Introduction
Itchgrass [Rottboellia cochinchinensis (Lour.) W.D. Clayton] is a C4 annual grass that infests disturbed areas and competes with corn (Zea mays L.), peanut (Arachis hypogaea L.), soybean [Glycine max (L.) Merr.], and sugarcane (Saccharum spp. interspecific hybrids) in more than 40 countries throughout the world (CABI 2020; Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977). Mature plants may reach a height of 3 m and are supported by prop roots that descend from lower nodes (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977). Erect fiberglass-like hairs protrude outward from the primary stem and tillers. Hairs dislodge from plants when handled and irritate unprotected skin. Seed introduced into Florida and Louisiana likely occurred in the early to mid-1900s at ports, and several infestations thereafter were reported near railroad tracks (Hall and Patterson Reference Hall and Patterson1992; Millhollon Reference Millhollon1983). A recent report suggested R. cochinchinensis infestations have expanded southward along roadsides in Santa Catarina state, southern Brazil, and in Nigeria, Maize yellow mosaic virus was reported infecting R. cochinchinensis in sugarcane (Funez et al. Reference Funez, Ferreira, Hassemer and Trevisan2016; Yahaya et al. Reference Yahaya, Al Rwahnih, Dangora, Gregg, Alegbejo, Lava Kumar and Alabi2017). Rottboellia cochinchinensis has become ubiquitous in Louisiana, particularly in areas under sugarcane cultivation. Drainage ditch banks, floodwater, infested seed cane fields, and agricultural machinery are common sources of R. cochinchinensis seed introduction to noninfested areas (Freshwater et al. Reference Freshwater, Benson and Hall1986; Millhollon Reference Millhollon1980). Although R. cochinchinensis has negligible impact on yield of soybean in the Midwest and northern United States due to limited distribution and effective POST soybean herbicides, the weed is problematic in Louisiana where sugarcane is cultivated (Griffin Reference Griffin1991; Millhollon Reference Millhollon1992, Reference Millhollon1993; Nester et al. Reference Nester, Harger and Geaghan1984). Rottboellia cochinchinensis competition with newly planted sugarcane for 30 and 60 d during summer months resulted in 7% and 17% reduction in sucrose yield, respectively, and greater yield loss ensued when R. cochinchinensis competed until sugarcane harvest (Millhollon Reference Millhollon1992). Rottboellia cochinchinensis competition during the entire sugarcane cropping season resulted in 43% less sucrose yield (Lencse and Griffin Reference Lencse and Griffin1991). Sequential herbicide applications in dry direct-seeded rice (Oryza sativa L.) systems in the Philippines were necessary to maximize rice yield and reduce R. cochinchinensis biomass by 97% (Chauhan and Bajwa Reference Chauhan and Bajwa2015).
