Introduction
Urocystis prolifer Villot, 1880 (Cyclophyllidea: Hymenolepididae) is one of the smallest cestodes parasitizing shrews of the genus Sorex. It has attracted particular attention due to its wide distribution in the Palaearctic and high rates of infection in definitive hosts (Vaucher, Reference Spassky and Andrejko1971; Genov, Reference Genov1984; Binkiene, Reference Binkiene2006; Binkiene et al., Reference Binkiene, Kontrimavichus and Hoberg2011; Shimalov, Reference Sato, Kamiya and Ohbayashi2012). The high abundance of U. prolifer is associated with a peculiarity of its ontogenesis – the asexual reproduction of larvae by budding in the intermediate host.
Villot (Reference Stammer1880) discovered a polycephalic larva in the diplopod Glomeris lumbatus, which was named U. prolifer Villot, 1880. The adult form of this cestode was described considerably later. Moreover, different authors have described the cestode under various names (Stammer, Reference Skrjabin and Mathevossian1955; Zarnowski, Reference Wardle and McLeod1955; Rybicka, Reference Rawson and Rigby1958; Baer & Della Santa, Reference Baer and Della Santa1960; Kisielewska, Reference Kisielewska1960). Spassky & Andrejko (Reference Skolka, Fãgăras and Paraschiv2004) and Vaucher (Reference Spassky and Andrejko1971) considered the forms described by all previous authors to be the same species, Hymenolepis prolifer, and believed that the main diagnostic character, the number of rostellar hooks (80–190), is widely varying in this species. The name U. prolifer Villot, 1880 was adopted for this form in the recent taxonomic surveys (Czaplinski & Vaucher, Reference Czaplinski, Vaucher, Khalil, Jones and Bray1994; Georgiev et al., Reference Georgiev, Bray, Littlewood, Morand, Krasnov and Poulin2006; Mariaux et al., Reference Lefebvre, Georgiev, Bray and Littlewood2017).
Despite the long history of studies on this species, its status as the only member of the genus, wide distribution in the Palaearctic and the detailed descriptions of adults (Zarnowski, Reference Wardle and McLeod1955; Rybicka, Reference Rawson and Rigby1958), there are significant differences in the data provided by various authors, including differences in the number of testes, proglottides in the strobila and rostellar hooks (table 1). Several articles have presented information on the stages of its metacestode development (Villot, Reference Stammer1880; Joyeux, Reference Jones and Alicata1922; Kisielewska, Reference Kisielewska1960).
Table 1. Comparison of the main taxonomic characters of gravid strobila of Urocystis prolifer.

The aims of the present work were to study the distribution of U. prolifer in the Palaearctic and to identify its geographical range as well as to redescribe the adult stage, provide an amended generic diagnosis and a detailed description of the metacestode development.
Materials and methods
New collections and specimens examined
The adult specimens of U. рrolifer used in the present study were collected in 1990–2018 from different regions of Russia, detailed in the following.
Rostov Oblast’ (27 specimens): Sholokhovsky District, the vicinity of the Stanitsa Vyoshenskaya in 2018 (49°37′N, 41°43′E; seven specimens of Sorex araneus Linnaeus, 1758 and one specimens of Sorex minutus Linnaeus, 1766), the vicinity of the town Belaya Kalitva in 2015 (48°10′N, 40°47′E; 12 specimens of S. araneus and seven specimens of S. minutus).
North Caucasus (172 specimens): Republic of Adygeya, Maykopsky District, the vicinity of the village Nikel in 2014–2015 (44°10′N, 40°09′E; 31 specimens of Sorex raddei Satunin, 1895, 31 specimens of Sorex volnuchini Ognev, 1922 and 21 specimens of Sorex satunini Ognev, 1922); the Karachay–Cherkess Republic, Teberda Nature Reserve in 2016 (43°21′N, 41°42′E; 31 specimens of S. raddei, six specimens of S. volnuchini and 14 specimens of S. satunini); the Republic of North Ossetia–Alania, Tseysky Nature Reserve in 2017 (43°11′N, 44°14′E; 16 specimens of S. volnuchini and 22 specimens of S. satunini).
Eastern Siberia (107 specimens): Krasnoyarsk Kraj, the vicinity of the village Tanzibey in 2009 (53°08′N, 92°56′E; one specimen of Sorex isodon Turov, 1924, five specimens of S. minutus and 21 specimens of S. araneus); Republic of Buryatia, Baikal Nature Reserve in 1990 (51°20′N, 105°09′E; 18 specimens of S. minutus, 29 specimens of S. isodon, 15 specimens of S. araneus and 18 specimens of Sorex caecutiens Laxmann, 1785).
