sensitivity × Age × Sex interaction effect. Post hoc analyses (Table 7) show that in daughters from less sensitive fathers, amygdala–mPFC connectivity was related to age (b = 0.23, p < .001 and b = 0.18, p = .001, right and left amygdala, respectively). There was no association between age and amygdala–mPFC connectivity in daughters from more sensitive fathers (b = –0.11, p = .10 and b = –0.07, p = .28, for right and left amygdala, respectively) or in sons (less sensitive fathers: b = –0.04, p = .45 and b = –0.05, p = .36, for right and left amygdala, respectively; highly sensitive fathers: b = 0.00, p = .99, and b = 0.10, p = .07, for right and left amygdala, respectively).
Contrasting analyses: Amygdala–lateral PFC connectivity
After correction for multiple testing, we did not find any combined Parental Sensitivity × Age or Combined Parental Sensitivity × Age × Sex interaction effects on amygdala–lateral PFC connectivity.
Discussion
In the present study we found that the association between age and amygdala–mPFC connectivity from age 6 to 10 years was modulated by normal variation in parental care. In children of less sensitive parents, age was related to stronger amygdala–mPFC connectivity, while in children from more sensitive parents, age was not associated with amygdala–mPFC connectivity. To our knowledge, the only study on amygdala resting-state functional connectivity that examined prepubertal children reported no evidence for connectivity between the amygdala and mPFC in children younger than 10.5 years, while for children older than 10.5 years, age was associated with stronger amygdala–mPFC connectivity (Gabard-Durnam et al., Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014). Our results, therefore, suggest that less sensitive parenting accelerates amygdala–mPFC circuit development. However, due to the age range of our sample, we are unable to test whether the increase in amygdala–mPFC coupling as displayed in children from less sensitive parents before the age of 10 years will also occur in children from highly sensitive parents after the age of 10 years.
Both animal and human research suggests that adverse caregiving experiences, such as maternal deprivation, may accelerate amygdala–mPFC connectivity (Callaghan & Richardson, Reference Callaghan and Richardson2011; Gee, Gabard-Durnam, et al., Reference Gee, Humphreys, Flannery, Goff, Telzer, Shapiro and Tottenham2013). Here, we show that in a population-based sample, variation in caregiving experience modulates the development of amygdala–mPFC coupling, such that lower levels of parental care seem to accelerate amygdala–mPFC circuit development. This suggests that even in family-reared children, those experiencing suboptimal parenting may prematurely activate the development of neural structures important for emotion regulation, which may enable them to self-regulate instead of needing to rely on their parents for emotion regulation.
Gee, Gabard-Durnam, et al. (Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) showed that institutionalized children who display a more mature amygdala–mPFC are less anxious than children who display a more immature amygdala–mPFC connectivity pattern, but are still more anxious than their family-reared peers. Thus, the more mature amygdala–mPFC connectivity pattern of children from less sensitive parents may support their regulation capacities, but they are still generally less well regulated than children who experienced more sensitive care (Manning, Davies, & Cicchetti, Reference Manning, Davies and Cicchetti2014; van der Voort et al., Reference van der Voort, Linting, Juffer, Bakermans-Kranenburg, Schoenmaker and van IJzendoorn2014). Life history theory predicts accelerated neurobiological development in children and preadolescents from less privileged backgrounds who have to be prepared for adaptation to nonoptimal environments by using a fast reproductive strategy (Hochberg & Belsky, Reference Hochberg and Belsky2013). Neurobiological and behavioral development might not be synchronized at all developmental time points, and in some cases neurobiological changes might prepare behavioral adaptations that seem to lag behind, a form of developmental “decalage” (van IJzendoorn, Juffer, & Poelhuis, Reference van IJzendoorn, Juffer and Poelhuis2005).
