Trisomy 18 is the second most common numerical chromosomal disorder with poor mortality and morbidity.Reference Muneuchi, Yamamoto and Takahashi1 Although trisomy 18 is frequently associated with multiple anomalies, including CHD, definitive surgical repair is not offered in most centers owing to its poor prognosis.Reference Kosiv, Gossett, Bai and Collins2, Reference Peterson, Kochilas, Catton, Moller and Setty3 Generally, parents of children with trisomy 18 aim to meet their child and have them discharged home and be a part of the family.Reference Janvier, Farlow and Barrington4
CHD with ductus-dependent circulation is relatively common in neonates with trisomy 18.Reference Muneuchi, Yamamoto and Takahashi1–Reference Peterson, Kochilas, Catton, Moller and Setty3 In patients with ductus-dependent circulation, free prostaglandin E1 (prostaglandin E1-CD) or lipid microspheres (lipo-prostaglandin E1) are indicated to maintain the ductus.Reference Akkinapally, Hundalani and Kulkarni5 However, patients who are treated with prostaglandin E1 find it difficult to be discharged from the hospital because of the need for continuous intravenous administration of medications.
Here, we report the case of a neonate with trisomy 18 diagnosed with hypoplastic left heart syndrome variant. The patient needed a patent ductus arteriosus to maintain systemic circulation. Oral prostaglandin E1 derivative successfully maintained ductus arteriosus, which led to discharge of the patient from our hospital.
Case report
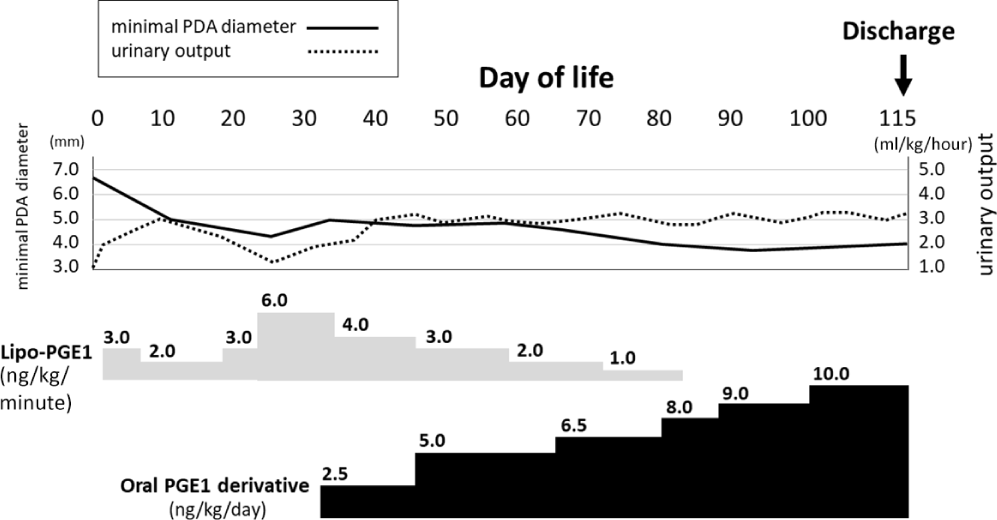
A female Japanese infant was born at 39 weeks of gestation via vaginal delivery, with a birth weight and Apgar scores of 1985 g and 6–9, respectively. The patient was transferred to our neonatal ICU because of low birth weight. Upon admission, she was suspected to have trisomy 18 owing to low birth weight, specific countenance, hip joint contracture, and rocker-bottom feet. Her vital signs were as follows: blood pressure, 50/28 mmHg; pulse rate, 160 beats/minute; respiratory rate, 60 breaths/minute; and body temperature, 37.0°C. Percutaneous oxygen saturation was 96%. Heart murmur was detected, and transthoracic echocardiography revealed hypoplastic left heart syndrome variant with a relatively hypoplastic left ventricle, bicuspid aortic valve, ventricular septal defect, atrial septal defect, and patent ductus arteriosus. Echocardiographic findings prompted us to start continuous intravenous infusion of lipo-prostaglandin E1 at 3.0 ng/kg/minute. Low-concentration oxygen therapy using nitrogen gas was started on day of life 1. The dose of lipo-prostaglandin E1 was increased up to 6.0 ng/kg/minute based on the diameter of patent ductus arteriosus (Fig 1). Trisomy 18 was confirmed by G-banding. The patient’s family and medical caregiver discussed the therapeutic strategy and decided against definitive surgical repair. Reduction of lipo-prostaglandin E1 dose was attempted because the patient’s parents desired for her to be discharged. However, the urine output had decreased owing to narrowing of the patent ductus arteriosus. Hence, an oral prostaglandin E1 derivative, Limaprost alfadex (Opalmon®, ONO PHARMACEUTICAL CO., LTD, Osaka, Japan), was administered as an alternative to lipo-prostaglandin E1. Informed consent was obtained from the patient’s parents, and off-label use of the drug was approved by the Institutional Review Board in our institute (approval number 479). Oral prostaglandin E1 derivative was administered at 2.5 ng/kg/day on day of life 32 and increased to 10.0 ng/kg/day based on the ductus arteriosus along with gradual decrease in lipo-prostaglandin E1 (Fig 1). We administered oral prostaglandin E1 to the patient every 6 hours through a feeding tube. Before administration of oral prostaglandin E1, we performed a simple suspension method in which tablets were disintegrated in hot water to form a suspension without grinding because prostaglandin E1 in tablet form is inactivated by porphyrisation.Reference Morita, Yamaguchi and Kimura6 She had no fever or apnea due to oral prostaglandin E1. The baseline percutaneous oxygen saturation gradually decreased to 80% possibly owing to the progression of pulmonary vasculature obstructive disease.Reference Muneuchi, Yamamoto and Takahashi1 Low-concentration oxygen therapy was discontinued on day of life 107 without worsening of the heart failure because of pulmonary high flow. She was discharged from our hospital on day of life 115 at 2726 g body weight. Mildly thickened periosteum was noted, but the patient lived a normal family life for 3 months without any serious complications.