Western Siberia (234 specimens): Altai Republic, Turochakskiy District, the vicinity of the village Artybash in 2015–2018 (51°47′N, 87°18′E; 151 specimens of S. araneus, 16 specimens of S. caecutiens, 35 specimen of S. isodon, one specimen of Sorex minutissimus, 21 specimens of S. minutus and ten specimens of Sorex tundrensis Merriam, 1900).
Far East: Primorsky Kraj (201 specimens): Kedrovaya Pad Nature Reserve in 2002 (43°06′N, 131°30′E; 50 specimens of S. caecutiens, 48 specimens of S. isodon, ten specimens of Sorex unguiculatus Dobson, 1890, one specimens of Sorex gracillimus Thomas, 1907 and 24 specimens of Sorex sp.); Lazovski Nature Reserve in 2003 (43°14′N, 133°24′E; 23 specimens of S. unguiculatus and 45 specimens of S. caecutiens); Khabarovsk Kraj (573 specimens): Bolshekhekhtsirsky Nature Reserve in 2003 (48°12′N, 134°51′E; 42 specimens of S. caecutiens, nine specimens of S. unguiculatus, 14 specimens of S. isodon, two specimens of S. gracillimus and one specimen of S. minutus); Solnechny District, the vicinity of the village Berezovka in 2004 (51°39′N, 135°40′E; 21 specimens of Sorex daphaеnodon Thomas, 1907, eight specimens of S. unguiculatus, 430 specimens of S. caecutiens, five specimens of S. minutissimus Zimmermann, 1780, nine specimens of S. gracillimus, 29 specimens of S. isodon and three specimens of Sorex roboratus Hollister, 1913); Kamchatka Kraj, the vicinity of the town Yelizovo in 2002 (53°11′N, 158°23′E; 13 specimens of S. caecutiens, 11 specimens of S. isodon and one specimen of S. daphaеnodon).
Islands of the Far East (352 specimens): Sakhalin Island, Poronayskiy Nature Reserve in 2005 (48°55′N, 144°30′E; 130 specimens of S. unguiculatus, 16 specimens of S. caecutiens, nine specimens of S. minutissimus, ten specimens of S. gracillimus, five specimens of S. isodon, 17 specimens of Sorex sp.), the vicinity of the town Yuzhno-Sakhalinsk in 2006–2007 (46°57′N, 142°44′E; 42 specimens of S. unguiculatus, three specimens of S. caecutiens, one specimen of S. minutissimus and 11 specimens of S. gracillimus); Kunashir Island, Kurils Nature Reserve in 2006 (44°05′N, 145°59′E; 103 specimens of S. unguiculatus, one specimen of S. caecutiens and 15 specimens of S. gracillimus).
In addition, the analysis included shrews caught in Japan, Hokkaido Island (69 specimens), the vicinity of the city Tomakomai in 2005 (43°30′N, 143°00′E; 41 specimens of S. unguiculatus, 17 specimens of S. caecutiens and 11 specimens of S. gracillimus).
The levels of infection were assessed using the following indicators (Fedorov, Reference Fedorov1986): P, prevalence (percentage of individuals of host population infected with a certain helminth species) and its standard error (±SE); I, intensity range (the minimum and maximum number of cestodes of a certain species in infected individuals in the host population); MI, mean intensity (average number of cestodes of a certain species per one infected individual of the host population); MA, mean abundance (average number of cestodes of a certain species per one studied individual of the host population).
For examination of the metacestode development, a stock culture of millipedes Julus ghilarovi Gulička, 1963 was obtained from the upper layer of soil and litter and kept in the laboratory in the Institute of Systematics and Ecology of Animals, Novosibirsk, Russia (ISEA) since 2018. The millipedes were kept at room temperature (20–25°С) in cages filled with an upper layer of soil, birch and aspen litter.
Adult specimens of U. рrolifer were collected from the small intestines of S. araneus in the summer of 2019 in the vicinity of the village Artybash, Turochakskiy District, Altai Republic, Russia. Host specimens were dissected immediately after death. The collected worms were rinsed quickly in mammalian Ringer's balanced salt solution at room temperature. Some cestodes were fixed in 70% ethanol and then stained with Ehrlich's haematoxylin, differentiated in a 3% aqueous solution of ferric ammonium sulphate 12-hydrate, dehydrated in an ascending ethanol series, cleared in clove oil and mounted in Canada balsam. Some scoleces were mounted in Berlese's medium to facilitate the observation of rostellar hooks. Slides of mounted specimens were studied using standard light and phase-contrast microscopy.
