Introduction
Borderline personality disorder (BPD) is a mental disorder characterized by increased impulsivity and, as a consequence, by increased risk-taking behaviour (Links et al. Reference Links, Heslegrave and van Reekum1999; APA, 2000). Executive dysfunctions are assumed to underlie the phenotypic features of BPD, especially increased impulsivity (Bazanis et al. Reference Bazanis, Rogers, Dowson, Taylor, Meux, Staley, Nevinson-Andrews, Taylor, Robbins and Sahakian2002; Lenzenweger et al. Reference Lenzenweger, Clarkin, Fertuck and Kernberg2004). Neuropsychological studies in BPD suggest impairments in multiple cognitive domains, with decision making or planning most frequently affected (Ruocco, Reference Ruocco2005; LeGris & van Reekum, Reference LeGris and van Reekum2006). Neuroimaging studies also support the notion that brain regions involved in impulse control and decision making are altered in BPD. Impairments include volume loss and hypometabolism of the orbitofrontal cortex (OFC) and the anterior cingulate cortex (ACC; De La Fuente et al. Reference De La Fuente, Goldman, Stanus, Vizuete, Morlan, Bobes and Mendlewicz1997; Tebartz van Elst et al. Reference Tebartz van Elst, Hesslinger, Thiel, Geiger, Haegele, Lemieux, Lieb, Bohus, Hennig and Ebert2003). OFC lesions have been associated with reduced performance in reinforcement learning because of a potential inability to modify behaviour in response to feedback (Rolls et al. Reference Rolls, Hornak, Wade and McGrath1994; Berlin et al. Reference Berlin, Rolls and Iversen2005). The ACC plays a pivotal role in the detection and evaluation of unfavourable outcomes (Ridderinkhof et al. Reference Ridderinkhof, Ullsperger, Crone and Nieuwenhuis2004). It is highly associated with risk prediction, including signalling the extent of risk and the severity of consequences (Brown & Braver, Reference Brown and Braver2007).
Decision making and impulsivity have been repeatedly examined with the Iowa Gambling Task (IGT; Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994). The IGT is a complex paradigm that is supposed to reflect real-life decision making in the way it considers uncertainty, rewards and punishment. It allows the investigation of persistent learning difficulties, and it has been suggested that deficits reflect a reduced ability to avoid negative feedback information (Bechara et al. Reference Bechara, Damasio and Damasio2000). Recent studies using the IGT showed that BPD patients, relative to healthy controls, made fewer advantageous and goal-directed decisions and exhibited reduced learning (Haaland & Landro, Reference Haaland and Landro2007; Maurex et al. Reference Maurex, Zaboli, Wiens, Asberg, Leopardi and Ohman2009). Specifically, decision-making deficits have been interpreted as a deficit to use feedback information from previous trials to make current decisions (Bechara et al. Reference Bechara, Damasio and Damasio2000). To our knowledge, alterations in feedback evaluation have not been examined yet in BPD. Therefore, the main objective of the present study was to investigate feedback processing in BPD to further understand decision-making dysfunctions in these patients. Event-related brain potentials (ERPs) are used to elucidate the relationship between neural responses to feedback and decision making. Previous research on performance monitoring has identified negative-going ERP components that occur shortly after incorrect responses or after negative performance feedback. The error-related negativity (ERN; Falkenstein et al. Reference Falkenstein, Hohnsbein, Hoormann, Blanke, Brunia, Gaillard and Kok1990; Gehring et al. Reference Gehring, Goss, Coles, Meyer and Donchin1990) arises following the execution of erroneous responses and the feedback-related negativity (FRN; Miltner et al. Reference Miltner, Braun and Coles1997) is elicited by negative performance feedback when outcomes are worse than expected (Holroyd et al. Reference Holroyd, Coles and Nieuwenhuis2002). Frank et al. (Reference Frank, Woroch and Curran2005) showed that the FRN magnitude predicts the degree to which participants learn about the negative consequences of their decisions. ERN and FRN are both assumed to originate in the ACC (Gehring & Willoughby, Reference Gehring and Willoughby2002; Debener et al. Reference Debener, Ullsperger, Siegel, Fiehler, von Cramon and Engel2005) and to reflect neural processes in reinforcement learning and behavioural adjustment (Holroyd & Coles, Reference Holroyd and Coles2002).
