INTRODUCTION
Capitulum mitella (Linnaeus, 1758), formerly assigned to the genus Pollicipes (Leach, 1817), is a predominant intertidal cirripede (Lee et al., Reference Lee, Shim and Kim2000). Capitulum mitella grows from Korea through India to the West Pacific Ocean. It settled extensively on rock cracks with strong wave action off the coast in the south of the Changjiang River Estuary in China. The soft part of C. mitella has been a source of food in China, and there is a strong market demand for Pollicipes in Spain and Portugal (Borja et al., Reference Borja, Muxika and Bald2006; Jacinto et al., Reference Jacinto, Cruz, Silva and Castro2010). The economic value of C. mitella in China has led to the decline of its population in recent years. However, limited literature on the biology and reproduction of C. mitella is currently available.
In general, the larval development of Cirripedia comprises six naupliar instars and a non-feeding cyprid instar. The final cypris larva plays a crucial role in its life cycle. In the barnacle life cycle, the cypris larva is the transitory stage between the free-swimming and sessile life. Its sole task is to search for a suitable substratum and carry out the actual settlement (Lagersson & Høeg, Reference Lagersson and Høeg2002). Although the attachment and metamorphosis of the cypris larva of barnacles have been the subject of intensive study (Glenner & Høeg, Reference Glenner and Høeg1998; Anil et al., Reference Anil, Khandeparker, Desai, Baragi and Gaonkar2010; Høeg et al., Reference Høeg, Maruzzo, Okano, Glenner and Chan2012; Maruzzo et al., Reference Maruzzo, Aldred, Clare and Høeg2012), inducing the attachment and metamorphosis of the cyprid of the genus Pollicipes in the laboratory is challenging (Kugele & Yule, Reference Kugele and Yule1996). Similarly, C. mitella cannot complete its life cycle under laboratory culture because cyprids cannot settle on a substratum, which impedes further research.
Jensen et al. (Reference Jensen, Høeg and Al-Yahaa1994) first described the carapace and lattice organs of the cypris larva of C. mitella using scanning electron microscopy (SEM). Moyse et al. (Reference Moyse, Høeg, Jensen, Al-Yahaa, Schram and Høeg1995) showed both the attachment disc and the carapace length of C. mitella. Lee et al. (Reference Lee, Shim and Kim2000) described the morphology of nauplius and outlines of the cyprid of C. mitella using light microscopy. In the last two decades, our studies have focused on the ecology, reproduction, embryonic development, larval development, spermatogenesis and culture conditions of C. mitella. No detailed studies on the cyprids of C. mitella using electron microscopic (SEM) methods have been reported thus far. Thus, we observed the external morphology of the cyprids of C. mitella using SEM and compared it with that of Balanus amphitrite (=Amphibalanus amphitrite) (Clare & Høeg, Reference Clare and Høeg2008) and Megabalanus rosa. We aim to determine the mechanisms by which the cyprids of C. mitella attach and metamorphose, and to provide a basis for further phylogenetic analysis of cypris morphology.
MATERIALS AND METHODS
Collection and rearing
Specimens of Capitulum mitella were collected in August 2011 in the intertidal zone of Dinghai, Fuzhou, Fujian, China (26°16′N 119°48′E). Egg masses obtained from the capitulum cavity of adults were cultured through air-bubbling at 25°C. The seawater was changed every day. After hatching, Nauplii I were transferred to plastic barrels (one to two larvae ml−1). The larval culture was maintained under the same conditions as the egg masses. An algal diet of Dicratelia zhanjiangensis was supplied at a density of 3 × 104 cells ml−1 after each change of seawater of 28‰. After approximately 10 d, Nauplii VI moulted into cyprids, which were then collected for sample preparation.
After induced to finished metamorphosis, moulting exuvias of cyprids were collected. Moulting thorax exuvias were observed using a light microscope to describe segmentation and setation of cypris thoracopods.
SEM preparation
Cyprids of C. mitella were relaxed with 0.4 M MgSO4 and then fixed in 2.5% glutaraldehyde (seawater base) for 2 h. The larvae were rinsed in distilled water for 30 s for SEM analysis. The specimens were post-fixed in 1% OsO4, dehydrated in a graded series of ethanol and critical-point dried with liquid CO2. Dried cyprids were mounted on carbon stubs with conducting carbon cement and then sputter-coated with gold. Observations were made with a JEOL JSM-6380LV scanning electron microscope operated at 15 kV.
RESULTS
The terminology of cypris morphology follows the scheme used by Jensen et al. (Reference Jensen, Høeg and Al-Yahaa1994), Glenner & Høeg (Reference Glenner and Høeg1995) and Blomsterberg et al. (2009).
General morphology
The general morphology of Capitulum mitella is obvious under light microscopy (Figure 1A). The cypris larva reared under our conditions measures 420–485 µm in length and 190–235 µm in height. The interior of the whole body exhibits a wine-red colour. The carapace has a pronounced reticulate outer surface. A pair of antennules can be observed protruding anteriorly, whereas six pairs of thoracopods and a pair of furcal rami can be observed posteriorly. A pair of compound eyes and a nauplius eye are situated at one-third of the body length from the anterior end. Ventrally, a pair of frontal filaments can be observed protruding between the carapace valves behind the antennules. The anterior end of the cyprids is filled with a large amount of yellow oil droplets.