The specimens of U. рrolifer from the present study (slide numbers 18.15.1–18.15.13) have been deposited in the collection of ISEA, Novosibirsk, Russia.
For the experimental infection, approximately 300–350 millipedes were used. Prior to infection, the millipedes were not fed for two days. A fully developed strobila of U. рrolifer with gravid proglottides was placed on a substrate in a cage with millipedes. Infected millipedes were maintained at 20–22°C. The dissection of millipedes was carried out in physiological saline (0.7–0.9%), with 10–12 specimens dissected per day. The examination of millipedes started on the ninth post-infection day (DPI). Measurements and photomicrographs of live metacestodes were obtained using a standard Ringer solution for poikilothermic organisms, an Axiolab phase-contrast microscope and an МС-80 microphotocamera. Measurements were in micrometres (μm) unless otherwise stated. The terminology used to describe the stages of hymenolepidid metacestode development in this paper follows that proposed by Skrjabin & Mathevossian (Reference Shimalov1942) and Chervy (Reference Chervy2002).
Results
Adult stage
Urocystis prolifer Villot, 1880
Synonyms: Hymenolepis prolifer Villot, 1880, Hymenolepis curiosa Stammer, 1955, Pseudodiorchis multispinosa Žarnowski, 1955, Pseudodiorchis kampinosi Rybicka, 1958, Echinoproboscilepis kedroviensis Sadovskaja, 1965, Coronacanthus parvihamata Sawada & Harada, 1990.
Redescription
(Based on specimens from intestines of S. araneus from Artybash village, Turochakskiy District, Altai Republic, Russia; figs 1 and 2.) Small-sized tapeworm (fig. 1). Gravid specimens 1.2–1.5 mm (1.4 mm, n = 11) long. Strobila flat, consisting of 35–50 (38, n = 11) proglottides: 6–9 juveniles (with primordia of male gonads), 6–9 with mature testes (with primordia of female gonads), 6–9 with hermaphroditic (with testes and female gonads), 8–15 postmature and 2–4 gravid. Strobilation gradual. Proglottides acraspedote, transversely elongate, in process of maturation increase somewhat in length (fig. 2a). Development and maturation of strobila follows pattern of typical protandry: primordia and maturation of male gonads appearing before primordia and maturation of female gonads. Female gonads appear in proglottides with mature testes and an almost formed male copulative apparatus. In the first female proglottis, only rudiment of the aporal testis remaining, internal seminal vesicle and seminal receptacle empty. Cirrus sac persisting in pregravid and gravid proglottides. In the last two female proglottides, young vesicular uterus distinct.