Decision making and feedback evaluation have also been linked to the feedback-related P300. This ERP component is suggested to reflect the activity of a noradrenergic system associated with motivational processes (Nieuwenhuis et al. Reference Nieuwenhuis, Aston-Jones and Cohen2005). In gambling tasks, such as the IGT, the P300 varied with outcome magnitude, regardless of whether the outcome is a gain or a loss (Sato et al. Reference Sato, Yasuda, Ohira, Miyawaki, Nishikawa, Kumano and Kuboki2005; Polezzi et al. Reference Polezzi, Sartori, Rumiati, Vidotto and Daum2009). Further, its amplitude is modulated by expectations, with enhanced amplitudes to unexpected feedback than to expected feedback (Hajcak et al. Reference Hajcak, Holroyd, Moser and Simons2005, Reference Hajcak, Moser, Holroyd and Simons2007). In sum, the P300 amplitude might index feedback salience (De Bruijn et al. Reference De Bruijn, Mars, Hulstijn, Ullsperger and Falkenstein2004; Yeung & Sanfey, Reference Yeung and Sanfey2004) and thus is associated with the motivational significance of feedback. In contrast, the FRN reflects whether the feedback is consistent with expectations and is associated with the efficacy of learning.
Individual differences in impulsivity and risk-taking behaviour have been linked to modulations in ERN amplitudes. Highly impulsive individuals and BPD patients exhibit smaller ERN amplitudes (De Bruijn et al. Reference De Bruijn, Grootens, Verkes, Buchholz, Hummelen and Hulstijn2006a; Ruchsow et al. Reference Ruchsow, Walter, Buchheim, Martius, Spitzer, Kachele, Gron and Kiefer2006). The ERN attenuation is explained by reduced action monitoring, which might suggest altered ACC functioning in these patients. As a result, BPD patients might not learn from errors and thus maintain their impulsive response style. In healthy individuals, smaller ERN amplitudes were associated with increased risk-taking behaviour during a card gambling task (Hewig et al. Reference Hewig, Trippe, Hecht, Coles, Holroyd and Miltner2007) and increased risk-taking traits in adolescents (Santesso & Segalowitz, Reference Santesso and Segalowitz2009).
In the current study, we examined whether impairments of decision making are related to alterations in feedback processing in BPD. Individuals with BPD and matched healthy controls underwent a modified version of the IGT while ERPs were recorded. The FRN and feedback-related P300 were examined to elucidate the neural mechanisms of feedback processing in patients. At the behavioural level, we expected that BPD patients would show more risky decisions in the IGT and reduced learning throughout the task. Further, we assumed that higher levels of impulsivity are associated with increased risk-taking behaviour in the IGT. At the neurobiological level, we aimed at comparing ERPs following positive and negative feedback within groups. We expected diminished FRN amplitudes in BPD reflecting reduced performance monitoring. With regard to previous ERP findings, we predicted that the FRN would be related to impulsivity and risk-taking behaviour. In addition, we investigated the P300 as an indicator for the motivational significance of feedback information in BPD.
Method
Participants
Eighteen BPD patients (16 women) and 18 healthy controls (16 women) participated in this experiment. Table 1 presents the demographic and clinical measures of the study sample. All participants had normal or corrected-to-normal vision and reported no history of head trauma or neurological disease. The groups were matched with regard to age, sex and verbal intelligence, as measured with a vocabulary test (Wortschatztest; Schmidt & Metzler, Reference Schmidt and Metzler1992). Patients were recruited from an outpatient therapy project (Berliner Borderline Versorgungsstudie, Borderline Netzwerk Berlin, Germany). Clinical diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-I and SCID-II; Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997). Patients with a current or lifetime diagnosis of psychotic disorder or substance dependence were excluded. Although BPD was the primary diagnoses in all cases, 16 patients met DSM-IV criteria for one or more current co-morbid diagnoses, including anxiety disorder (n=15), somatoform disorder (n=5), substance abuse (n=5) and eating disorder (n=4). The severity of depression was assessed using the Beck Depression Inventory (BDI) within patients (Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961). Even though patients with current co-morbid affective disorders were excluded, 12 patients reported BDI total scores that exceeded the cut-off for clinical significance (BDI total scores >18). Further, eight patients were taking antidepressant medication at the time of testing (amitriptyline n=1, citalopram n=4, fluoxetine n=1, mirtazapine n=1, paroxetine n=1).