Fig. 1. Cypris larvae of Capitulum mitella: (A) light microscopy micrograph of a cyprid in lateral view; (B) lateral view of a whole cyprid; (C) detail of carapace showing socketed setae and hinge-line; (D) ventral view of anterior end showing distal part of first antennular segment, frontal horn pore and frontal filaments; (E) dorsal view of a whole cyprid showing lattice organs and posterior carapace slit. Ant, antennules; CE, compound eye; CS, posterior carapace slit; FF, frontal filament; FP, frontal horn pore; FR, furcal rami; HL, hinge-line; LO1-5, lattice organs 1–5; NE, naupliar eye; S1, first antennular segment; SS, socketed setae; Tho, thorax; Thp, thoracopods.
Carapace
The cypris body is strongly compressed laterally (Figure 1B). The carapace covering the entire body has a spindle shape with a rounded anterior end and a narrower, truncated posterior end. The dorsal margin of the carapace is distinctly curved, with the ventral margin being practically straight. The carapace has a distinct mid-dorsal hinge-line. The anterior slit of the carapace is almost at the anterior end, whereas the posterior slit is approximately a sixth of the body length from the posterior end (Figure 1E). The entire carapace surface, on which a few small socketed setae are scattered, is sculptured by slender ridges demarcating rectangular or irregular polygonal areas with very fine pores (Figure 1C).
The carapace has a pair of frontal horn pores situated near the anterioventral margin, approximately 80 µm from the anterior end (Figure 1D). These horn pores are oval and 13 µm in diameter, with no special features (Figure 2A). A pair of frontal filaments protrude ventrally, midway between the antennules and the thorax (Figure 1E).
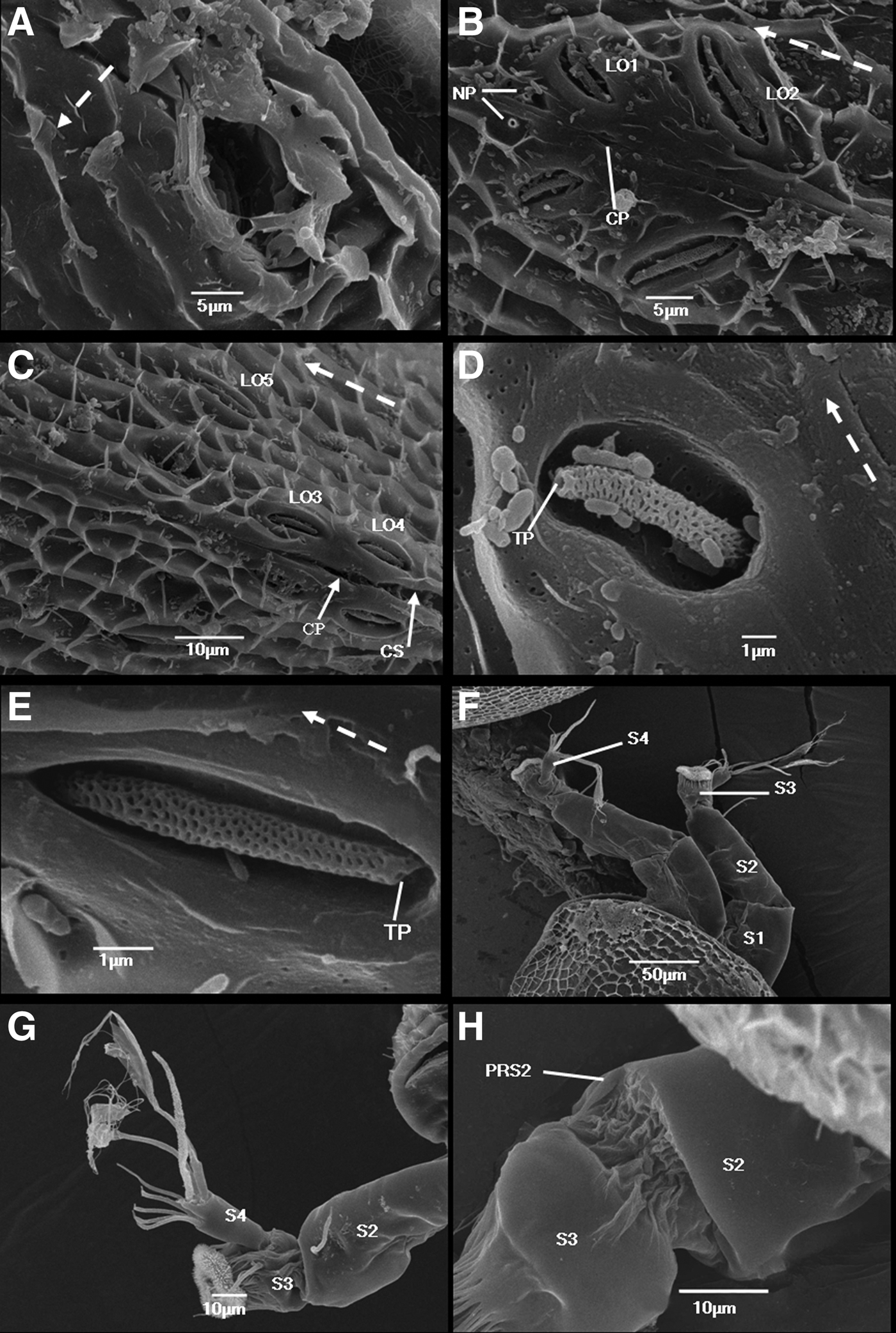
Fig. 2. Cypris larvae of Capitulum mitella: (A) detail of a frontal horn pore located anterioventrally on each carapace valve. A dotted arrow indicates dorsal side; (B) dorsal view of anterior lattice organs. Note central pore and nozzle-like pores; (C) dorsal view of posterior lattice organs. Note central pore and posterior carapace slit; (D) detail of left lattice organ 1; (E) detail of left lattice organ 4. (F) Lateral view of cypris anterior end showing a pair of protruding antennules; (G) lateral view of left antennule showing fourth, third and part of second segment; (H) second antennular segment showing postaxial setae 2. Note that the arrow points to a terminal pore. A dotted arrow indicates anterior direction; CP, central pore; CS, posterior carapace slit; NP, nozzle-like pores; PS2, postaxial setae 2; S1, first antennular segment; S2, second antennular segment; S3, third antennular segment; S4, fourth antennular segment; TP, terminal pore.