Fig. 1. General view of Urocystis prolifer Villot, Reference Stammer1880.
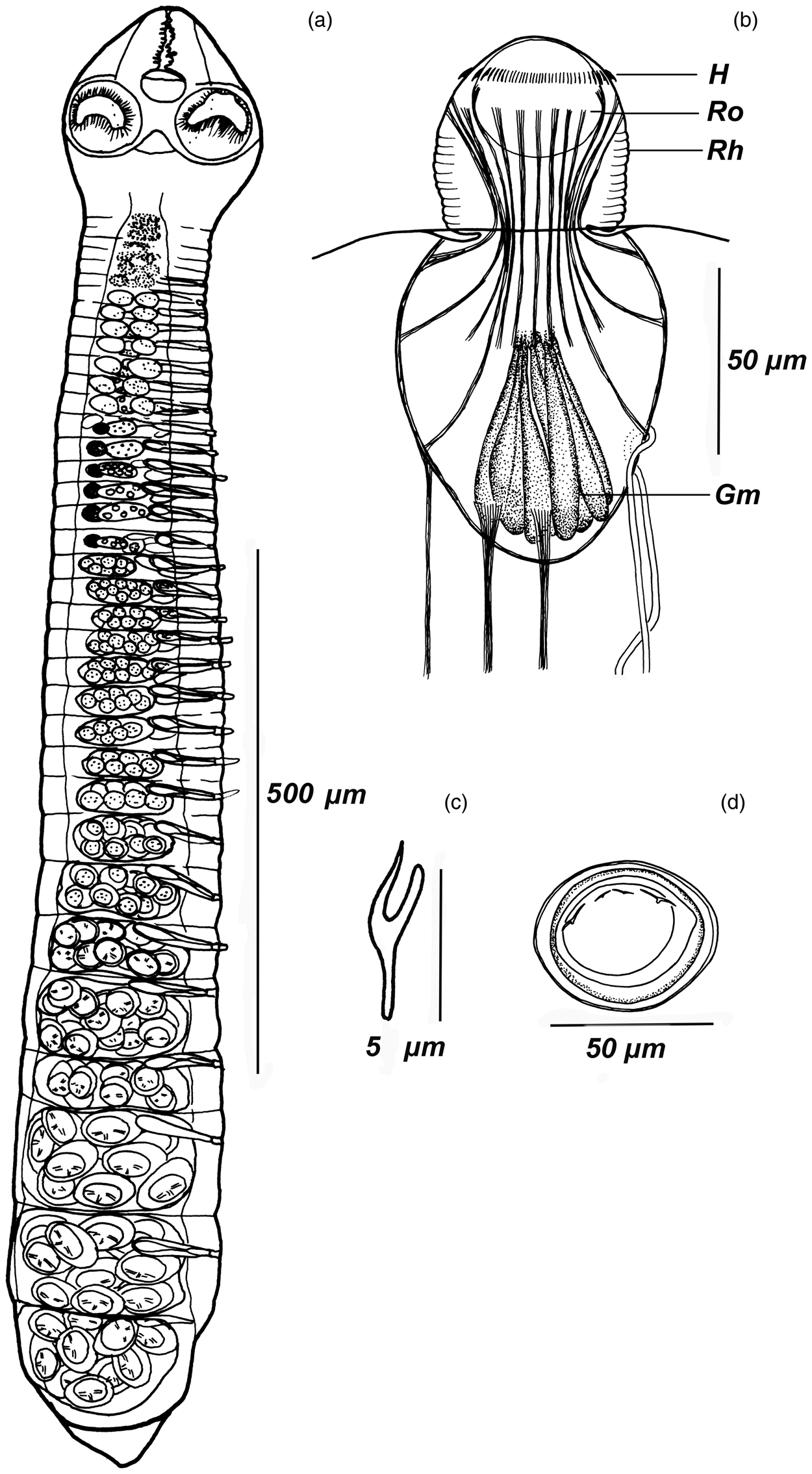
Fig. 2. Urocystis prolifer: (a) whole strobila; (b) rostellar apparatus complex; (c) hook; (d) egg. Abbreviations: Ro, rostellum; Rh, rhynchus; H, hooks; Gm, glandular matrix.
Scolex small, conical, 150–170 × 160–180 (161 × 167, n = 11), with rostrum (fig. 2a). Suckers subspherical, 80–83 × 80–84, with well-developed musculature, widely spaced and shifted to corners of scolex. Rostellar apparatus complex. Rhynchus small, 58–68 long, 55–57 wide, with well-developed own musculature consisting of circular and retractor muscle system. Rhynchus retractors extending from its top, divided into two bundles: one fixed at top of rostellar pouch, second at its equator. Surface of rhynchus corrugated (fig. 2b).
Rostellum subspherical, 30–35 × 35–40 (32 × 38, n = 8), with invagination, deeply immersed in rostellar pouch. Rostellar hooks 120–130 in number, very small, arranged in single row, 4–6 (5, n = 25) long (fig. 2c). Each hook associated with muscle bundle. Muscle fibres from several rostellar hooks merge, thus forming 14–16 retractors. Rostellar pouch voluminous, 80–83 × 100–140 (81 × 128, n = 8), reaching posterior margins of suckers. Ten bundles of retractor muscles extending from bottom of rostellar pouch and passing into thin layer of longitudinal musculature of strobila in neck region. Basal part of rostellar pouch filled with glandular matrix (fig. 2b). Neck clearly distinct from scolex.
Two pairs of osmoregulatory canals without transverse anastomoses. Ventral osmoregulatory canals 5–7 (5, n = 8) in diameter, dorsal canals 2–3 (3, n = 8) in diameter. Genital pores unilateral, dextral, opened in middle or somewhat anterior to middle of proglottis margin. Genital atrium simple, cylindrical, 8–16 deep (14, n = 8), surrounded by large glandular cells.
Male mature proglottides 15–20 × 135–150 (15 × 142, n = 11). Testes two, 15–18 × 20–28 (16 × 26, n = 11), oval, in one row, one poral and one antiporal, situated symmetrically in median field of proglottis (fig. 3a). Testes of neighbouring male proglottides adjacent. Cirrus sac cigar-shaped, elongate, thin-walled, 13–15 × 55–62 (13 × 57, n = 11), crossing poral osmoregulatory canals; not reaching midline of proglottis (fig. 3a). Cirrus short, 20−22 (22, n = 11), armed with small spines, covering only its proximal part (fig. 3b). Internal seminal vesicle ovoid, elongated, 10–12 × 15–25 (10 × 26, n = 11). External seminal vesicle 15–18 × 23–25 (16 × 26, n = 11), connected to cirrus sac by long and curved duct.

