INTRODUCTION
The major goal achieved by the 1992 Rio Earth Summit was that it drew international concern to issues of global biological diversity and transformed this concept into a matter of public awareness and into an important component of the political debate (Magurran, Reference Magurran2004). One of the implied challenges was to determine the extent to which changes in biodiversity may induce reduction of the potential of ecosystems to perform well and to provide the citizens of the world with products and services (Worm et al., Reference Worm, Barbier, Beaumont, Emmett Duffy, Folke, Halpern, Jackson, Lotze, Micheli, Palumbi, Sala, Selkoe, Stachowicz and Watson2006). The latter may have severe consequences since it can be translated into several trillions of dollars, lost forever (e.g. Costanza et al., Reference Costanza, d'Arge, de Groot, Farberk, Grasso, Hannon, Limburg, Naeem, O'Neill, Paruelo, Raskin, Suttonkk and van den Belt1997). Recently, scientific concerns about biological diversity have been brought to the forefront in the area of biodiversity–ecosystem functioning, in an attempt to challenge fundamental issues such as the relationship between the decline in biological diversity and the functioning and stability of ecosystems (e.g. Loreau et al., Reference Loreau, Naeem and Inchausti2002). The introduction of approaches capable of successfully addressing large-scale patterns relevant to the complexity of the ecosystem and to socio-economic issues (Rafaelli, Reference Rafaelli2006) has become another issue in the current agenda, useful for the management and conservation of marine ecosystems, in particular those which receive major anthropogenic impacts such as the coastal ones.
Perhaps, the major effect of the Rio Summit was that it changed the scientists' view of the biomes on Earth by forcing them to think on multiple scales, either concerning biological organization (e.g. genes, species and ecosystems) or geographical (or any other type) observations (e.g. from local to global) in which alterations may occur. These changes in focus helped scientists to take the logical step towards the development of new methodologies using information deriving from many of the previously-mentioned levels or scales. The standard procedure followed until recently was to take and explore information only from a single level or scale or even from several but exploring their results separately. This practice has only partially changed through the development of the taxonomic relatedness concept which makes use of the information at taxonomic (phylogenetic) levels higher than the species.
The theory, on the other hand, predicts that identification of organisms to the species level may not always be necessary to describe spatial patterns, especially when patterns are strong such as along established pollution gradients (Pearson & Rosenberg, Reference Pearson and Rosenberg1978; Boesch & Rosenberg, Reference Boesch, Rosenberg, Barrett and Rosenberg1981; Warwick, Reference Warwick1988; Ferraro & Cole, Reference Ferraro and Cole1990; Olsgard et al., Reference Olsgard, Somerfield and Carr1997, Reference Olsgard, Somerfield and Carr1998). Therefore, multivariate patterns deriving from higher phylogenetic/taxonomic categories may reflect these gradients more closely than those based on species abundances, which are considered to be more affected by natural variability (the ‘hierarchic-response-to-stress’ hypothesis). Consequently, the taxonomic level most highly correlated with environmental variables indicative of anthropogenic impacts will increase with increasing levels of stress (Olsgard et al., Reference Olsgard, Somerfield and Carr1997, Reference Olsgard, Somerfield and Carr1998; Olsgard & Somerfield, Reference Olsgard and Somerfield2000). Comparisons of patterns derived from the species level with those from higher taxonomic categories show that patterns are more closely related in polluted areas than in pristine ones (Olsgard et al., Reference Olsgard, Somerfield and Carr1998; Olsgard & Somerfield, Reference Olsgard and Somerfield2000). However, this approach was never applied on different components of the ecosystem, reflecting different levels of its function such as primary production, primary and secondary consumption, and facilitating decomposition of the organic material.
One of the most challenging habitats for the experimental application of these approaches is the coastal lagoons. Coastal lagoons are characterized by a limited range of species, compared with other marine habitats. This low diversity is induced by the naturally severe fluctuations in their important environmental variables which act as a filter against the establishment of many populations (Gray, Reference Gray1974). Low values of diversity, coupled with a few highly tolerant and dominant species are, thus, the common community trend (Guelorget & Michel, Reference Guelorget and Michel1979; Nicolaidou et al., Reference Nicolaidou, Bourgoutzani, Zenetos, Guelroget and Perthuisot1988; Arias & Drake, Reference Arias and Drake1994; Reizopoulou et al., Reference Reizopoulou, Thessalou-Legaki and Nicolaidou1996).