Lattice organs
The cyprid possesses five pairs of lattice organs (LOs) on the dorsal surface of the carapace (Figure 1E). These LOs are arranged as two anterior (LO1 and LO2) and three posterior (LO3 to LO5) pairs straddling the dorsal hinge-line of the carapace (Figure 2B, C). A distinctive and symmetrically arranged pair of nozzle-like pores exists on the carapace surface in front of LO1 (Figure 2B). The morphology of the five pairs of lattice organs is halfway between the crest-in-a-trough and pore-field types (Figure 2D, E). A crest of a distinctive seta-shaped structure lies in an elongated deep trough. The crest with numerous deep pores tapers slightly to a large terminal pore. The trough is perforated by pores similar to those on the general carapace surface. A terminal pore of the crest is situated anteriorly in LO1 and LO2 but posteriorly in LO3 to LO5 (Figure 2D, E). The size of the lattice organs is slightly different. The LO1 is the shortest (~7 µm), whereas LO2 is the longest (~14 µm). LO3 and LO4 have almost the same length (~9 µm), whereas LO5 is slightly longer than LO3 and LO4 (~11 µm). LO1 and LO2 are about a third of the body length from the anterior end, converging distinctly posteriorly and parallel to each other. LO3 and LO4 are oriented parallel to the hinge-line, almost straddling the proximal end of the posterior slit in the carapace. LO5, converging slightly posteriorly, lies more laterally and more anteriorly than LO3 and LO4. A slot-like central pore lies in the dorsal midline of the carapace between LO1 and LO2 as well as between LO3 and LO4 (Figure 2B, C).
Antennules
The general morphology of the antennules resembles that of the cyprids of other thoracican Cirripedia (Figure 2F, G). The antennules consist of four segments, but the first segment is always positioned within the carapace. Thus, we cannot describe the proximal parts of the antennule (Figure 1D).
The second segment is an elongated cylinder laterally compressed to some extent; it tapers slightly distally toward the articulation with the third segment (Figure 2F). An extensive band of thin arthrodial membrane can be observed in the joint to the third segment (Figure 2H). The second segment is approximately 90 µm long. The postaxial seta 2 (PS2) sits distally on the ventral side of the second segment; however, it is still somewhat removed from the articulation with the third segment. PS2 is ~30 µm long, smooth, slightly curved and tapering towards the end (Figure 3A). A distinctly narrower part with a terminal pore can be observed near its apex. The second segment carries another short (~8 µm), smooth preaxial seta 2 (PRS2) (Figure 2H). PRS2 is on the dorsal side of the second segment and situated very close to the articulation to the third segment, but slightly medial of the midline. PRS2 tapers distally towards a terminal pore.
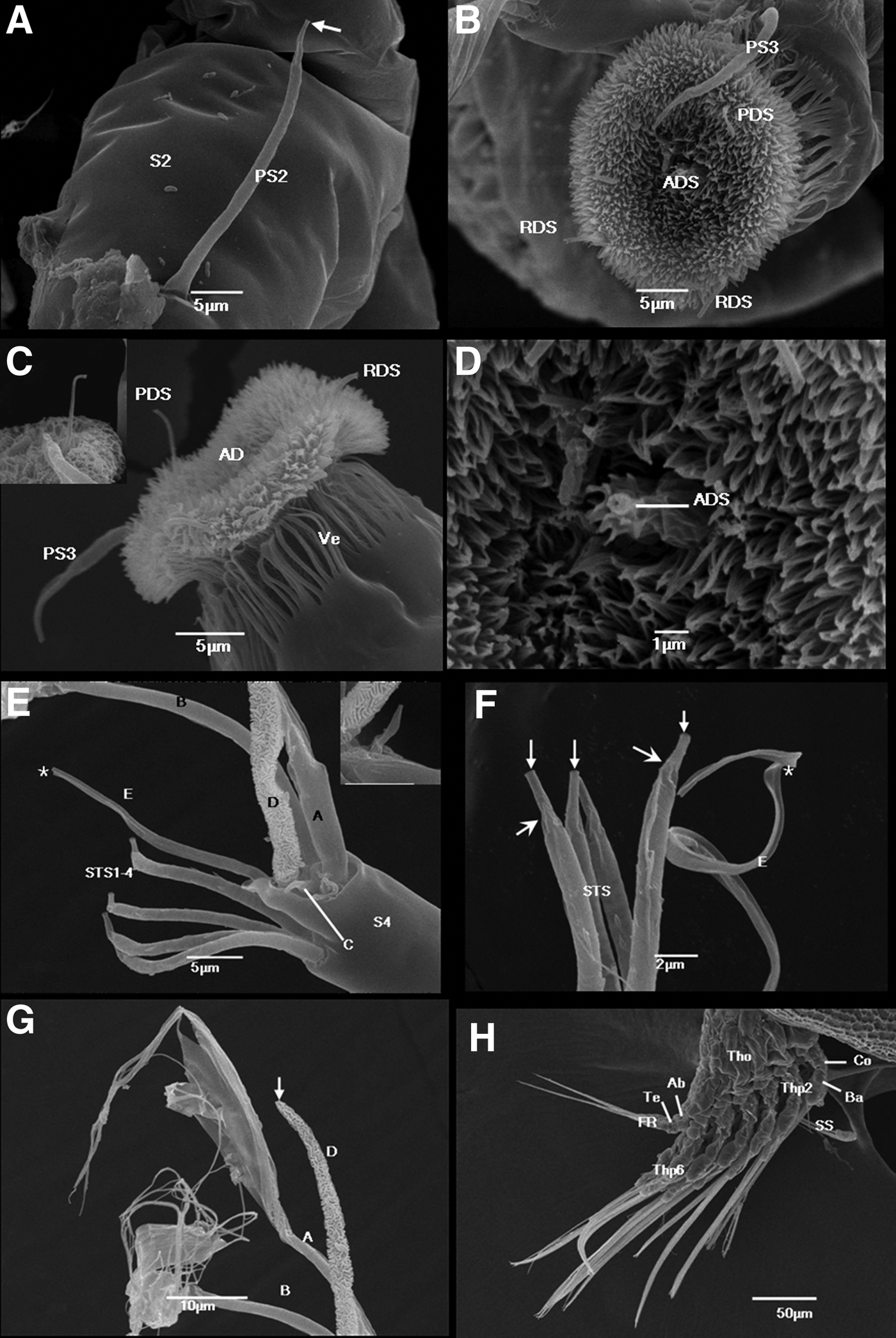
Fig. 3. Cypris larvae of Capitulum mitella: (A) lateral view of second and third antennular segment showing an open-ended preaxial seta 2 and arthrodial membrane; (B) attachment disc showing postaxial seta 3, axial disc seta, postaxial disc seta and two radial setae; (C) lateral view of third antennular segment showing postaxial seta 3 and attachment disc with postaxial disc seta and two radial disc setae; inset shows terminal pore of PDS; (D) close-up of Figure 3B showing axial disc seta with a small pore; (E) close-up