Table 1. Demographic and clinical characteristics of 18 healthy controls (16 women) and 18 BPD patients (16 women)
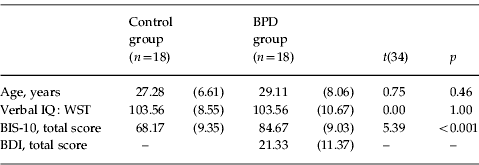
BPD, Borderline personality disorder; IQ, intelligence quotient; WST, Wortschatztest (German Vocabulary Test); BIS-10, Barratt Impulsivity Scale version 10; BDI, Beck Depression Inventory.
Data are given as mean (standard deviation).
Healthy controls were recruited using advertisements in local newspapers. SCID-I and SCID-II interviews revealed no past or current psychiatric diagnoses. In the entire group, impulsivity was measured using the Barratt Impulsivity Scale version 10 (BIS-10; Barratt, Reference Barratt, Spence and Izard1985). Relative to healthy controls and in line with previous research (Rentrop et al. Reference Rentrop, Backenstrass, Jaentsch, Kaiser, Roth, Unger, Weisbrod and Renneberg2008), BPD patients described themselves as significantly more impulsive [t(34)=5.39, p<0.001]. Following a detailed description of the study, all participants received verbal and written explanations of the purpose and procedures of the study, and gave written informed consent. The study was approved by the local ethics committee of the Charité University Hospital, Berlin and was conducted in accordance with the Declaration of Helsinki. All participants received financial compensation (€8 per h) for their participation.
Task and procedure
Participants underwent a computerized version of the IGT (Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994) that was modified for ERP recordings (Fig. 1). Participants were presented with four decks of cards (A, B, C and D) in a horizontal line for 1000 ms. Cards were selected by pressing one of four corresponding response buttons. After 700 ms, participants were shown the outcome associated with the card selection for 1000 ms. A red frowny face together with a negative amount indicated a disadvantageous choice, while a green smiley face together with a positive amount indicated an advantageous choice. When responses were slower than 1000 ms, participants were instructed to respond more quickly. Each trial started with an adaptive feedback bar that informed participants about their current total score. The next trial was presented at random intertrial intervals between 650 and 950 ms. Participants were instructed to win as much virtual money as possible, and they were told that some decks were worse than others, but that they could still win if they avoided the worst decks (Bechara et al. Reference Bechara, Damasio, Damasio and Lee1999). Decks A and B were associated with large magnitude outcomes, while decks C and D were associated with low magnitude outcomes. In the long run, decks A and B were disadvantageous (referred to as being ‘risky’) as they led to a net loss over time. Decks C and D were advantageous (referred to as being ‘safe’) as they led to net gains throughout the task.

Fig. 1. Schematic depiction of the modified Iowa Gambling Task. Participants were instructed to select one card by pressing one of four response buttons corresponding to the four decks (A, B, C and D). After 700 ms, they were shown the outcome associated with the selected card for 1000 ms. A red frowny face together with a negative amount indicated a disadvantageous choice, while a green smiley face together with a positive amount indicated an advantageous choice. Information about the current score was indicated by a green bar, which represented a positive score or by a red bar, which represented a negative score. The experiment lasted about 40 min.
Participants underwent 12 blocks of 60 trials each with short breaks between blocks (720 total trials in the modified IGT v. 100 trials in the original IGT; Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994). Table 2 presents an overview of the reinforcement schedule on each deck. Decks A and C yielded losses on 50% of the trials, while decks B and D yielded losses on 20% of the trials. The lengthening was necessary to obtain a sufficient number of negative feedback trials for ERP analyses in all conditions. In order to maintain motivation throughout the experiment, each block began with 2000 points of starting credit (Bechara et al. Reference Bechara, Damasio and Damasio2000). Blocks were characterized by different levels of gains and losses and were presented in pseudo-randomized order. Furthermore, deck positions were pseudo-randomized at the beginning of each block.
Table 2. Reinforcement schedule in the modified Iowa Gambling TaskFootnote a

a Participants completed 12 blocks with 60 trials each (720 total trials). Blocks consisted of 12 different levels of gains and losses and were presented in pseudo-randomized order. At the beginning of each block, deck positions were pseudo-randomized. Decks A and B were associated with large magnitude outcomes, while decks C and D were associated with low magnitude outcomes. Decks A and C yielded losses on 50% of the trials, while decks B and D yielded infrequent losses on 20% of the trials. In the long run, decks A and B were disadvantageous (referred to as being ‘risky’) as they led to a net loss over time. Decks C and D were advantageous (referred to as being ‘safe’) as they led to net gains throughout the task.