A suite of methods for the assessment of the effects of disturbance on species assemblages and especially those of the brackish-water ecosystem has been developed over the past years, based primarily on: (i) univariate and multivariate techniques applied to all benthic and pelagic communities (Clarke & Green, Reference Clarke and Green1988; Clarke & Warwick, Reference Clarke and Warwick1994); (ii) taxonomic relatedness (applied to macro- and meiobenthic communities; Warwick & Clarke, Reference Warwick and Clarke2001); (iii) body size or size spectra (e.g. Holling, Reference Holling1992; Robson et al., Reference Robson, Barmuta and Fairweather2005); (iv) biomass distribution among functional groups (e.g. Pearson, Reference Pearson2001; Gerino et al., Reference Gerino, Stora, Francois-Carcaillet, Gilbert, Poggiale, Mermillod-Blondin, Desrosiers and Vervier2003); (v) functional diversity (e.g. Diaz & Cabido, Reference Diaz and Cabido2001; Petchey & Gaston, Reference Petchey and Gaston2002; Bremner et al., Reference Bremner, Rogers and Frid2003); and (vi) biomass or productivity measures (e.g. Pearson & Rosenberg, Reference Pearson and Rosenberg1978). Recently, Mouillot et al. (Reference Mouillot, Dumay and Tomasini2007) have developed a methodology for the testing of the niche filtering hypothesis, with promising results in two Mediterranean lagoons. Additionally, Bremner (Reference Bremner2008) has reviewed biological traits analysis in benthic communities and its potential use in conservation and management. Almost all of the above methods have been applied to coastal Mediterranean lagoons with a varying degree of success (e.g. Basset et al., Reference Basset, Galuppo and Sabetta2006; Reizopoulou & Nicolaidou, Reference Reizopoulou and Nicolaidou2007).
Therefore, the present study makes use of information patterns derived from four benthic components of the lagoonal habitat: macrophytes, macrozoobenthos, megafaunal decapods and demersal fish. The main idea behind this approach is to explore the potential for change in the interrelationships between these four components as indicative of the degree of disturbance. As a measure of these interrelationships we use the inter-distance (/resemblance) values between the components as calculated by standard resemblance coefficients. The multivariate pattern comparisons are based on existing methodology, namely the second-stage non-metric multidimensional scaling (MDS), as suggested by Clarke et al. (Reference Clarke, Somerfield, Airoldi and Warwick2006a). The basic assumption in this approach comes from the Pearson & Rosenberg (Reference Pearson and Rosenberg1978) model along a stress gradient: disturbance is a pressure that induces homogeneity in the structure of communities through the selection of closely related species, tolerant enough to respond to the increased levels of stress. This environmental structuring (filtering) inevitably leads the brackish-water communities to be composed of species rather similar also in terms of their functioning traits. We assume, therefore, that these inter-component relationships are indicative of their functioning potential. Consequently, the hypothesis which is tested by this mathematical experiment is that changes in the relationships between the multivariate patterns of the benthic components may mirror the degree of environmental stress imposed on their corresponding communities. The pattern anticipated is that inter-component dissimilarities would be significantly lower in the stations experiencing more stress than in those with less stress, mirroring the ‘hierarchical-response-to-stress’ hypothesis (Olsgard et al., Reference Olsgard, Somerfield and Carr1998): stations with more stress impose rigorous environmental structuring on the organisms allowing only those tolerant species to dominate the substrates and forcing all benthic components to show a similar pattern throughout the year. Conversely, in the stations with less stress, components are anticipated to be richer in species with a more stochastic fluctuation in their abundance or biomass values, hence larger distances should be anticipated among them.
MATERIALS AND METHODS
Study area
The Evros Delta is located at the north-eastern part of the Aegean Sea (Figure 1). Fresh water reaches the delta area from the eastern branch of the Evros River and also, usually from late autumn through to early summer, from the western branch of the Evros River and associated streams. Three islets and three lagoons are formed in the delta area. Laki Lagoon, with an area of about 1.0 km2, communicates directly with the sea through two openings. Monolimni (or Paloukia) Lagoon, with an area of about 1.12 km2, communicates with the sea mainly through a 15 m wide opening. Drana Lagoon covers an area of 2.2 km2. In the past, Drana Lagoon was connected to Laki lagoon by a narrow opening 4 m wide and, through that opening, to the sea. In order to drain the lagoon in 1987, its opening had been closed and its indirect communication with the sea had ceased. During the sampling period, Drana Lagoon dried out from late August to the end of September 1998. Therefore, their connection to the sea as the major factor affecting the benthic components was studied. The sampling sites were selected for their different state of sea connectivity that reflects a different stress gradient (see next sub-section).

Fig. 1. Map indicating stations of the Mediterranean lagoonal systems taken into account in the present study.