of Figure 2G showing position of four subterminal setae and five terminal setae (A–E) on segment 4 (note that TS-E seems to be broken in this specimen (asterisk)); inset shows TS-C; (F) detail of distal part of fourth antennular segment, showing tip of four subterminal setae and TS-E (TS-E is broken in this specimen (asterisk)); note that the arrows point to terminal pores, arrowheads designate the constriction near their apex; (G) close-up of Figure 2G showing distal part of TS-A, B and D; note that the arrow points to terminal pore of TS-D; (H) lateral view of cypris posterior end showing protruding thorax, adomen and telson. Ab, abdomen; AD, attachment disc; ADS, axial disc seta; AM, arthrodial membrane; Ba, basis; Co, coax; FR, furcal rami; PDS, postaxial disc seta; PS3, postaxial seta 3; PRS2, preaxial seta 2; RDS, radial setae; S2, second antennular segment; S3, third antennular segment; S4, fourth antennular segment; SS, stout seta; STS, subterminal setae; Te, telson; Tho, thorax; Thp, thoracopods; A-E, terminal setae A–E; Ve, velum.
Third segment
The third segment is a cylinder approximately ~25 µm long, with the attachment disc located on the morphologically ventral surface (Figure 2F). On the third segment, the only seta outside the attachment disc is the single postaxial seta 3 (PS3) (Figure 3B). It sits proximoventrally in the middle of the segment. PS3 resembles PS2 in size, shape and orientation.
Attachment disc
Front view: the attachment disc is almost circular, ~ 5 µm in diameter, and is covered with a moderately dense carpet of microcuticular projections or ‘villi’ (Figure 3B). The length of the dense villi is at least 5 µm. Lateral view: the attachment disc is somewhat bell shaped (Figure 3C). A series of slender cuticular flaps forms a distinct ‘velum’ around the base of the disc, rather than the margin of the disc (Figure 3C). The axial disc seta (ADS) sits at the centre of the disc (Figure 3D) and emerges directly from the microcuticular projections, preventing us from observing its basal-most part. The ADS is inconspicuous, not protruding from the disc surface; however, it has a clear small pore. The postaxial disc seta (PDS) is a distinct yet simple seta that is 5–6 µm beyond the surface of the disc. The PDS projects in the midline at the proximal end of the disc and terminates in a small pore (Figure 3C). Two radial disc setae (RDS) are situated symmetrically near the distal perimeter of the disc. They are 3–4 µm beyond the surface of the disc and slightly shorter than the PDS. The RDS are simple setae with a terminal pore (Figure 3B, C).
Fourth segment
The fourth segment sits proximolaterally on the third segment and has an inverted cone shape, being rather narrow at the base but increasing in width towards the apical end (Figure 2G). This segment is approximately 26 µm long, shorter than the third segment. The fourth segment carries four subterminal setae (STS) and five terminal setae (TS-A to TS-E). The STS are situated in a transverse row on a ledge near the apex of the segment (Figure 3E). They are morphologically identical and approximately equal in length (~22 µm). They are slightly shorter than the segment itself, and each tapers distally and terminates in a pore (Figure 3F). A short, narrower part near the apex setting off from the rest of the seta can be observed. The STS lack setules but carry a few distinct spines on the surface.