Electroencephalogram (EEG) recording and data analyses
The EEG was recorded from 64 electrodes sites including Cz as recording reference by using an equidistant electrode system (EASYCAP GmbH, Germany). Additional electrodes were placed below the right and left eye (IO1, IO2) to record vertical eye movements and the activity from distant muscles (neck electrode). The ground electrode was located below the left mastoid (T1). Electrode impedances were kept below 5 kΩ. During recording, all activity was sampled digitally at a rate of 500 Hz, using a time constant of 10 s and a low-pass filter of 250 Hz. Individual electrode positions were digitized based on the run-time measurement of ultrasonic pulses using ELPOS (zebris Medical GmbH, Germany). Off-line, the EEG data were re-referenced to average reference and corrected for eye-movement artifacts using the multiple source eye correction method as implemented in BESA 5.1 (Brain Electrical Source Analysis; MEGIS Software GmbH, Germany). Raw data were filtered with a low-pass filter of 40 Hz and a notch filter of 50 Hz. Feedback-locked epochs were obtained for each trial, starting 200 ms prior to feedback onset and continuing for 1000 ms post-feedback. Individual averages were baseline corrected to an average activity between 200 and 0 ms before feedback onset. Feedback-locked epochs were excluded from further analyses if they still contained artifacts. For each participant, ERPs were averaged separately for positive and negative feedback. Grand average ERPs were filtered with a 15 Hz low-pass filter.
As FRN and P300 amplitudes did not always show clear peaks in individual waveforms, ERP analyses were based on mean amplitudes instead of peak amplitudes. For statistical analysis, FRN amplitudes were determined in a time window between 240 and 310 ms following feedback onset at electrodes Fz and FCz. The P300 was quantified at CPz and Pz and defined as the mean amplitude within 300 to 400 ms after feedback presentation. ERP time windows were based on the visual inspection of the grand-average waveforms and were centred around peaks (for FRN: Fz, for P300: Pz).
For behavioural analysis, deck choices were classified as advantageous (decks C and D) or disadvantageous (decks A and B). IGT net scores were calculated as the difference between the number of advantageous and disadvantageous choices (Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994). IGT net scores served as the dependent variable and were compared between the groups using two-tailed t tests. Due to the a priori hypotheses, IGT learning was examined by dividing the 12 blocks into terciles, each containing four blocks (blocks 1–4, 5–8, 9–12). IGT net scores were calculated for each of these terciles to identify changes in the pattern of choices (Bechara et al. Reference Bechara, Damasio and Damasio2000). Learning was then analysed by comparing the first and the final IGT terciles. To examine whether the high number of trials within the total experiment (n=720) might have caused a motivational decline, all analyses were repeated for the first 60 trials (i.e. block 1).
ERP data were analysed by repeated-measurement analysis of variance with the between-subjects factor ‘group’ (BPD patients v. healthy controls) and the within-subject factors ‘electrode’ (for FRN: Fz and FCz, for P300: CPz and Pz) and ‘feedback valence’ (positive feedback v. negative feedback). Since the FRN amplitude was largest at the Fz electrode, correlation coefficients (Pearson's r) were used to examine the association between FRN magnitude at Fz and self-reported impulsivity and risky decisions in the IGT. To assess whether the FRN following negative feedback was affected by elevated levels of depressive symptoms (BDI total score), in patients additional bivariate correlations were computed within BPD patients (n=18). Behavioural analyses were conducted with ‘deck choices’ as the within-subject factor and ‘electrode’ and ‘feedback valence’ as within-subject factors for the ERP analyses, respectively.
Results
Behavioural findings
In BPD, deck choices resulted in a negative mean IGT net score of −53.4 (s.d.=144.6), whereas healthy controls showed a positive mean IGT net score of 78.2 (s.d.=193.3). BPD patients performed significantly worse compared with healthy controls, as reflected in lower mean IGT net scores [t(34)=2.23, p<0.05]. Fig. 2 presents the mean IGT net scores within terciles for healthy controls and BPD patients. Across the blocks, BPD patients showed lower mean IGT net scores compared with healthy controls (p<0.05). Furthermore, there was no improvement within patients (p=0.36), but learning was observed in healthy controls (p=0.05). To control for motivational effects, IGT performance was additionally analysed within the first 60 trials (i.e. block 1). BPD patients tended to show lower mean IGT net scores compared with controls [t(34)=1.78, p=0.08]. Again, there was no learning in BPD (p=0.37), whereas the controls tended to enhance performance throughout the first 60 trials (p=0.09).