When the quality of parental care is low, it may be beneficial to switch from parent-regulated emotional control to self-regulated emotional control. In the long term, however, early termination of the immature period may have negative consequences (Callaghan & Tottenham, Reference Callaghan and Tottenham2016b). A long childhood period is beneficial for development. For example, a prolonged period of cortical thickness growth has been associated with superior intelligence in children (Shaw et al., Reference Shaw, Greenstein, Lerch, Clasen, Lenroot, Gogtay and Giedd2006). Low levels of parental care may terminate the immature period before the circuit has acquired the characteristics necessary for adequate self-regulatory control. Early life stress has been associated with increased amygdala–mPFC resting-state connectivity in adulthood (Philip et al., Reference Philip, Sweet, Tyrka, Price, Bloom and Carpenter2013). In adults, aberrant amygdala–mPFC functional connectivity has been associated with anxiety and depression (Hahn et al., Reference Hahn, Stein, Windischberger, Weissenbacher, Spindelegger, Moser and Lanzenberger2011; Townsend et al., Reference Townsend, Torrisi, Lieberman, Sugar, Bookheimer and Altshuler2013), psychological problems that are more prevalent in individuals with adverse caregiving experiences (Norman et al., Reference Norman, Byambaa, De, Butchart, Scott and Vos2012).
One way in which parental care may influence development of the amygdala–mPFC circuit is via regulation of a sensitive period of plasticity (Callaghan & Tottenham, Reference Callaghan and Tottenham2016a). Within the visual system, the balance between excitatory and inhibitory signaling appears to regulate the onset of sensitive period plasticity. Continued development then results in formation of structural brakes that terminate the sensitive period (Hensch, Reference Hensch2004). In the case of amygdala–mPFC development, child caregiver independence may regulate the sensitive period for environmental input. Callaghan and Tottenham (Reference Callaghan and Tottenham2016a) suggest that during childhood, relative caregiver dependence may allow for environmental input in the amygdala–mPFC circuit. Over development, the child's independence grows, which may cause the sensitive period to close. When the child reaches independence early in development, this may prematurely close the sensitive period, resulting in accelerated development of the amygdala–mPFC circuit (Callaghan & Tottenham, Reference Callaghan and Tottenham2016a).
Our exploratory analyses suggest that parental sensitivity affects the development of amygdala–mPFC connectivity in daughters only. Compared to boys, girls may generally rely more on others for emotion regulation (Quiroga, Willis, Lopez-Rodriguez, & Moreno, Reference Quiroga, Willis, Lopez-Rodriguez and Moreno2015; Zimmermann & Iwanski, Reference Zimmermann and Iwanski2014). If this is true, the effect of parental care on the development of neural networks associated with emotion regulation may be expected to be larger in girls than in boys. However, there is also evidence suggesting that compared to boys, girls are more likely to seek contact with mother and stay in closer proximity to her regardless of distress, while boys’ proximity seeking is associated with distress specifically (Buss, Brooker, & Leuty, Reference Buss, Brooker and Leuty2008). Alternatively, the different effect of parental sensitivity on sons’ and daughters’ amygdala–mPFC circuit development may be explained by sex differences in neurodevelopment. The social brain has been reported to develop earlier in girls than in boys (Mutlu et al., Reference Mutlu, Schneider, Debbane, Badoud, Eliez and Schaer2013). It is, therefore, possible that in boys the interaction effect of parental sensitivity and age occurs later in development and was thus not visible in the present sample. Moreover, although Gabard-Durnam et al. (Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014) reported no sex differences in amygdala–mPFC connectivity development, one study suggests that in adolescent girls, amygdala–dorsal mPFC connectivity increased with age, while in adolescent boys amygdala–ventral mPFC connectivity increased with age (Alarcon, Cservenka, Rudolph, Fair, & Nagel, Reference Alarcon, Cservenka, Rudolph, Fair and Nagel2015). Although we did not find such evidence in our study, a similar moderating effect of sensitivity on amygdala–mPFC circuit development may be evident in boys, but in a different region of the mPFC.
The results of the exploratory three-way interaction analyses are only partly in accordance with our hypothesis. Consistent with the sex-controlled analyses, daughters of less sensitive parents show an increase in functional connectivity over age. However, contrary to our hypothesis, daughters from highly sensitive parents show a decrease in functional connectivity over age. One possible explanation for this unexpected finding is that the regions we find are located somewhat more dorsally than the regions reported in the Gabard-Durnam et al. (Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys, Telzer and Tottenham2014) and Gee et al. (Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) studies. The development of amygdala–mPFC connectivity in this specific region may follow a different trajectory. Moreover, as three-way interactions require large sample sizes, the present study may not have enough power to properly examine three-way interaction effects. Results of the three-way interaction analyses should thus be regarded as exploratory and await replication.