Fig. 3. Urocystis prolifer: (a) strobila fragment with male and female mature proglottids; (b) copulatory apparatus.
Hermaphroditic mature proglottides 15–20 × 150–175 (16 × 168, n = 11). Ovary sacciform, transversely elongate, 15–19 × 58–63 (16 × 61, n = 11), in centre of proglottis. Vitellarium compact, subspherical, 11–13 × 15–18 (11 × 16, n = 11), aporal and dorsal to ovary. Vagina thin-walled, opening ventral to cirrus sac. Copulatory part of vagina 25–28 (26, n = 11). Seminal receptacle pear-shaped, large, 15–16 × 25–26 (16 × 26, n = 11) (fig. 3a).
Young uterus vesicular. Gravid uterus sac-like, occupying entire median field, does not cross osmoregulatory canals (fig. 2a), containing 12–20 (13, n = 10) eggs. Eggs large, 45–48 × 56–60 (46 × 56, n = 11), with a sclerotized outer embryonic membrane (fig. 2d). Embryonated eggs lie freely in uterine cavity, not contacting closely with uterine epithelium, scattered one by one through breaks in uterine wall.
Amended generic diagnosis (modified after Czaplinski & Vaucher, Reference Czaplinski, Vaucher, Khalil, Jones and Bray1994). Proglottides much broader than long. Scolex with retractile, armed rostellum. Hooks numerous (more than 100), very small. Testes two or three, in transverse row, more or less overlapping female gonads. Ovary sacciform, transverse, in centre of proglottis. Vitellarium compact, subspherical, aporal to ovary. Cirrus sac not reaching middle of proglottis. Cirrus armed with small spines. Uterus sacciform, containing few eggs. Type-species U. prolifer Villot, Reference Stammer1880.
Distribution of the species in the Palaearctic
The monotypic genus Urocystis is included in many checklists of helminths of shrews from the western to eastern areas of the Palaearctic. The species has been recorded mostly in the taiga and forest zones (in temperate broadleaf deciduous and mixed forests) (fig. 4) (Vaucher, Reference Spassky and Andrejko1971; Prokopič & Matsaberidze, Reference Prokopic1972; Genov, Reference Genov1984; Sato et al., Reference Ryšavý and Prokopič1988; Anikanova et al., Reference Anikanova, Bespyatova, Bugmyrin and Yeshko2001; Kornienko, Reference Korneva, Kornienko and Jones2001; Irzhavsky & Gulyaev, Reference Haukisalmi, Hardman, Foronda, Feliu, Laakkonen, Niemimaa, Lehtonen and Henttonen2002; Dokuchaev et al., Reference Dokuchaev, Melnikova, Gulyaev and Chernyagina2003; Ribas et al., Reference Quentin and Beaucournu2003; Skolka et al., Reference Sheykina and Zhigileva2004; Binkiene, Reference Binkiene2006; Kornienko et al., Reference Kornienko and Ishigenova2008; Zubova et al., Reference Yushkov2008a, Reference Zarnowskib; Binkiene et al., Reference Binkiene, Kontrimavichus and Hoberg2011). Despite Vaucher's statement that U. prolifer is absent from Scandinavia (Vaucher, Reference Spassky and Andrejko1971), there are records of it in southern Finland (on the coast of the Gulf of Finland) (Haukisalmi et al., Reference Haukisalmi2010; Haukisalmi, Reference Haukisalmi2015). In addition, we have found this species in Sweden and Finland (Västernorrland County, Sweden, 62°45′N, 17°52′E; northern Savo, Finland 62°53′N, 27°40′E). According to our data and data in the literature, this species has not been recorded in the tundra and arid zones and some forest zone regions of the Palaearctic (north-east of the East European Plain, Russian Far East (Chukotka Peninsula), eastern Siberia (Yakutia and Tuva), south of the Lesser Caucasus (Armenia) and northern Kazakhstan) (Yushkov, Reference Villot1995; Movsessian et al., Reference Mariaux, Tkach, Vasileva, Caira and Jensen2006; Kirillov et al., Reference Joyeux2017; Kornienko et al., Reference Kornienko, Zubova, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2018; Sheykina & Zhigileva, Reference Sadovskaja, Leonov, Mamaev and Oshmarin2018). The lack of records in these regions is likely due to the absence of intermediate hosts of the cestode (diplopods). Novikov (Reference Movsessian, Chubarian and Nikogosian1995) found three specimens of U. prolifer in S. isodon caught in the Selemdzha River basin (Magadan Oblast, Russian Far East). However, the coordinates of the cestodes (60°18′N) provided by the author do not correspond to the location of this river. The site of this record requires clarification.