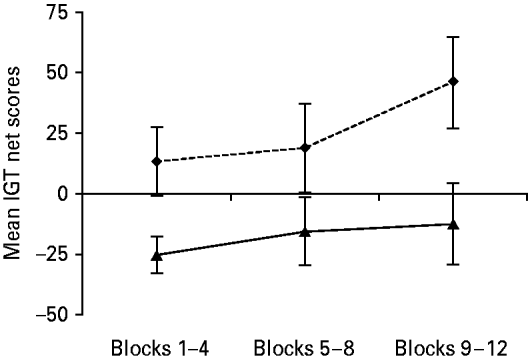
Fig. 2. Iowa Gambling Task (IGT) learning for borderline personality disorder patients (n=18, –▴–) and healthy controls (n=18, - -◆- -). The x-axis represents the consecutive terciles, each containing four blocks. The y-axis represents the mean IGT net score (total number of choices from decks C and D minus total number of choices from decks A and B), with standard errors represented by vertical bars.
ERP findings
Feedback-locked ERP waveforms following positive and negative feedback presentation are displayed in Fig. 3. With regard to the FRN, no main effect for ‘feedback valence’ was found (F<1), but a significant interaction between ‘feedback valence’ and ‘group’ was obtained [F(1, 34)=4.16, p=0.05]. In line with previous studies, healthy controls showed significant amplitude differences between positive and negative feedback (p<0.02). In contrast, this amplitude difference was not found in patients (p=0.60). Relative to healthy controls, patients had reduced (i.e. more positive) FRN amplitudes following negative feedback (p<0.03) and tended to have reduced mean amplitudes following positive feedback (p=0.08, Fig. 4a). Additionally, a significant main effect of electrode emerged [F(1, 34)=60.26, p<0.001], due to larger amplitudes at Fz compared with FCz.

Fig. 3. Averaged feedback-locked event-related brain potential (ERP) waveforms for the feedback-related negativity (FRN) and the P300 of (a) healthy controls (n=18) and (b) borderline personality disorder patients (n=18) at Fz, FCz, CPz and Pz for positive (- - - -) and negative (–––) feedback.

Fig. 4. Amplitudes of feedback-locked event-related brain potentials plotted as a function of group [healthy controls, n=18; borderline personality disorder (BPD) patients, n=18] and feedback (□, positive; ▪, negative). (a) Mean feedback-related negativity (FRN) amplitudes across electrodes Fz and FCz relative to a pre-stimulus baseline. It should be noted that the FRN is a negative-going deflection but positive (μV). The larger the FRN, the less positive the deflection. (b) Mean P300 amplitudes across electrodes CPz and Pz relative to a pre-stimulus baseline. * Mean value was significantly different from that for positive feedback (p<0.05).
The P300 is also depicted in Fig. 3. A main effect for ‘feedback valence’ was found [F(1, 34)=31.09, p<0.001], indicating that the P300 was larger following negative feedback compared with positive feedback. Importantly, there was a significant interaction between ‘feedback valence’ and ‘group’ [F(1, 34)=13.65, p<0.01]. Fig. 4b demonstrates that this interaction was caused by larger P300 amplitudes following negative feedback compared with positive feedback in the patient group (p<0.001). Healthy controls, however, did not show significant P300 amplitude differences between positive and negative feedback (p=0.18). For the P300, no main effect for electrode was found (F<1).
Correlational findings
Across groups (n=36), bivariate correlations were computed between the FRN amplitude (at Fz electrode) and impulsivity (BIS-10 total score) or mean IGT net scores. To control for the direction of correlation coefficients, mean FRN amplitudes following negative feedback were multiplied with minus 1, so that a positive correlation indicates an increase in FRN amplitude (i.e. a more negative potential). First, a significant negative correlation between FRN amplitude and impulsivity was found (r=–0.34, p<0.05), indicating that higher levels of impulsivity were associated with reduced (i.e. more positive) FRN amplitudes. Second, a significant positive correlation between FRN amplitude and IGT net score was observed (r=0.36, p<0.05), reflecting that larger FRN amplitudes were associated with higher IGT net scores (i.e. fewer risky choices). Last, a significant negative correlation between impulsivity and IGT net score was found (r=−0.46, p<0.02), indicating that higher levels of impulsivity were associated with lower IGT net scores. Additionally, no significant correlation between FRN amplitude and depressive symptoms (BDI total score) was found in the patient group (r=0.32, p=0.20)Footnote 1.