Studies on caregiving and child development tend to focus on the role of mothers more so than on the role of fathers. In the present study, the Sensitivity × Age interaction effect for the combined parental sensitivity measure had a smaller p value (on face value) than the interaction effect for the maternal sensitivity measure, implying that both maternal and paternal sensitivity contribute to the observed effect. Moreover, results of the exploratory three-way interaction analysis suggest similar effects for mothers and fathers. Our results, therefore, imply that the quality of parental caregiving is more important for brain development than the sex of the person providing this care. However, statistical tests to examine the significance of the difference in maternal versus paternal contributions to the connectivity are difficult to conduct on imaging data, and power constraints prohibited adding parent gender as an additional interaction term. These considerations need to be taken into account, and make our suggestions speculative.
Several limitations should be noted. The present study is based on cross-sectional MRI data, with sensitivity assessed 2 to 6 years earlier. Although such studies can provide information on development, longitudinal studies with both variables measured at both time points are of course better equipped to answer developmental questions. As we are unable to test whether children from more sensitive parents will show a similar increase in amygdala–mPFC coupling after the age of 10 years as displayed by children from less sensitive parents before the age of 10 years, assumptions about accelerated development are made based on the literature and cannot be conclusively inferred from our own data. Moreover, resting-state fMRI data were collected for 5 min and 20 s. While a 5- to 6-min acquisition time should provide adequate sampling to produce stable spatial maps even in children (White et al., Reference White, Muetzel, Schmidt, Langeslag, Jaddoe, Hofman and Tiemeier2014), longer acquisition time are better to distinguish subtle individual differences in the strength of functional connections within networks (Birn et al., Reference Birn, Molloy, Patriat, Parker, Meier, Kirk and Prabhakaran2013). In line with research on previously institutionalized children (Gee, Gabard-Durnam, et al., Reference Gee, Gabard-Durnam, Flannery, Goff, Humphreys, Telzer and Tottenham2013) on which we based our hypothesis, we examined quality of the caregiving environment in relation to brain development. A related but somewhat different question is the association between parent–child attachment and amygdala–mPFC development, or brain development in general. Repeating the analyses with attachment instead of parental sensitivity as predictor would increase the number of tests (with an increased risk of false positives), which is the reason why we focused on parental sensitivity in the current study. Future studies may add to this line of research by examining parent–infant attachment quality as a precursor of optimal brain development.
Although our findings are preliminary, they point to potentially fruitful directions for the study of the long-term effects of attachment-based interventions aiming at the enhancement of parental sensitivity. Previous studies have shown that attachment-based interventions can get “under the skin,” which might explain the persistence of their influence on the parents and their children. For example, Dozier, Peloso, Lewis, Laurenceau, and Levine (Reference Dozier, Peloso, Lewis, Laurenceau and Levine2008) found that their attachment and bio-behavioral catch-up intervention led to a reduction in basal cortisol in children in foster care. In a sensitivity-focused video-feedback intervention for toddlers at risk of behavioral problems, Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, and Juffer (Reference Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman and Juffer2008) also found long-term improvement of cortisol regulation, in particular in carriers of dopamine receptor D4 seven-repeat alleles. Cortisol secretion is regulated in brain regions such as the hippocampus, and the search for attachment-based intervention effects on brain structure, connectivity, and function might shed further light on the cascade of neurobiological changes in children participating in such interventions even if the intervention effects are relatively small, and certainly not as dramatic as the influence of adoption or foster care of previously institutionalized children (Nelson, Fox, & Zeanah, 2015). Our nonexperimental, correlational study with typically developing children and their parents may be considered a first step toward elucidating this cascade in clinical and nonclinical populations (see Fearon et al., Reference Fearon, Groh, van IJzendoorn, Bakermans-Kranenburg, Roisman and Cicchetti2016).
In conclusion, the present population-based study suggests that parenting quality may moderate the development of amygdala–mPFC coupling. Not only extreme caregiving experiences, but also normal variation in caregiving appears to be associated with development of amygdala–mPFC functional connectivity. The present study provides insight into how children adapt their developmental strategies to suboptimal parental care, which may shed light on the mechanisms through which suboptimal parental care increases the risk of emotional problems and psychopathology.