Fig. 4. Localities of Urocystis prolifer in the Palaearctic. Own data are indicated by circles, literature data by triangles (see chapter “Distribution of the species in the Palaearctic”).
The cestode U. prolifer is characterized by not only an extensive geographical range but also wide host specificity. Definitive hosts of U. prolifer include S. araneus, S. isodon, S. minutus, S. caecutiens, S. gracillimus, S. daphaеnodon, S. roboratus, S. satunini, S. raddei, S. volnuchini, S. minutissimus, S. tundrensis, S. unguiculatus and Sorex shinto Thomas, 1905.
The characteristics of cestode infection (prevalence, abundance and intensity) vary significantly among different species of shrews in different parts of the Palaearctic. In a study of S. araneus in Bulgaria, no more than 5% of the shrews were infected by this tapeworm (Genov, Reference Genov1984). In a study in the territory of Belarus, only 2% of the studied common shrews were found to host U. prolifer (Shimalov, Reference Sato, Kamiya and Ohbayashi2012). In a study in Lithuania, approximately 20% of the examined common and pygmy shrews were infected with U. prolifer (Binkiene, Reference Binkiene2006). In our investigations in the European part of Russia and West and East Siberia, approximately a third of the studied shrews were infected with U. prolifer; in mainland and insular part of the Russian Far East, the prevalence of the cestode is lower. Despite the large regional variation in the prevalence of infection with this cestode, the intensity of infection is high across the study regions (table 2). There is some difference in infection levels between different species of shrews. In the Caucasus, S. raddei has the highest infection rate with U. prolifer. In Siberia (both western and eastern), all shrews species (except tundra shrew in the western, and pygmy shrew in eastern Siberia) have high level of infection with U. prolifer: the prevalence, abundance and intensity. At the same time, S. caecutiens has the highest infection rate with U. prolifer in all of the researched regions (table 2). About 30% of the common shrews from the European part of Russia and Siberia are infected with cestode. The prevalence of U. prolifer in S. minutus is low: single specimens of pygmy shrew are infected. In mainland and insular part of the Far East, the prevalence of U. prolifer is no higher than 20%. The lowest infection with U. prolifer is detected in S. daphaenodon, S. gracillimus and S. minutissimus. Some specimens of S. roboratus, S. minutissimus and S. minutus caught in the Khabarovsk Kraj are not infected with U. prolifer. Among the Japanese shrews, only the long-clawed shrew is infected with U. prolifer (table 2).
Table 2. Infection in shrews of the genus Sorex with Urocystis prolifer.

P, prevalence; SE, standard error; MA, mean abundance; I, intensity range; MI, mean intensity.
Larvogenesis of cysticercoid of U. prolifer in millipedes J. ghilarovi
Megalospheres lying in the body cavity of millipedes were found on the ninth DPI. The megalosphere is a morula-like structure covered with a thin fibrillar membrane (fig. 5a), reaching 52 in diameter.

Fig. 5. Postembryonic development of Urocystis prolifer: (a) megalosphere; (b) elongation and differentiation; (c) the formation of primary blastogens, leading to the formation of a saccular colony (d); (e, f) formation of a primary lacuna in secondary blastogens. Scale bars: (a) 20 μm; (b, c) 50 μm; (d–f) 30 μm.
In the following six days (10th–16th DPI), larvae were at the stage of elongation. From the megalosphere, a saccular maternal individual (blastomere) is formed (fig. 5b) with a primary lacuna, which increases in proportion to the growth of the larva and reaches 57 in length. The length of the elongated larva is 71–75.
On the 22nd DPI, metacestodes at stages of budding of primary blastogens were found. The maternal individual begins to bud off primary blastogens (fig. 5c), and a saccular colony of larvae appears (fig. 5d). The maximum colony length is 800 and the width ranges from 38 to 100. Each colony contains several dozen spherical and elongated larvae with transverse constrictions. Young blastogens of the colony do not have primary lacuna. During the period of growth and formation of the primary lacuna, daughter buds detach from the wall of the maternal individual (fig. 5e, f) and are capable of a new cycle of budding (fig. 6a). Blastogens with a primary lacuna reach 11–17 in diameter (fig. 5e).

Fig. 6. Postembryonic development of Urocystis prolifer: (a) secondary blastogens; (b) detached secondary blastogen at the stage of early morphogenesis of the scolex; (c) formation of suckers and rostellar apparatus; (d) morphogenesis of the scolex in undetached secondary blastogens; (e) late morphogenesis of the scolex and primordium of rostellar hooks; (f) fully developed urocyst. Scale bars: (a–f) 40 μm.