The apical end of the fourth segment is blunt, forming a sloping circular platform surrounded by a high cuticular skirt (Figure 3E). The five TS are projected distally from the platform and comprise two similar complicated TS-A and TS-B, a very short and simple TS-C, a conspicuously ornamented TS-D and a long and simple TS-E. TS-A and TS-B sit adjacent to each other, whereas TS-D is situated between setae TS-A, TS-B and TS-E. TS-C is located between TS-A and TS-D, whereas TS-E sits slightly apart from the other setae.
The TS-A and TS-B are the longest among the TS, i.e. over 100 µm long (Figure 2G). They have a cylindrical proximal part, approximately a third of the seta length, which forks into several straps extending to approximately 30 µm length. At the distal part of each strap, a strap splits a few threadlike setules that are at least 30 µm long (Figure 3E, G). TS-A and TS-B do not completely splay out; thus, the exact number of straps and setules of the setae cannot be counted. Both setae lack a terminal pore. Although TS-C is short (only 4 µm), it tapers rapidly and has a terminal pore (Figure 3E). TS-D is easily identifiable because it is the stoutest of the TS (Figure 3E). TS-D is covered with highly convoluted cuticular ridges throughout its whole length (~52 µm). It tapers towards the tip and terminates in a pore (Figure 3G). TS-E consists of a cylindrical proximal part (approximately 10 µm long) and a flattened, band-like apical part (approximately 25 µm long). The entire length of TS-E is slightly shorter than that of TS-D. It distinctly curves and lacks a pore at the tip (Figure 3E, F).
Thoracopods
Similar to other thoracican cyprids, those of C. mitella bear six pairs of biramous natatory thoracopods, which consist of a protopod (coxa + basis), a two-segmented exopod and a two-segmented endopod (Figure 3H).
Thoracopod 1 is described first (Figure 4A). The first segment of exopod is an elongated cylinder laterally compressed to some extent, approximately 30 × 14 µm. Latero-distally it carries a single, stout, serrate seta (34 µm), extending beyond the tip of the ramus. The second segment of exopod (32 × 13 µm) is cylindrical, slightly curved on the medial side. It carries four long setae (~125–140 µm long ) on its medial side: three (Nos. 1, 2, 3) sited subterminally and one (No. 4) at one-third of its length from the proximal end. The first segment of endopod (30 × 12 µm) is slightly slender than its exopod counterpart, with a big spine mid-medially. A long seta is located on the medial side of the distal end (No. 8). The second segment of endopod (14 × 8 µm ) is much shorter and smaller than its exopod counterpart. It carries three long setae (~125–140 µm long): one sited mid-medially (No. 7) and two terminally on the segment (Nos. 5, 6). There are minor differences in size and shape among these eight setae. The setae length is slightly different owing to their situated location. Setae 2–8, setule length of which is up to 50 µm, are plumose, whereas the first seta is biserrate.
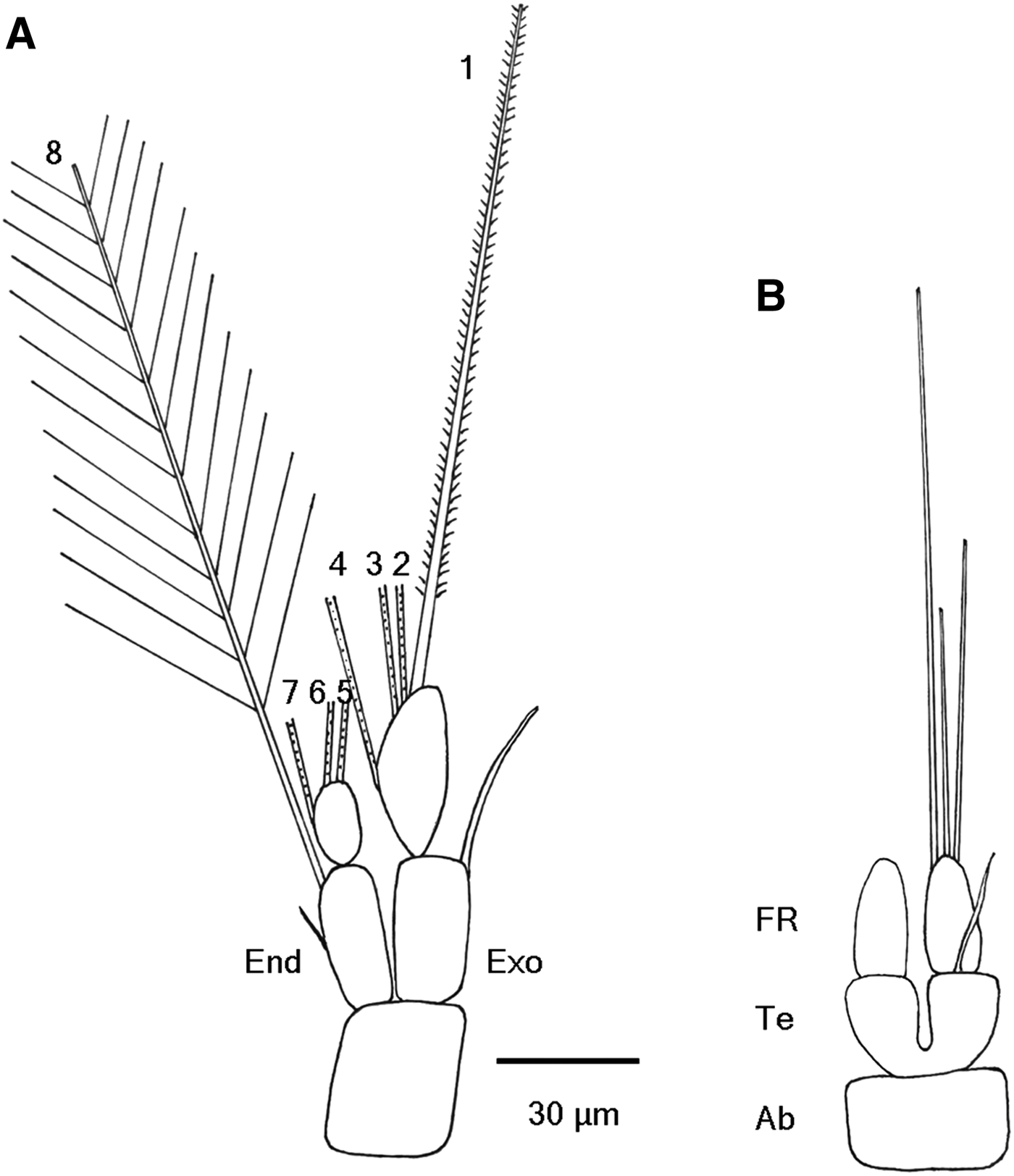
Fig. 4. Schematic drawing of cyprid of Capitulum mitella under a light microscopy: (A) thoracopod 1; (B) dorsal view of abdomen and telson with a pair of one-segmented furcal rami. Ab, abdomen; End, endopod; Exo, exopod; FR, furcal ramus; Tel, telson.
Morphology and setation of thoracopods 2–6 almost resemble the first thoracopod. Compared to the first thoracopod, thoracopods 2–6 exhibit an obvious difference in their exopods. The second segment of their exopods carries an additional plumose seta, situated near the middle of the medial side. The number difference of exopod setae between the first thoracopod and thoracopods 2–6 in C. mitella is also present in Balanus amphitrite (Glenner & Høeg, Reference Glenner and Høeg1995).