Discussion
This study focused on decision making in individuals with BPD using a modified IGT. Simultaneously, EEG recordings were assessed to clarify how feedback evaluation was related to decision making in these patients. In agreement with previous research on IGT performance, BPD patients were less likely to develop a preference for advantageous decks and preferred the risky decks (Haaland & Landro, Reference Haaland and Landro2007; Maurex et al. Reference Maurex, Zaboli, Wiens, Asberg, Leopardi and Ohman2009). Further, the number of risky decisions correlated with enhanced impulsivity in the entire sample. Decision-making deficits in the IGT have been found in patients with several neurological or psychiatric disorders. For instance, IGT impairment was shown in patients with OFC/ventromedial cortex lesions or with amygdala damage (Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994, Reference Bechara, Damasio, Damasio and Lee1999). In addition, individuals with substance dependence (Bechara et al. Reference Bechara, Dolan, Denburg, Hindes, Anderson and Nathan2001) as well as patients with impulse spectrum disorders (e.g. pathological gambling; Cavedini et al. Reference Cavedini, Riboldi, Keller, D'Annucci and Bellodi2002) exhibit abnormal decision making in the IGT. Consequently, decision-making disturbances seem to be a neurobiological hallmark of diminished impulse control and may be a trademark of impulse spectrum disorders (Hollander & Rosen, Reference Hollander and Rosen2000). In the present study, correlation analysis provided further evidence for an association between decision-making deficits and enhanced impulsivity. Thus, findings are in line with previous neuropsychological studies showing that diminished impulse control in BPD plays a pivotal role in decision making and planning (Bazanis et al. Reference Bazanis, Rogers, Dowson, Taylor, Meux, Staley, Nevinson-Andrews, Taylor, Robbins and Sahakian2002; Lenzenweger et al. Reference Lenzenweger, Clarkin, Fertuck and Kernberg2004).
With regard to the FRN and in agreement with previous findings (Miltner et al. Reference Miltner, Braun and Coles1997), healthy controls exhibited enhanced FRN amplitudes following negative feedback as opposed to positive feedback. In BPD patients, however, these FRN modulations by feedback valence were not observed. BPD patients exhibited diminished FRN amplitudes and at trend level reduced amplitudes following positive feedback, suggesting general alterations of feedback processing in BPD. The FRN is assumed to reflect ACC activity (Holroyd & Coles, Reference Holroyd and Coles2002), a brain region that plays a key role in feedback evaluation and learning about the consequences of actions to select more appropriate future behaviours (Ridderinkhof et al. Reference Ridderinkhof, Ullsperger, Crone and Nieuwenhuis2004). When outcomes are worse than expected, the FRN is elicited by a phasic decrease in activity of mesencephalic dopaminergic neurons (Holroyd & Coles, Reference Holroyd and Coles2002; Schultz, Reference Schultz2002). Research on the biological basis of BPD has revealed a deficit in serotonergic activity (Silk, Reference Silk2000; Skodol et al. Reference Skodol, Siever, Livesley, Gunderson, Pfohl and Widiger2002), although there is evidence that dopamine dysfunction may also be associated with BPD (Friedel, Reference Friedel2004). ERP results are consistent with structural as well as functional ACC alterations in BPD. Previous research has shown decreased baseline metabolism in subregions of the ACC (De La Fuente et al. Reference De La Fuente, Goldman, Stanus, Vizuete, Morlan, Bobes and Mendlewicz1997; Tebartz van Elst et al. Reference Tebartz van Elst, Hesslinger, Thiel, Geiger, Haegele, Lemieux, Lieb, Bohus, Hennig and Ebert2003) as well as smaller grey matter volume in BPD (Hazlett et al. Reference Hazlett, New, Newmark, Haznedar, Lo, Speiser, Chen, Mitropoulou, Minzenberg, Siever and Buchsbaum2005). Further, studies using emotional, stressful and sensory stimuli have consistently shown deactivation of ACC in BPD (Donegan et al. Reference Donegan, Sanislow, Blumberg, Fulbright, Lacadie, Skudlarski, Gore, Olson, McGlashan and Wexler2003; Schmahl et al. Reference Schmahl, Vermetten, Elzinga and Bremner2004).