The stage of budding of secondary blastogens was found on 24th–25th DPI. This blastogen has narrow tail-like outgrowth connecting it to the maternal individual during the early stages of morphogenesis (fig. 6b). Subsequently, the secondary blastogen detaches from the maternal individual and develops separately.
On the 26th–28th DPI, metacestodes were at the stage of elongation and early morphogenesis of the scolex. At this stage, the larva measures 38–40 in width by 54–56 in length, and the size of the primordium of the scolex is 17–18 × 16–18 (fig. 6b). Subsequently, suckers are formed and rostellar hooks start their development on the rhynchus (fig. 6c). The scolexogenesis of secondary blastogens can occur in unseparated (fig. 6d) and separated individuals (fig. 6c). Thus, at least two asexual larval generations are observed in the ontogeny of U. prolifer. Characteristic features of this stage of larvogenesis are the absence of the primary lacuna and cercomer and the small size of the rudiments of the strobila and cysts (fig. 6c, d). There is no formation of excretory atrium.
At the stage of late morphogenesis of the scolex (29th–30th DPI), the measurements of larvae increase to 69–71 × 69–75; suckers are 25–33 × 24–28 (fig. 6e). Rhynchus, 25–30 wide in 24–26 long, is retracted before the encysting of the larva (fig. 6e). Encystation occurs without invagination of the scolex; the primordium of the cyst actively advances on the scolex. The two-layered cyst wall of the encysted larva is very thin and mobile. The cyst of the definitive urocyst lacks anterior and posterior obturator valves (fig. 6f). In addition, excretory bodies are completely absent (a unique feature among metacestodes).
The fully developed cysticercoids measure 54–80 × 46–75 (fig. 6f).
Discussion
Our study and the analysis of the data by previous authors (see above) allow us to clarify the morphological characteristics of U. prolifer, and to provide a generic diagnosis of Urocystis and the geographical range of this cestode species. Despite the inclusion of the genus Urocystis in many checklists of helminths from shrews in different regions of the Palaearctic, we believe that the northern boundary of the geographical range of U. prolifer is no higher than the 62nd parallel north and that the southern boundary is no lower than the 42nd parallel north.
The cestode U. prolifer is characterized by an extensive geographical range, wide host specificity and high characteristics of infection (prevalence, abundance and intensity). The high infection levels of U. prolifer are due to the generalized diet and high abundance of some species of shrews (Haukisalmi, Reference Gulyaev, Ishigenova and Kornienko1989; Binkiene, Reference Binkiene2006).
The characteristics of larval development and intermediate hosts are known for only a sixth of the 70 species of tapeworms from Sorex: Ditestolepis Cholodkowsky, 1906, Mathevolepis Spassky, 1948, Staphylocystis Villot, 1877, Lineolepis Spassky, 1959, Vigisolepis Mathevosyan, 1945, Neoskrjabinolepis Spassky, 1947, Monocercus Villot, 1882 and Urocystis Villot, 1880 (e.g. Joyeux, Reference Jones and Alicata1922; Kisielewska, Reference Kirillov, Kirillova, Krasnobaev YuP, Vekhnik, Evlanov and Pelgunov2017, Reference Kisielewska1959, Reference Kisielewska1960; Rawson & Rigby, Reference Prokopič and Matsaberidze1960; Ryšavý & Prokopič, Reference Rybicka1965; Quentin & Beaucournu, Reference Prokopic1966; Prokopic, Reference Prokopic1968a, Reference Prokopicb; Procopič et al., Reference Prokopič and Matsaberidze1970; Obushenkov & Rudzhanskaite, Reference Novikov1984; Ryšavý, Reference Ribas, Casanova, Feliu, Fons and Magnanous1989; Gulyaev & Kornienko, Reference Gulyaev and Kornienko1998; Lefebvre et al., Reference Kornienko, Dokuchaev and Odnokurtsev2009a, Reference Lefebvre, Georgiev, Bray and Littlewoodb; Gulyaev et al., Reference Gulyaev and Kornienko2010; Kornienko & Ishigenova, Reference Kornienko2012; Ishigenova & Kornienko, Reference Ishigenova and Gulyaev2013).