Many unique weed biotypes have been reported in the literature in recent years. Many biotypes exhibited resistance to herbicides frequently applied POST to control weeds in corn and soybean (Bradley et al. Reference Bradley, Wu, Hatzios and Hagood2001; Foes et al. Reference Foes, Vigue, Stoller, Wax and Tranel1999; Sarangi et al. Reference Sarangi, Stephens, Barker, Patterson, Gains and Jhala2019; Spaunhorst and Johnson Reference Spaunhorst and Johnson2017). However, some authors reported waterhemp [Amaranthus tuberculatus (Moq.) Sauer], R. cochinchinensis, and Palmer amaranth (Amaranthus palmeri S. Watson) biotypes that exhibited diverse morphological traits, growth, sex ratio frequencies, and inflorescence emergence timings when grown in a common environment (Alloub et al. Reference Alloub, Juraimi, Rajan, Kadir, Saad and Sastroutomo2005; Christopher et al. Reference Christopher, Kumari and Mini1989; Heneghan and Johnson Reference Heneghan and Johnson2017; Millhollon and Burner Reference Millhollon and Burner1993; Pamplona and Mercado Reference Pamplona and Mercado1981; Spaunhorst et al. Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018). Biotypes with fecundate reproduction and prolific growth may become more abundant and difficult to manage. Alloub et al. (Reference Alloub, Juraimi, Rajan, Kadir, Saad and Sastroutomo2005) reported that three R. cochinchinensis biotypes exist throughout Malaysia, and a biotype found in banana (Musa acuminata Colla), cocoa (Theobroma cacao L.), and oil palm (Elaeis guineensis Jacq.) fields was 12% taller and had 14% and 39% fewer leaves and seeds, respectively, than a biotype present in corn, upland rice, rubber [Hevea brasiliensis (Willd. ex A. Juss.) Müll Arg.], sugarcane, and tobacco (Nicotiana tabaccum L.) crops. Genetic variability among R. cochinchinensis populations across geographic locations in São Paulo, Brazil, as determined using an amplified fragment length polymorphism technique, was 22% and partially explained unsatisfactory control reported with herbicides (Schiavetto et al. Reference Schiavetto, Perecin, Pinto, Azania, Zera and Melloni2016). Millhollon and Burner (Reference Millhollon and Burner1993) reported two R. cochinchinensis biotypes that infested sugarcane in Louisiana: Louisiana-1 is daylength neutral and Louisiana-2 is a short-day biotype. Louisiana-1 is the predominant biotype currently found in sugarcane fields (EC Petrie, personal communication). The two biotypes have distinct phenotypic characteristics. The primary culm diameter for Louisiana-1 was 15% smaller, produced 58% fewer secondary culms, and flowered 17 d earlier when exposed to a 12-h photoperiod compared with the Louisiana-2 biotype (Millhollon and Burner Reference Millhollon and Burner1993).
Several weed species, including R. cochinchinensis, exhibited discontinuous emergence stimulated by rainfall, cultivation, and radiant energy that allowed weeds to establish at various stages of crop development (Anderson and Nielsen Reference Anderson and Nielsen1996; Bagavathiannan et al. Reference Bagavathiannan, Norsworthy, Smith and Burgos2011; Bolfrey-Arku et al. Reference Bolfrey-Arku, Chauhan and Johnson2011; Roberts and Potter Reference Roberts and Potter1980; Zimdahl et al. Reference Zimdahl, Moody, Lubigan and Castin1988). Establishment timing influenced weed biomass accumulation, seed production, sex ratio frequency, and for some weed biotypes, seed size (Heneghan and Johnson Reference Heneghan and Johnson2017; Spaunhorst et al. Reference Spaunhorst, Devkota, Johnson, Smeda, Meyer and Norsworthy2018). Because two distinct R. cochinchinensis biotypes exist in Louisiana sugarcane fields and there have been anecdotal reports of emergence occurring earlier in the growing season, there is a need to test the hypothesis that March-established R. cochinchinensis will grow taller, accumulate more biomass, and produce more racemes than later-established plants and that enhanced control strategies are needed to prevent seed introduction for Louisiana-1, because reproduction is not daylength conditional. These two biotypes were investigated because they are two known biotypes found infesting sugarcane in Louisiana. To our knowledge Louisiana-1 was most widespread throughout the industry, because of its quick vegetative to reproductive life cycle, but recent observations indicated unique R. cochinchinensis phenotypes coexisting in a particular region of the sugarcane industry where Louisiana-1 was initially discovered that warrant further investigation. Due to the array of R. cochinchinensis biotypes reported across the globe and further geographic expansion, ecophysiological studies are needed to understand the growth and reproductive parameters for unique biotypes present in diverse environments where sugarcane is cultivated (Patterson Reference Patterson1985). Furthermore, ecophysiological studies play a vital role in determining stages of pest development at which the weed may be particularly vulnerable to management practices. The objectives of this research were to establish both biotypes in a common garden experiment at five establishment timings during critical periods of sugarcane development and field preparation to evaluate plant growth and developmental characteristics and determine whether management practices need tailored to biotype.