Recent ERP investigations reported an association between the magnitude of the FRN and performance adjustment and demonstrated that the FRN was more negative when participants had learned from feedback information (Frank et al. Reference Frank, Woroch and Curran2005). In the current study, diminished FRN amplitudes were related to deficient IGT performance and heightened impulsivity. These associations suggest that BPD patients may not learn from feedback, as reflected by the absence of developing a preference for the advantageous decks. The results of our study are in line with previous findings linking diminished inhibition as a possible mediating process to reinforcement learning deficiencies in BPD (Bornovalova et al. Reference Bornovalova, Lejuez, Daughters, Zachary Rosenthal and Lynch2005). Incarcerated females with BPD (Hochhausen et al. Reference Hochhausen, Lorenz and Newman2002) and healthy individuals high in BPD symptoms (Chapman et al. Reference Chapman, Leung and Lynch2008) demonstrated reduced avoidance of punishment (e.g. financial penalties).
Although there is evidence for decision-making deficits in BPD, neural correlates of these processes have not yet been investigated. ERP research has focused on error processing and has demonstrated diminished ERN amplitudes in impulsive individuals (Potts et al. Reference Potts, George, Martin and Barratt2006, but Santesso & Segalowitz, Reference Santesso and Segalowitz2009) as well as individuals with BPD (De Bruijn et al. Reference De Bruijn, Grootens, Verkes, Buchholz, Hummelen and Hulstijn2006a; Ruchsow et al. Reference Ruchsow, Walter, Buchheim, Martius, Spitzer, Kachele, Gron and Kiefer2006). Further, smaller ERNs were associated with increased risk-taking in a card gambling task (Hewig et al. Reference Hewig, Trippe, Hecht, Coles, Holroyd and Miltner2007) and risk-taking traits in adults (Santesso & Segalowitz, Reference Santesso and Segalowitz2009). The current study expands on previous ERP findings regarding action-monitoring alterations in BPD in that it also demonstrates deficits in feedback evaluation in these patients.
To elucidate the salience of feedback information in BPD, the P300 was investigated. The results reveal that the P300 was insensitive to feedback valence in controls, while in patients the P300 was increased following negative feedback compared with positive feedback. Previous studies also found that the P300 was insensitive to feedback valence in healthy individuals (Yeung & Sanfey, Reference Yeung and Sanfey2004). Possibly, healthy controls already had processed feedback valence earlier, as reflected in the FRN modulation. In BPD, however, the processing of feedback valence might be delayed, and thus be reflected by the P300. Since the P300 is modulated by expectations (Hajcak et al. Reference Hajcak, Holroyd, Moser and Simons2005, Reference Hajcak, Moser, Holroyd and Simons2007), the increased P300 in BPD may also indicate that negative outcomes were relatively unexpected to the patients. Altered processing of negative reinforcement information in BPD is also provided by a study using a binary-outcome gamble (Kirkpatrick et al. Reference Kirkpatrick, Joyce, Milton, Duggan, Tyrer and Rogers2007). Individuals with BPD demonstrated dysfunctional processing of loss information when the probability of gains was high. In that study, risky decision making in BPD is explained by problems using feedback information, suggesting an imbalance between the appetitive and aversive motivational states excited by available reinforcement signals. Further, ERP studies demonstrated that the P300 responded more strongly to negative feedback than to positive feedback (Frank et al. Reference Frank, Woroch and Curran2005) and that the amplitude increased in individuals who attributed more meaning to feedback information (De Bruijn et al. Reference De Bruijn, Mars, Hulstijn, Ullsperger and Falkenstein2004). In this regard, controls may evaluate positive and negative feedback information as being equally meaningful. Perhaps, enhanced P300 amplitudes following negative feedback in BPD suggest that these patients attributed more meaning to negative feedback information. This is supported by the hypothesis that the P300 may reflect motivational processes linked to noradrenergic transmission (Nieuwenhuis et al. Reference Nieuwenhuis, Aston-Jones and Cohen2005), which play an important role in modulating the reactivity and sensitivity to environmental feedback and is considered to be associated with affective instability in BPD (Steinberg et al. Reference Steinberg, Trestman, Siever and Silk1994).