Several studies have examined the structure of the fully developed larva of U. prolifer or the stages of larval development (Villot, Reference Stammer1880; Joyeux, Reference Jones and Alicata1922; Stammer, Reference Skrjabin and Mathevossian1955; Baer & Della Santa, Reference Baer and Della Santa1960; Kisielewska, Reference Kisielewska1960; Ishigenova, Reference Irzhavsky, Gulyaev and Gulyaev2009). According to the literature, the intermediate hosts of U. prolifer are the millipedes (Diplopoda) Glomeris limbata Lutz, Glomeris conspersa Koch and Craspedosoma alemanicum Verhoeff (Stammer, Reference Skrjabin and Mathevossian1955; Villot, Reference Stammer1880; Baer & Della Santa, Reference Baer and Della Santa1960). We performed an experimental infection of millipedes J. ghilarovi Gulička, 1963 (Julida: Julidae). Millipedes are common terrestrial invertebrates, and most are slow-moving detritivores that inhabit the upper layer of soil and litter. Millipedes may accidentally ingest eggs of U. prolifer with decaying leaves and become intermediate hosts. The shrews willingly eat infected millipedes, as evidenced by the high prevalence of the cestode in shrews (table 2).
According to previous authors, the larva of U. prolifer is a polycephalic cysticercoid that is produced by budding (blastogenesis) and detaches from the maternal tissue. The process of cysticercoid budding (asexual larval reproduction) was first mentioned by Jones & Alicata (Reference Ishigenova and Kornienko1935), who indicated that fully developed larvae of Hymenolepis (=Staphylepis) cantaniana resemble mycelium from which cysticercoids bud and reproduce. Wardle & McLeod (Reference Vaucher1952) termed this type of cysticercoid ‘urocyst’. Subsequently, Chervy (Reference Chervy2002) modified the nomenclature of the stages of larval development and the larvae of different taxonomic groups of cestodes. This author suggested that cysticercoids similar to U. prolifer be called urocysticercoid or urocyst.
The cysticercoid of U. prolifer developed by blastogenesis occurs over the entire surface of the maternal tissue. Within the intermediate host, a colony of metacestodes forms, consisting of individuals of different generations: the maternal saccular larva (blastomeres), which arise by sexual reproduction, and daughter cysticercoids (blastogens), formed by asexual larval reproduction. The body cavity of infected millipedes becomes filled with single larvae and groups of colonial larvae of various shapes: from morula-like solitary individuals to budding ones (fig. 5). The urocyst is a multilobed parenchymatous mass, from which cysticercoids develop; the cysticercoid has a long pedicel and separates before the completion of its development.
Some cestode species in which blastogenesis is observed (e.g. Polycercus paradoxa (Rudolphi, 1802) (Gulyaev, Reference Gulyaev2000) have a small number of proglottides in the strobila, and the small size of the uterus limits their fecundity. Similar traits are apparent in the adults of U. prolifer (see above). These traits likely result in slow rates of ontogeny and the maturation of invasive eggs (Gulyaev & Kornienko, Reference Gulyaev and Kornienko2009). Invasive eggs have a sclerotized outer embryonic membrane (Korneva et al., Reference Kisielewska2012), which probably enables the eggs to persist in the litter for an extended period. The intermediate hosts, eating invasive eggs, become infected with the larvae of U. prolifer, each of which gives rise to a clone. Asexual reproduction of the cestode in the intermediate host, through several cycles of budding, results in a large number (table 2) of cysticercoids in the host. Each oncosphere of U. prolifer forms numerous and the smallest among the members of Cyclophyllidea cysticercoids. A shrew that has eaten even a small number of intermediate hosts containing larvae becomes infected with multiple cestodes, which leads to a high infection intensity and, thus, high densities of micropopulations of these cestodes. The infection intensity of U. prolifer ranges from 1 to 10,000 individuals per infected shrew (table 2).
The large number of U. prolifer larvae that enter the intestines of one definitive host from several intermediate hosts provides a high probability of cross-fertilization between adults, which may increase the level of heterozygosity of the parasite population (Ishigenova, Reference Irzhavsky, Gulyaev and Gulyaev2009).
Acknowledgements
This study was made on the basis of the Teletsky Research Field Station of the Institute of Systematics and Ecology of Animals of the Siberian Branch, Russian Academy of Sciences. We thank the anonymous reviewers for their constructive feedback.
Financial support
This work was supported by the Russian Foundation for Basic Research (grant number 19-54-18015 Bolg_a) and the Federal Fundamental Scientific Research Programme for 2021–2025 (FWGS-2021-0004).
Conflicts of interest
None.
Ethical standards
The authors carefully reviewed the ethical standards of the journal and hereby certify that the procedures used with the investigated species comply fully with those standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals in accordance with the legislation of the Russian Federation. The methods used in the current study were approved by the ethics committee of the Institute of Systematics and Ecology of Animals, Novosibirsk, Russia.