Materials and Methods
Experimental Design
Field studies were conducted in 2017 and 2018 at the USDA-ARS Sugarcane Research Unit in Houma, LA (29.5850°N, 90.7299°W). The experimental design was a randomized complete block with four replications. Two R. cochinchinensis biotypes were evaluated: Louisiana-1 is daylength neutral and Louisiana-2 is a short-day biotype (Millhollon and Burner Reference Millhollon and Burner1993). Each year five planting dates were used to simulate a natural discontinuous emergence pattern during critical periods of sugarcane development and field preparation: sugarcane emergence from winter dormancy (March), spring cultivation (April), fertilization (May), layby (June), and sugarcane grand growth stage (July). Cultivation is an intermittent practice that begins in late February to remove established winter annual weeds, incorporate postharvest residue, repair equipment ruts from the previous year’s harvest, and reestablish eroded rows. The operations cease when equipment can no longer pass over sugarcane without injuring stalks. The grand growth stage of sugarcane development, typically from July to September, is a period of rapid growth and elevated water consumption (Gascho Reference Gascho1985).
Average monthly precipitation and air temperature for each year are given in Table 1 and the dates for R. cochinchinensis seed being planted in the greenhouse and transplanted to the field, raceme emergence, and biomass harvest are presented in Table 2. Rottboellia cochinchinensis height and number of leaves per plant when transplanted to the field are presented in Table 3. Both biotypes were of similar size and growth stage when transplanted. Louisiana-1 seed was collected from the Ardoyne Research Farm near Schriever, LA (29.6372°N, 90.8395°W). Seed germination for Louisiana-1 was poor for both years; therefore, germinated plants with no more than one leaf were exhumed from the same sugarcane field at the Ardoyne Research Farm where Louisiana-1 seed was originally collected and transplanted into seedling trays 2 to 3 d after Louisiana-2 seed was planted in the greenhouse and watered daily. Day and night greenhouse air temperatures were set at 34 and 26 C, respectively. Louisiana-2 seed was collected in the late 1970s from sugarcane fields near Larose, LA (29.5724°N, 90.3817°W) and stored in a cooler at 4 C. Each plot contained plants of the same biotype, and the plot size was 1.8 by 9.1 m, with three rows of R. cochinchinensis that were spaced 0.3 m apart, and spacing between individual plants measured 0.9 m for a total of 27 plants per plot (Figure 1). Rottboellia cochinchinensis were watered at the time of transplanting, and plots were weed-free at trial initiation and remained weed-free once plants were established.
Table 1. Monthly precipitation (mm), average monthly air temperatures (C), and daylength (h:min) from March through September in comparison to the 30-yr average at the Sugarcane Research Unit in Houma, LA.a

a Thirty-year average (1981 to 2010) obtained from the National Climatic Data Center (2019).
b Weather data for 2017 and 2018 were obtained from a weather station located at the Ardoyne Research Farm in Schriever, LA.
c Day-length for Houma, LA, was recorded on the 15th of each month.
Table 2. Dates of planting in the greenhouse, transplanting to field, raceme emergence, and biomass harvest of two Rottboellia cochinchinensis biotypes at five establishment timings in a field study conducted in 2017 and 2018 at the Sugarcane Research Unit in Houma, LA.

a Abbreviations: Feb, February; Oct, October; Sept, September.
b The Louisiana-1 biotype was excavated from the field 2 to 3 d after the Louisiana-2 biotype was seeded in the greenhouse. The April establishment timing in 2018 was delayed until April 12 for the Louisiana-2 biotype, because seed failed to germinate when seeded in the greenhouse on March 27.
c Raceme emergence was not observed.
d Seed failed to germinate in the greenhouse.