Sampling and laboratory techniques
Four ecosystem components, representative of the major trophic levels and of their associated functions at the macro-scale (individuals retained between 0.5 mm and 2 mm), were sampled: (a) macrophytes, as primary producers; (b) macrozoobenthos as primary and secondary consumers; (c) fish, primarily as secondary consumers or top predators for the benthic system; and (d) decapods as ‘benthic cleaners’, or scavengers.
Samples were collected monthly from February 1998 to February 1999 at Station A1 (40o48′59″N and 26o00′39″E, 0.5 m deep) located in the innermost part of Laki Lagoon, Station I1 (40o46′12″N and 26o03′12″E, 0.85 m deep) located in the outer part of Monolimni Lagoon, Station B2 (40o46′41″N and 26o03′42″E, 0.5 m deep) located in the innermost part of Monolimni Lagoon and Station C (40o48′26″N and 26o01′22″E, 0.65 m deep) located in Drana Lagoon (Figure 1). The sampling stations of Drana (C) and the inner part of Monolimni lagoon (B2) experience greater degrees of stress than the other two stations. Drana had an additional stress (because of the periodical drainage) than B2 with the limited communication with the sea. At each station four replicates samples of: (a) macroalgae using a 25 × 25 cm frame; (b) submerged angiosperms using a 20 cm diameter corer penetrating to a depth of 20 cm; (c) zoobenthos using a modified Van Veen grab covering a 400 cm2 surface (20 × 20 cm, penetrating to a depth of 20 cm); and (d) epibenthic decapods and demersal fish using a special net with a 40 × 40 cm opening towed on the bottom surface for a distance of 10 m, were collected. Macrozoobenthic samples were sieved through a 0.5 mm screen. Individuals were identified to the lowest possible taxon and counted. Angiosperm material was partitioned into rhizomes plus stems, leaves, roots and reproductive organs. Macrophytes, after being dried for five days at 70oC, were weighed to the nearest 0.1 mg (dry-weight biomass).
Sediment samples were taken with a small corer for particle size analysis and estimation of the organic matter content. Depth, salinity, temperature, dissolved O2 and pH of the water near the bottom were also measured. Sediment analysis and estimation of organic matter were determined according to the methods described by Buchanan (Reference Buchanan, Holme and McIntyre1984).
Data and data analyses
A part of the data derived from the above-described sampling plan was also used in some other papers (Kevrekidis, Reference Kevrekidis2004; Malea et al., Reference Malea, Kevrekidis and Mogias2004; Mogias & Kevrekidis, Reference Mogias and Kevrekidis2005). As for this study dry-weight biomass values for macrophytes and abundance values for the remaining components were averaged at each station and month (sampling period) and then extrapolated as per square metre. Subsequently, four species-by-monthly abundance (biomass) matrices were constructed for each of the four sampling stations, corresponding to the four benthic components sampled. Consequently, sixteen (4 stations × 4 components) matrices were totally constructed. Dry-weight biomass was considered to be the closest approximation for the measurement of the macrophyte multivariate pattern since their abundance or the surface covered by angiosperm fractions could not be adequately determined. In addition, since the focus of the comparisons is the multivariate pattern and not the absolute values of the community measures, this approximation for the macrophytes is not expected to greatly influence the results deriving from the methodology described below.
Non-metric multidimensional scaling was performed on resemblance matrices calculated using Bray–Curtis similarity coefficient (Clarke et al., Reference Clarke, Somerfield and Chapman2006b) on the quantitative data (standardized and fourth-root transformed) of the monthly samples from each component. Stress of 2-dimensional plots was measured by Kruskal's stress formula I (Clarke & Green, Reference Clarke and Green1988). Comparisons in pattern deriving from different benthic components in the four different lagoonal stations were assessed by means of second-stage MDS (Somerfield & Clarke, Reference Somerfield and Clarke1995; Clarke et al., Reference Clarke, Somerfield, Airoldi and Warwick2006a). According to the procedure described a Spearman's rank correlation coefficient was used to estimate the relationship between their corresponding elements. Thus, two kinds of second-stage resemblance matrices were constructed: (a) one matrix for each sampling station (4 × 4 components) in order to display interrelationships between patterns derived from different benthic components within the stations; and (b) an overall matrix including all multivariate patterns derived from all components in all lagoonal stations (16 × 16 components) in order to display component interrelationships both within and between the stations. These second-stage resemblance matrices were further processed by MDS (second-stage MDS) in which benthic components showing similar changes in pattern group together. The rationale and robustness of the application of the second-stage MDS in multi-factorial experiments has been discussed in detail by Clarke et al. (Reference Clarke, Somerfield, Airoldi and Warwick2006a).
The hypothesis tested is whether the multivariate patterns derived from the benthic inter-components in the stations experiencing more stress, that is the stations in the inner parts of the lagoons (C and B2), are more closely connected to each other than those from the stations receiving less environmental stress (A1 and I1), in compliance with the ‘hierarchical-response-to-stress’ hypothesis (Olsgard et al., Reference Olsgard, Somerfield and Carr1998).