Abdomen, telson and furcal rami
Cyprids of C. mitella possess a rudimentary, but distinct abdomen. The almost completely cleaved telson (~15 µm long) connects directly the abdomen (Figure 4B). A single seta (~27 µm long) sits terminally on the dorsal side of the telson halves. The telson itself bears a pair of one-segmented furcal rami (~25 µm long ). Each furcal ramus gradually tapers towards the end. The furcal ramus carries three simple setae with varying lengths at the apex. The longest seta (~100 µm) is located on the medial side of the apex, whereas the medium-length seta (~60 µm) is located on the lateral side of the apex. The shortest and smallest seta (only ~45 µm) is situated between the two setae.
DISCUSSION
Lee et al. (Reference Lee, Shim and Kim2000) described briefly the outline shape of the cyprid of Capitulum mitella under a microscope and measured its size (total length: 790 µm, shield width: 312 µm). Jensen et al. (Reference Jensen, Høeg and Al-Yahaa1994) described the morphology of carapace and lattice organ in detail with SEM (470 µm long). Moyse et al. (Reference Moyse, Høeg, Jensen, Al-Yahaa, Schram and Høeg1995) also showed the carapace length (460 µm). Apparently, the cypris length reported by Lee is different from that by Jensen, Moyse and this article. The account of Jensen agrees with our observations on the size, morphology of carapace and number and position of lattice organs in the cyprid of C. mitella reared in our laboratory.
Lattice organs are specialized chemoreceptors that develop from setae in the naupliar head shield (Rybakov et al., Reference Rybakov, Høeg, Jensen and Kolbasov2003). Jensen et al. (Reference Jensen, Høeg and Al-Yahaa1994) distinguished two distinct varieties: the crest-in-a-trough and pore-field types. Cyprids of some thoracican cirripedes (e.g. Capitulum and Pollicipes) have a morphological intermediate between the crest-in-a-trough and pore-field types, in which a distinct central crest traversing an elongated depression is perforated by numerous small pores (Jensen et al., Reference Jensen, Høeg and Al-Yahaa1994; Kolbasov et al., Reference Kolbasov, Grygier, Høeg and Klepal2008). Our observation confirms the morphology of the lattice organs in the cyprids of C. mitella. The central pore associated with both the anterior and posterior lattice organs is the exit of a special gland and apparently a ground pattern feature for Thecostraca (Høeg et al., Reference Høeg, Hosfeld and Jensen1998; Høeg & Kolbasov, Reference Høeg and Kolbasov2002). Similarly, central pores exist between LO1 and LO2 and between LO3 and LO4 in the cyprids of C. mitella.
In terms of general morphology, the cypris antennules of C. mitella fall well within the morphological spectrum known from thoracican cyprids. Cyprids of two model barnacles, Megabalanus rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009) and Balanus amphitrite (Clare & Nott, Reference Clare and Nott1994; Glenner & Høeg, Reference Glenner and Høeg1995; Lagersson et al., Reference Lagersson, Garm and Høeg2003; Maruzzo et al., Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011), are described in great detail. Although C. mitella and two model barnacles are not closely related and belong to two separate orders within Thoracica, the morphology and setation of the cypris antennules of C. mitella are close to those described for M. rosa by Bielecki et al. (Reference Bielecki, Chan, Høeg and Sari2009) and B. amphitrite by Maruzzo et al. (Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011). Thus, the cypris antennules of C. mitella are compared with the two model barnacles.