Table 3. Mean Louisiana-1 (daylength neutral) and Louisiana-2 (short-day) Rottboellia cochinchinensis height and leaf number when transplanted to the field in 2017 and 2018 at the Sugarcane Research Unit in Houma, LA.

a Seed failed to germinate in the greenhouse.

Figure 1. Photos were taken on July 14, 2017, of Rottboellia cochinchinensis plants established in (1) March, (2) April, (3) May, (4) June, and (5) July. The Louisiana-1 R. cochinchinensis biotype was daylength neutral (A), and Louisiana-2 flowered when daylength decreased (short-day) to 13 h (B).
Data Collection
Data were collected from five plants from the middle row of each plot that were marked at transplanting by placing a fluorescent pin flag adjacent to each plant. Measurements were not recorded from other plants. Seedlings were measured when transplanted and each week thereafter until biomass harvest. Plant height was determined by measuring plants from the soil surface to the last fully expanded leaf collar (Table 2). Racemes were removed by hand and counted weekly when three or more joints were present. The five plants from each plot were harvested in autumn by clipping plants from the soil surface, placing them in separate paper bags, and air-drying them at 40 C (Table 2). Plant biomass was determined 2 wk later.
Data Analysis
Cumulative growing degree days (GDDs) were calculated daily for each establishment timing using the following equation:
 $${\rm{GD}}{{\rm{D}}_{T{\rm{base}} = 11{\rm{\;}}C}} = \mathop \sum \nolimits_{i = 1}^n ({T_{{\rm{mean}}}} - {T_{{\rm{base}}}})$$
$${\rm{GD}}{{\rm{D}}_{T{\rm{base}} = 11{\rm{\;}}C}} = \mathop \sum \nolimits_{i = 1}^n ({T_{{\rm{mean}}}} - {T_{{\rm{base}}}})$$
where T mean represents the daily mean air temperature in C, and T base is the minimum temperature at which R. cochinchinensis grows. Patterson et al. (Reference Patterson, Meyer, Flint and Quimby1979) reported maximum and minimum growth for R. cochinchinensis at 32 and 11 C day and night temperatures, respectively. GDD 0 represents when R. cochinchinensis were transplanted to the field. Maximum R. cochinchinensis height and GDDs to maximum height data were not transformed, whereas racemes produced and dry weight data were square-root transformed, and GDDs to 20-cm height data were log transformed. Means were back transformed for presentation. Growth to 20 cm was selected, because this is the maximum labeled height at which asulam and trifloxysulfuron were expected to control this species. The year by establishment date interaction was significant for all dependent variables; therefore, years were analyzed separately.
Rottboellia cochinchinensis height data were converted to a percentage of the maximum recorded height for each biotype by establishment timing combination and fit to several regression models using SigmaPlot (v. 12.5, Systat Software, San Jose, CA). Parameter estimates for each model are presented in Table 4. A three-parameter sigmoid model,
 $$\;y = a/\left\{ {1 + \exp {\rm{ }}\left[ {{{x - {\rm{GD}}{{\rm{D}}_{50}}} \over b}} \right]} \right\}$$
$$\;y = a/\left\{ {1 + \exp {\rm{ }}\left[ {{{x - {\rm{GD}}{{\rm{D}}_{50}}} \over b}} \right]} \right\}$$
Table 4. Parameter estimates of the three-parameter sigmoid model, linear model, and quadratic models fit to the growth of two Louisiana Rottboellia cochinchinensis biotypes established at different timings in the field in 2017 and 2018 at the Sugarcane Research Unit in Houma, LA.a

a Abbreviation: Est, establishment.
b Values in parentheses represent the standard error of the mean.
c Louisiana-1 plants were daylength neutral; and Louisiana-2 plants were short-day.
d Seed failed to germinate in the greenhouse.