This study has some limitations. Eight patients were taking psychotropic medication at the time of testing. However, patients were only taking antidepressant medication which does not alter action-monitoring processes (De Bruijn et al. Reference De Bruijn, Sabbe, Hulstijn, Ruigt and Verkes2006b). Although patients with co-morbid affective disorders were excluded, 12 patients reported elevated symptom scores for depression as measured with the BDI (Beck et al. Reference Beck, Ward, Mendelson, Mock and Erbaugh1961). However, no significant correlation between FRN amplitude and depressive symptoms was found in patients. Although, decision-making deficits in BPD patients are in line with earlier studies, depressive symptoms might also have influenced IGT performance. However, previous studies employing the IGT with depressed patients have found inconclusive results, showing impaired, unaltered or superior performance (Dalgleish et al. Reference Dalgleish, Yiend, Bramham, Teasdale, Ogilvie, Malhi and Howard2004; Must et al. Reference Must, Szabo, Bodi, Szasz, Janka and Keri2006; Smoski et al. Reference Smoski, Lynch, Rosenthal, Cheavens, Chapman and Krishnan2008). It might be interesting to compare our findings with those based on BPD samples without any co-morbidity or depressive symptoms. But it remains questionable whether a ‘pure’ BPD sample would be representative considering the high co-morbidity rate for BPD in general (Zanarini et al. Reference Zanarini, Frankenburg, Hennen, Reich and Silk2004). Finally, there are possible behavioural confounds which might account for group differences. It remains unclear whether altered decision making in patients is a consequence of altered feedback processing or whether it leads to these FRN alterations. Recent empirical findings suggest that the IGT is a complex paradigm, and task complexity may also interfere with the ability to distinguish the different component processes that are implicated in task performance. IGT impairments are not simply related to reinforcement learning deficits, but may also reflect the disability to attend to, synthesize and remember complex reinforcement histories and to resolve the approach–avoidance conflict that arises when a deck is associated with both reward and punishment (Fellows & Farah, Reference Fellows and Farah2005; Dunn et al. Reference Dunn, Dalgleish and Lawrence2006). Another mechanism that could explain IGT deficits is lack of motivation. Rather than being unable to make adequate decisions, impaired patient groups may simply not care enough about the negative outcomes to actively avoid them. But, in the present study enhanced P300 following negative feedback in BPD argues against motivation deficits in patients. Nonetheless, behavioural results should be interpreted cautiously since controls also showed some difficulties within the first block. It is possible that task modifications complicated learning also within controls and that learning occurred more implicitly compared with the original IGT.
Reduced performance monitoring is not specific to BPD, and FRN reductions have also been reported in other groups, such as schizophrenia patients (Morris et al. Reference Morris, Heerey, Gold and Holroyd2008) and older adults (Pietschmann et al. Reference Pietschmann, Simon, Endrass and Kathmann2008). Additional research should include adequate psychiatric control groups to determine the specific impairment of reinforcement learning in BPD. Last, it should be mentioned that the current study relies on a relatively small sample size and replication in a larger sample is needed. Notwithstanding these limitations, the present study confirms previous findings regarding altered decision making in BPD and sheds new light on cognitive impairments by combining behavioural and ERP measures.
Learning from feedback is highly important for successfully making future decisions. This study indicates that BPD patients are impaired in decision making, which might be related to a dysfunctional use of feedback information. Specifically, BPD patients did not learn to avoid disadvantageous selections, albeit they attended to negative consequences. Altered neural correlates of reinforcement learning are consistent with problems faced by individuals with BPD; namely, continued engagement in certain behaviours despite negative consequences (e.g. risky sexual behaviour, alcohol/drug use, self-harm). Impulsive decision making is a core feature of BPD, so understanding the underlying mechanisms involved in feedback evaluation could greatly have an impact on the treatment of individuals with BPD.
Acknowledgements
We thank the following people for their contributions to this work: S. Röpke, M.D. (Charité-Universitätsmedizin Berlin, Germany); T. Pinkpank, Dipl.-Psych, R. Kniesche, Dipl.-Ing, R. Blasiak, M.A., H. Groth and S. Kessler-Scheil. We also thank J. Beyer, Dipl.-Psych., C. Steffens, Dipl.-Psych., D. Spretz, Dipl.-Psych. and T. Wagner, Dipl.-Psych. of the Borderline Netzwerk Charité, Campus Benjamin Franklin, Berlin, for their assistance in patient recruitment.
Declaration of Interest
None.