The first segment lacks setae, whereas the second segment carries PRS2 and PS2. The third segment carries PS3 outside the attachment disc, whereas PDS, ADS and RDS are all located inside the disc. The fourth segment carries four STS (STS1 to STS4) and five TS-A-E (TS-A to TS-E). However, a few morphological differences exist among the three thoracican cyprids in relation to both attachment disc and antennular setation.
The morphology of the attachment disc is important in understanding both the temporary adhesion during surface exploration and final cementation (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009). The morphology of the attachment disc varies considerably among thoracican cyprids. The bell-shaped variety, which is considered ‘typical’ of all thoracicans, is probably a specialization in species that settle in high-energy habitats of the rocky intertidal (Moyse et al., Reference Moyse, Høeg, Jensen, Al-Yahaa, Schram and Høeg1995). The attachment disc of C. mitella is more or less a bell-shaped type similar to that of B. amphitrite (Glenner & Høeg, Reference Glenner and Høeg1995) and M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009). The attachment disc is encircled by a velum. The velar flaps of C. mitella are narrower than those of M. rosa, which split apically into small fringes. However, the morphology of the velar flaps in B. amphitrite has not been described in detail.
The axial disc seta is part of a prominent feature near the centre of the attachment disc. The seta itself is placed on top of a small hump in B. amphitrite (Maruzzo et al., Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011) and M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009); however, it is inconspicuous, not protruding from the disc surface in C. mitella. The surface of the attachment disc is covered with a dense carpet of cuticular villi. The RDS are more or less obscured by the cuticular villi, making their accurate number hard to determine by SEM. Bielecki et al. (Reference Bielecki, Chan, Høeg and Sari2009) presumed that nine RDS (RDS0–RDS8) are present in cirripedes. This presumption is based on their studies on the cyprids of M. rosa and the TEM observations on the cyprids of Semibalanu balanoides by Nott & Foster (Reference Nott and Foster1969). However, only seven setae (RDS0–RDS6) were actually observed in M. rosa described by Bielecki et al. (Reference Bielecki, Chan, Høeg and Sari2009). Previously, Glenner & Høeg (Reference Glenner and Høeg1995) identified two RDS on the attachment disc in B. amphitrite. Recently, Maruzzo et al. (Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011) have confirmed through detailed observation that five setae (RDS1–RDS5) are present in B. amphitrite. However, only two setae, equivalent to RDS3 and RDS4, were found in C. mitella.
The morphology of the TS in C. mitella differs in some features from that found in the two model barnacles. TS-A and TS-B are similar; they are normally the longest and setulated. Both setae lack a terminal pore. Although TS-A and TS-B in C. mitella possess setules, they are composed of three parts. The morphology of TS-A and TS-B in C. mitella is obviously different from that in M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009) and B. amphitrite (Glenner & Høeg, Reference Glenner and Høeg1995). TS-C is always diminutive and has a terminal pore. The TS-C in M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009) and B. amphitrite (Glenner & Høeg, Reference Glenner and Høeg1995) sits on a small hump, whereas that in C. mitella has no evident hump. TS-D is normally the stoutest; it is thin-walled with a sculptured surface. The decoration of TS-D is similar among the three barnacles. TS-E is somewhat laterally compressed into a band shape in the distal part and has a terminal pore. The TS-E in M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009) and B. amphitrite (Maruzzo et al., Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011) bears a terminal pore, whereas that in C. mitella bears no terminal pore according to our observation.
In the cyprids of M. rosa (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009) and B. amphitrite (Maruzzo et al., Reference Maruzzo, Conlan, Aldred, Clare and Høeg2011), all antennular setae are speculated to be bimodal and have a chemo and mechano-receptive function, except for TS-A and TS-B, which are purely mechanoreceptive. The terminal pore of setae usually indicates a chemoreceptive function (Bielecki et al., Reference Bielecki, Chan, Høeg and Sari2009). The TS-E of C. mitella lacks a terminal pore; thus, it probably only has a mechano-receptive function. Except for TS-E, the functions of the other antennular setae of C. mitella should be similar to those of M. rosa and B. amphitrite.
The present species, formerly assigned to the genus Pollicipes (Leach, 1817), is classified as C. mitella after reviewing the relationships between the genera of Pollicipidae, Scalpellomorpha and Pedunculata (Pérez-Losada et al., Reference Pérez-Losada, Harp, Høeg, Achituv, Jones, Watanabe and Crandall2008). To date, little related data can be acquired to compare with the cypris characteristics of Pollicipidae. This study is the first to compare the morphology and setation of the cypris antennules of C. mitella with those of other pedunculate barnacles (Table 1). The morphology of the cypris antennules of C. mitella is similar to that of Octolasmis angulata and Ibla quadrivalvis, but is more different from that of Lepadidae. The antennule characteristics of Lepadidae are obviously different from the other three Pedunculata. Aside from PS2, a small seta exists, i.e. PRS2 lies on the second segment in the Lepadidae as in C. mitella and two model balanomorphan. However, the presence of PRS2 is not yet known in O. angulata and I. quadrivalvis, which may be attributed to its small size. The third segment is dwarfed by a rather characteristic and very bowl-shaped attachment disc in Lepadidae, but it is normal and distinct in all other thoracicans, including C. mitella. Within Thoracica, Lepadidae members are apomorphic in having up to eight PS3 setae on the third segment (Blomsterberg et al., Reference Blomsterberg, Høeg, Jeffries and Lagersson2004), but only single PS3 is found in all other Pedunculata and Sessilia.