was fit to the data for Louisiana-1 in 2017 and 2018, except for the July establishment timing in 2018, for which a linear model,
 $$y = {y_1} + ax$$
$$y = {y_1} + ax$$
was fit to the data. April and March establishments in 2017 and 2018, respectively, for Louisiana-2 were fit to the data using the three-parameter sigmoid model. Louisiana-2 established later in the season failed to adequately fit the three-parameter sigmoid model; therefore, the linear model was fit to the data for July-planted Louisiana-2 in 2017 and 2018. A quadratic model,
 $$y = {y_1} + ax + b{x^2}$$
$$y = {y_1} + ax + b{x^2}$$
was fit to the data for May and June establishments in 2017 and April, May, and June establishments in 2018 for Louisiana-2. In the three-parameter sigmoid model, y is the estimated plant height at the time x (GDD) after transplanting to the field, a is the maximum of the parameter, GDD50 is the GDDs to reach 50% of the final plant height, and b is the slope. For the linear and quadratic models, y is the estimated plant height; a, b, and y 1 are constants; and x is GDDs after transplanting to the field.
Results and Discussion
Maximum R. cochinchinensis height for Louisiana-1 was 57% to 169% greater than for Louisiana-2 for plants established synchronously in May or later in 2017 (Table 5). A similar trend was observed in 2018, when Louisiana-1 plants were 18% to 94% taller than Louisiana-2 plants for plants established from April to June (Table 5). Maximum height difference between biotypes was generally greater in 2017 than 2018. This result could be due to greater precipitation and cooler air temperatures reported in May and June 2017 when compared with 2018 (Table 1). This could suggest that the Louisiana-1 biotype was more tolerant than Louisiana-2 to environmental stress, like ephemeral flooding, that occurred during months with above normal precipitation, especially when plants were small. Persistent exposure to saturated soils has reduced growth and seed production of R. cochinchinensis, junglerice [Echinochloa colona (L.) Link], and A. tuberculatus (Chauhan Reference Chauhan2013; Chauhan and Johnson Reference Chauhan and Johnson2010; Sarangi et al. Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015). Sarangi et al. (Reference Sarangi, Irmak, Lindquist, Knezevic and Jhala2015) reported growth and seed production were similar between two A. tuberculatus biotypes exposed to excessive water stress from Nebraska, but the authors did not allude to biotype susceptibility or resistance to herbicides or indicate whether biotypes differed in flower initiation to increasing or decreasing day length. Differences in herbicide tolerance between Louisiana R. cochinchinensis biotypes have not been investigated, and widespread control failures have not been reported to my knowledge.
Table 5. Effect of Rottboellia cochinchinensis biotype and establishment timing on maximum height and growing degree days (GDDs) to 20-cm height and maximum height.a
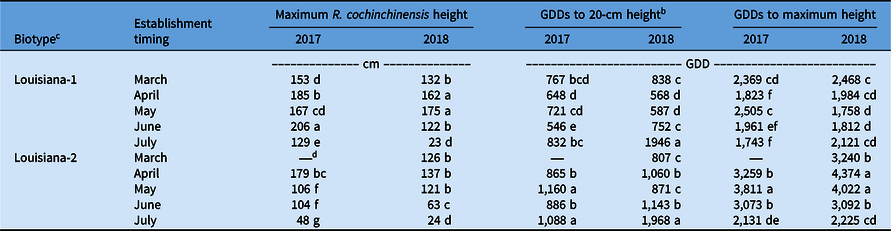
a Means presented within each column with no common letter(s) are significantly different according to an adjusted Tukey’s HSD at α ≤ 0.05.
b Cumulative growing degrees were calculated using the equation  ${\rm{GD}}{{\rm{D}}_{T{\rm{base}} = 11C}} = \mathop \sum \nolimits_{i = 1}^n \left( {{T_{{\rm{mean}}}} - {T_{{\rm{base}}}}} \right)$
, where T mean represents the daily mean air temperature in C, and T base is the minimum temperature at which R. cochinchinensis grew.