Figure 1. Clinical course of the patient. Oral prostaglandin E1 derivative (Limaprost alfadex [Opalmon®]) is started at a dose of 2.5 ng/kg/day and increased to 10.0 ng/kg/day based on the diameter of patent ductus arteriosus with gradual decrease in lipo-prostaglandin E1. PDA = patent ductus arteriosus; PGE1 = prostaglandin E1.
Discussion
We reported a case of trisomy 18 with ductus-dependent CHD treated using oral prostaglandin E1 derivative, which was found to be as effective as intravenous lipo-prostaglandin E1. Subsequently, the patient was able to discharge home without serious complications.
The use of oral prostaglandin E to maintain ductus arteriosus was firstly reported by Silove et al in 1985.Reference Silove, Roberts and de Giovanni7 They used oral prostaglandin E2 as an alternative medication for intravenous prostaglandin E. Although oral prostaglandin E2 was nearly effective, it required frequent administration and a substantial amount of the maintenance dose (27 µg/kg/hour) leading to several short- and long-term adverse effects.Reference Akkinapally, Hundalani and Kulkarni5, Reference Silove, Roberts and de Giovanni7 Therefore, oral prostaglandin E2 might not have been suitable for clinical use and thus has not been widely used. Six years after the first report of oral prostaglandin E use, Saji et al reported the successful use of oral prostaglandin E1 in an infant with ductus-dependent pulmonary circulation.Reference Saji, Matsuura, Hoshino, Yamamoto, Ishikita and Matsuo8 As a prostaglandin E1 analogue, Limaprost alfadex acts as an agonist at prostaglandin E2 receptors. It likely stimulates the adenylate cyclase coupled E2 subtype of these receptors to produce smooth muscle relaxation.Reference Ruiz Rubio and Hernández9 To date, only a few case reports (including Japanese articles) have evaluated the usefulness of oral prostaglandin E1 derivative.Reference Matsuura, Saji, Yamamoto, Ishikita, Aoki and Matsuo10 However, it has not been widely used because it is not approved for maintaining ductus arteriosus but thromboangiitis obliterans or lumbar canal stenosis in Japan.
Children with trisomy 18 have been considered to be incompatible with long-term survival, with death occurring frequently within 1 year.Reference Kosiv, Gossett, Bai and Collins2, Reference Peterson, Kochilas, Catton, Moller and Setty3 Historically, aggressive treatments for multiple anomalies in trisomy 18 had not been considered in children because of the expected short life span and low functional status.Reference Peterson, Kochilas, Catton, Moller and Setty3 However, recent studies suggested that interventions in patients with trisomy 18 may be beneficial with improved alive-discharge rates, low in-hospital mortality, or longer life span, if suitable patients are selected.Reference Muneuchi, Yamamoto and Takahashi1–Reference Janvier, Farlow and Barrington4 Based on this perspective, oral prostaglandin E1 derivative for ductus-dependent CHD may be one of the palliative options in children with trisomy 18.
In conclusion, oral prostaglandin E1 derivative for ductus-dependent CHD may be a beneficial and feasible option for palliative treatment, especially in infants with serious chromosomal disorders not suitable for definitive cardiac surgery. Further studies are warranted to evaluate the therapeutic effects of oral prostaglandin E1 in this population.
Acknowledgements
The authors thank Dr Reiji Hirano, Dr Shinnosuke Fukunaga, Dr Yusuke Okada, and Dr Takahiro Motonaga (all Department of Pediatrics, Saiseikai Shimonoseki General Hospital) for assistance of data collection.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and was approved by the Saiseikai Shimonoseki General Hospital Institutional Review Board with waiver of informed consent.
Contributors’ Statement
Dr Miyake analyzed and interpreted data and drafted the initial manuscript. Dr Okada led conceptualization and design of the analysis, supervised data collection, reviewed the manuscript critically, and approved the final manuscript as submitted. Dr Ishikawa contributed to collection of the data, reviewed the manuscript critically, and approved the final manuscript as submitted.