Table 1. Comparisons of characteristics for antennules in cyprids of pedunculate barnacles.

Based on present results, Blomsterberg et al. (Reference Blomsterberg, Høeg, Jeffries and Lagersson2004) and Høeg et al. (Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009). AD, attachment disc; ADS, axial disc seta; PDS, postaxial disc seta; PRS2, preaxial seta 2; PS2, postaxial seta 2; PS3, postaxial seta 3; RDS, radial disc setae; TP, terminal pore; TS-A, terminal seta A; TS-B, terminal seta B; TS-C, terminal seta C; TS-E, terminal seta E; ?, no information available.
The attachment disc has no skirt or velum in the Lepadidae, but it is encircled by a conspicuously swollen rim or a wavy margin. The attachment disc is encircled by a velum in C. mitella and I. quadrivalvis and by a skirt in O. angulata. In C. mitella a series of slender cuticular flaps forms the distinct velum, whereas the velum of I. quadrivalvis certainly does consist of small cuticular flaps (Hoeg et al., Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009). And the flaps in I. quadrivalvis do not run far down the side of the segment, therefore they differ from the long, narrow flaps in C. mitella and balanomorphans. The RDS in the Lepadidae have two rows, whereas those in the other three species of Pedunculata have only one row. Capitulum mitella and I. quadrivalvis have two RDS, whereas O. angulata has a few RDS.
The TS-A and TS-B in C. mitella, O. angulata and Lepadidae are setulated, whereas those in I. quadrivalvis are not. Although the TS-A and TS-B of O. angulata and Lepadidae bear setules, their detailed morphology has not been described. The morphology of TS-A and TS-B in C. mitella is unique. The surface ornamentation of TS-D is remarkably different among several pedunculate barnacles, whereas that of C. mitella is similar to that of B. amphitrite (Glenner & Høeg, Reference Glenner and Høeg1995). The TS-E is diminutive in Lepadidae, but its size in other pedunculate barnacles is normal.
The abdomen and telson cleavage of cirripedian cyprids exhibit diversity. Within the Cirripedia, only the Acrothoracica have a separate abdomen, Rhizocephalan and thoracian cyprids have no free abdomen, and the cleaved telson (or abdomen–telson) connects directly with the thorax (Kolbasov et al., Reference Kolbasov, Høeg and Elfimov1999; Høeg et al., Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009). Although there is no trace of an independent, abdominal body part in cyprids of I. quadrivalvis (Høeg et al., Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009), cypris larvae of the family Poecilasmatidae have a rudimentary abdomen which seems to consist of two small segments (Kolbasov et al., Reference Kolbasov, Elfimov and Hoeg2013). Cyprids of C. mitella also have a rudimentary abdomen according to our observation. The process of telson cleavage seems to have occurred gradually in the evolution of the Cirripedia (Kolbasov et al., Reference Kolbasov, Høeg and Elfimov1999; Kolbasov & Høeg, Reference Kolbasov and Høeg2007). Cypris larvae of the Acrothoracica exhibit different degrees in telson cleavage. For example, the telsonic cleft is shallow in Trypetesa, of intermediate depth in Armatoglyptes, while it almost cleaves the telson in cyprids of Weltneria and Kochlorine (Kolbasov & Høeg, Reference Kolbasov and Høeg2007). Rhizocephalan cyprids have a shallow telsonic cleft (Rybakov et al., Reference Rybakov, Korn, Høeg and Walossek2002), and the telson is almost completely cleaved in thoracican cyprids (Kolbasov et al., Reference Kolbasov, Høeg and Elfimov1999; Høeg et al., Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009). Because the telson divided into two equal parts looks like basal segments of the furcal rami in B. amphitrite cyprids, Glenner & Høeg thought they had a two-segmented furcal rami (1995). Much evidence reveals that the cleaved body part is a true telson rather than the basal part of a two-segmented furcal ramus (Grygier, Reference Grygier1987; Kolbasov et al., Reference Kolbasov, Høeg and Elfimov1999; Kolbasov & Høeg, Reference Kolbasov and Høeg2007; Høeg et al., Reference Høeg, Achituv, Chan, Chan, Jensen and Pérez-Losada2009), hence the distinct telson carries a pair of one-segmented furcal rami. The telson is almost completely cleaved in C. mitella, although its base is still complete. Cyprids of C. mitella possess a rudimentary abdomen and an almost completely cleaved telson, with one-segmented furcal rami.
In short, the morphology and setation of cypris antennules share more similarities with C. mitella and the two model barnacles, although they belong to different orders of Thoracica. These similarities may be linked to the nature of the substrata on which they settle; C. mitella settles on intertidal rock, similar to B. amphitrite.
ACKNOWLEDGEMENT
We are very grateful to Professor Jens Thorvald Høeg of the University of Copenhagen for his comments, which helped us improve this paper.
FINANCIAL SUPPORT
This work was supported by the Natural Science Foundation of Fujian Province, PR China (2011J01147).