${\rm{GD}}{{\rm{D}}_{T{\rm{base}} = 11C}} = \mathop \sum \nolimits_{i = 1}^n \left( {{T_{{\rm{mean}}}} - {T_{{\rm{base}}}}} \right)$
, where T mean represents the daily mean air temperature in C, and T base is the minimum temperature at which R. cochinchinensis grew.
c Louisiana-1 plants were daylength neutral; and Louisiana-2 plants were short-day.
d Seed failed to germinate in the greenhouse.
Vegetative growth for the Louisiana-2 biotype occurred until autumn, because the biotype was photoperiod sensitive, and raceme production began when daylength shortened (Figures 1 and 2). This result was most apparent when R. cochinchinensis plants were established in May or later (Figure 2). In a greenhouse experiment, Millhollon and Burner (Reference Millhollon and Burner1993) found that the Louisiana-2 biotype flowered when the photoperiod decreased to 13 h. Because the Louisiana-1 biotype was daylength neutral, flower initiation occurred 5 to 6 wk after plants were established in the field, and plants continued to grow and produced additional racemes until late summer (Table 2; Figure 2). The Louisiana-2 biotype exhibited season-long growth and required 22% to 78% more GDDs than Louisiana-1 to reach maximum height when biotypes were established concurrently in 2017 (Table 5). Louisiana-1 March- and May-established plants needed 2,369 to 2,505 GDDs, and Louisiana-2 required 3,811 GDDs for maximum height when established in May (Table 5). Cooler air temperatures likely contributed to reduced plant growth for the March establishment timing, but the combination of cool air temperatures and excessive precipitation slowed growth of both biotypes established in May 2017 (Tables 1 and 5). Monthly climatic conditions in 2018 resembled the 30-yr average more than conditions reported in 2017, with the exception of September, which was cool and wet (Table 1). The Louisiana-1 biotype established in May 2018 required 29% fewer GDDs to reach maximum height when compared with plants established in March (Table 5). However, the Louisiana-2 biotype established in May 2018 needed 24% more GDDs to reach maximum height when compared with March-established plants. Excessive soil moisture appeared to slow R. cochinchinensis growth and may prevent timely PRE herbicide applications. The majority of Louisiana hectares under sugarcane cultivation are on poorly drained soils and, combined with a shallow water table, herbicide application may not be feasible using ground-operated equipment.

Figure 2. Influence of establishment timing on Rottboellia cochinchinensis height (% of maximum) for (A) Louisiana-1 in 2017, (B) Louisiana-1 in 2018, (C) Louisiana-2 in 2017, and (D) Louisiana-2 in 2018 at the Sugarcane Research Unit located in Houma, LA. The Louisiana-1 biotype plants were daylength neutral; and Louisiana-2 plants were short-day. Louisiana-2 seed failed to germinate in the greenhouse for the March 2017 establishment timing. Growing degree day 0 represents the time of Rottboellia cochinchinensis planting. Symbols represent percent of maximum mean height, and vertical bars represent SEs. A three-parameter sigmoid model,  $y = a/\left\{ {1 + \exp \left[ { - {\frac{{x - {\rm{GD}}{{\rm{D}}_{50}}}}\over{b}}} \right]} \right\}$
, was fit to the data for Louisiana-1 in 2017 and 2018, except for the July establishment timing in 2018, where a linear model,
$y = a/\left\{ {1 + \exp \left[ { - {\frac{{x - {\rm{GD}}{{\rm{D}}_{50}}}}\over{b}}} \right]} \right\}$
, was fit to the data for Louisiana-1 in 2017 and 2018, except for the July establishment timing in 2018, where a linear model,  $y = {y_1} + ax$
was fit to the data. The linear model was also fit to the data for July-planted Louisiana-2 in 2017 and 2018. April and March establishments in 2017 and 2018, respectively, for Louisiana-2 were fit to the data using the three-parameter sigmoid model. A quadratic model,
$y = {y_1} + ax$
was fit to the data. The linear model was also fit to the data for July-planted Louisiana-2 in 2017 and 2018. April and March establishments in 2017 and 2018, respectively, for Louisiana-2 were fit to the data using the three-parameter sigmoid model. A quadratic model,  $y = {y_1} + ax + b{x^2}$
, was fit to the data for May and June establishments in 2017 and April, May, and June establishments in 2018 for Louisiana-2.
$y = {y_1} + ax + b{x^2}$
, was fit to the data for May and June establishments in 2017 and April, May, and June establishments in 2018 for Louisiana-2.
The soil-applied herbicides pendimethalin and trifluralin are labeled for control of R. cochinchinensis in sugarcane. Asulam and trifloxysulfuron applied separately or in a mixture are POST herbicides labeled for R. cochinchinensis no larger than 20 cm; however, crop injury resulted when sensitive sugarcane cultivars were treated or exposed to soil moisture stress, disease and insect pressure, and low soil fertility (Anonymous 2020a, 2020b). GDDs to 20-cm height in 2017 ranged from 546 to 832 and 865 to 1,160 for Louisiana-1 and Louisiana-2, respectively (Table 5). Louisiana’s subtropical humid climate favors rapid plant growth that typically begins in May and persists through September. Growth to 20 cm occurred most rapidly, within approximately 5 wk based on GDDs calculated for June and July, when Louisiana-1 was established in June. Generally, it is not advantageous to treat sugarcane using trifloxysulfuron or asulam during summer months, because these herbicides may reduce sugarcane biomass, a critical component determining final sucrose yield. Slower initial growth reported with Louisiana-2 would allow more time for growers to treat emerged plants with POST herbicides, and if plants are treated when small, there is an increased likelihood of plant death from optimal spray coverage (Legleiter et al. Reference Legleiter, Young and Johnson2018). Larger R. cochinchinensis plants treated with asulam and or trifloxysulfuron are often injured less than smaller-sized plants. Decreased herbicide performance is often reported when applications are made to large (30-cm) plants when compared with smaller (10-cm) sized plants (Meyer and Norsworthy Reference Meyer and Norsworthy2019).
Raceme initiation in the present study varied by year for Louisiana-2 and began on June 23 (2017) and September 24 (2018) for plants established in April. Millhollon and Burner (Reference Millhollon and Burner1993) reported Louisiana-2 planted in April flowered in August after transplanting in the greenhouse. The variable results were a product of environmental stress that promoted flowering, including excessive rainfall (297% above 30-yr average) and/or cooler air temperatures (15% below 30-yr average) present during May 2017 (Table 1). In Nigeria, Omolaja et al. (Reference Omolaja, Aikpokpodion, Oyedeji and Vwioko2009) reported 162% more cocoa flowers in May when rainfall was abundant (86.9-mm rainfall) compared with January when precipitation was scarce (1.00mm rainfall). Big bluestem (Andropogon gerardi Vitman), a prairie tallgrass, exposed to simulated rainfall had 30% more flowering stems when compared with the control, and in one year plants failed to flower when irrigation was not implemented (Knapp Reference Knapp1984). Chauhan (Reference Chauhan2013) investigated the effect of soil moisture level on R. cochinchinensis seed production in the greenhouse and reported soils at 50% to 100% field capacity produced more than 450 seeds plant−1 compared with fewer than 100 seeds plant−1 in soils at 25% field capacity or less.
Spring R. cochinchinensis control is necessary for reduced crop competition and to minimize additional soil seedbank recruitment. Total raceme production per plant increased when R. cochinchinensis were established earlier in the season during both years for Louisiana-1, with the fewest produced in July. Furthermore, both Louisiana biotypes established in July generally produced less biomass than earlier-established plants. Seed recruitment by autumn was not observed in 2017 when Louisiana-2 was established in May, and in 2018 only 1 raceme plant−1 was produced from April-established plants (Table 6). This result suggests season-long R. cochinchinensis competition with sugarcane occurs in fields infested with Louisiana-2, because plants emerging after April were unlikely to replenish the soil seedbank for the biotype to exist in the subsequent growing season.
Table 6. Effect of Rottboellia cochinchinensis biotype by establishment timing interaction on racemes produced and dry weight in a field study conducted in 2017 and 2018 at the Sugarcane Research Unit in Houma, LA.a

a Means presented within each column with no common letter(s) aresignificantly different according to an adjusted Tukey’s HSD at α ≤ 0.05. Mean separation for R. cochinchinensis racemes and dry weight is from square-root transformed data, presented means are from back-transformed data.
b Racemes were removed from the plant upon seed drop. Total raceme production represented the period from establishment timing until autumn each year.
c Louisiana-1 plants were daylength neutral; and Louisiana-2 plants were short-day.
d Seed failed to germinate in the greenhouse.
The present study demonstrated the importance of managing the Louisiana-2 biotype in March and April, but fields infested with the Louisiana-1 biotype were at greater risk for potential crop yield loss, because plants produced high quantities of seed when established over a wider period of time (March to June). Infestations warrant a zero-tolerance approach to limit crop competition and seedbank replenishment. Both biotypes infesting the same field have been observed on very few hectares. Biotype awareness is needed due to rapid initial growth of Louisiana-1 when compared with Louisiana-2, which may compromise POST herbicide performance, especially when the two biotypes coexist. Proliferation of R. cochinchinensis throughout the sugarcane belt and the limited number of newly released herbicides that control R. cochinchinensis should be alarming, considering that repeated applications of pendimethalin and trifluralin herbicides have been applied to manage the weed for several decades. Control failures rapidly replenish the soil seedbank due to high fecundity, especially in environments exhibiting high annual rainfall, such as Louisiana. This is especially problematic for Louisiana sugarcane producers where ephemeral flooding can limit field operations and timely herbicide application. The majority of Louisiana fields under sugarcane cultivation are precision graded for improved water drainage. Oftentimes, weed seed and crop residues from higher elevations are carried toward low-lying areas and remain after floodwater subsides (Skoglund Reference Skoglund1990). Few R. cochinchinensis seedlings (<5%) emerged from 8 cm below the soil surface, but 80% emerged at the soil surface 18 d after sowing (Bolfrey-Arku et al. Reference Bolfrey-Arku, Chauhan and Johnson2011). Rottboellia cochinchinensis seed deposited on the soil surface and subsequently covered with 2,300 to 9,100 kg ha−1 of postharvest residue reduced R. cochinchinensis density no more than 10% when compared with seeds on the soil surface without postharvest residue cover (Correia et al. Reference Correia, Gomes and Perussi2013). Burning postharvest crop residue by January not only reduced R. cochinchinensis seedling emergence at the soil surface 68% to 97%, but also increased ratoon sugarcane sucrose yield by 12% when compared with mulching the residue (Spaunhorst et al. Reference Spaunhorst, Orgeron and White2019; Viator et al. Reference Viator, Johnson and Richard2005). Postharvest residue from sugarcane harvested before burning, referred to as “green cane harvesting,” is deposited on the soil surface and “blankets” the soil surface with several centimeters of postharvest residue that is separated from billets by the extractor fan on the chopper harvester. The residue layer traps warm air and protects the stool during severe freezes (−4 C), but the opposite effect results (trapping of cool air) in springtime when the crop reemerges from winter dormancy. The reduction in sucrose yield often reported with postharvest residue retention was likely influenced by microclimatic factors and possible allelochemical uptake from postharvest residue retention that slowed sugarcane regrowth.
Acknowledgments
The authors thank Eric Petrie, Norris Matherne, Kader Wilson, and Blake Landry for their technical assistance in conducting this research. USDA is an equal opportunity provider and employer. No conflicts of interest have been declared. This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.