Two non-parametric tests were applied to check for homogeneity among multivariate patterns deriving from different benthic components in the sampling stations: analysis of similarities (ANOSIM, termed as second-stage ANOSIM by Clarke et al., Reference Clarke, Somerfield, Airoldi and Warwick2006a) and the Mann–Whitney test (Mann & Whitney, Reference Mann and Whitney1947; Sokal & Rohlf, Reference Sokal and Rohlf1981), both based on ranks rather than on absolute values. The former test takes into account all differences in distances between every possible pair of benthic components, both within and between the sampling stations. The latter, on the other hand, may well focus on selected pattern dissimilarities within the sampling stations and, based on these, to compare the second-stage patterns between the sampling stations. Therefore, it was possible with this test to compare only those dissimilarities in multivariate patterns which reflect the functional hierarchy in the benthic components: macrophytes to macrobenthos, macrobenthos to decapod scavengers, decapod scavengers to fish and, ultimately, fish to macrophytes, as depicted in Figures 2 & 3.

Fig. 2. Second-stage non-metric multidimensional scaling plot comparing benthic components within the lagoons. Letters representing sampling stations as in Figure 1.

Fig. 3. Second-stage non-metric multidimensional scaling plot comparing benthic components within and between the lagoons. Letters representing sampling stations as in Figure 1.
The PRIMER software (v. 6.1, Clarke & Warwick, Reference Clarke and Warwick1994) was used for the analysis.
RESULTS
Monthly variation in assemblage structure
The main characteristics of the faunal and floral communities are described in the following paragraphs, station by station. However, since the focus of the study lies in the second-stage multivariate pattern (comparisons), no cluster dendrograms or MDS plots deriving from the ‘first-stage’ resemblance matrices for each of the components at each sampling station are shown.
STATION A1
Station A1, located in the innermost part of Laki Lagoon, had a better connection to the sea than the Stations C and B2 and as a result the biotic components of this lagoon received the lowest stress from those of the remainder stations studied. A total of fifteen seaweed taxa were collected in this station during the sampling period. Macroalgae occurred throughout the annual cycle, except in March. The green seaweed Ulva rigida C. Agardh, 1823 and the red seaweed Gracilaria bursa-pastoris (S.G. Gmelin) P.C. Silva, 1952 were the most frequent and dominant. March samples were characterized by the absence of seaweed species, February and April by the absence of U. rigida while in August and September, samples were mainly characterized by the absence of G. bursa-pastoris. Sixteen macrozoobenthic taxa were collected in Station A1. The amphipod Corophium orientale Schellenberg, 1928 dominated the assemblage (mean monthly dominance of 54%). This amphipod, the polychaetes Steblospio shrubsolii (Buchanan, 1890) and Hediste diversicolor (O.F. Müller, 1776) and the bivalve Abra segmentum (Recluz, 1843) were always present, while four other taxa (Tubificidae, Ventrosia maritima (Milaschewitsch, 1916), Gammarus aequicauda (Martynov, 1931) and Cerastoderma glaucum (Poiret, 1789)) were frequently found. The macrozoobenthic community structure showed a clear seasonal periodicity, which was mainly characterized by seasonal variation in the density of the same species: it changed evenly from late winter–early spring to mid- and late spring, early and mid-summer, late summer and early autumn and mid- autumn and late autumn and winter. Four epibenthic decapod species were found in Station A1. The shrimp Crangon crangon (Linnaeus, 1758) was the dominant and the most frequently found decapod followed by the crab Carcinus aestuarii Nardo, 1847. Crangon crangon was collected from mid-spring to late winter and C. aestuarii during mid-spring to early winter, both having their highest abundances in early summer. One or two other decapod species were also found in July and November, correspondingly, while no decapods were collected in February–March 1998. Five demersal fish species were also found in Station A1. The goby Knipowitschia caucasica Kawrajsky, in Berg, 1916 was the dominant and the most frequently observed species; it was collected from late spring to late winter. The other four fish species were occasionally collected during summer and autumn, while no fish species were found from February to April 1998.
Epibenthic decapods were placed in the left part of the second-stage MDS plot close to macrophytes (Figure 2) indicating a similar monthly variation in their assemblages structure. The small fish component was placed in the upper part of the plot while macrozoobenthos was placed in the right bottom part.
STATION I1
Station I1 is located in the outer part of Monolimi Lagoon and resembles Station A1 in that its biotic components receive much less stress than those of the Stations C and B2. Sixteen seaweed taxa occurred in Station I1 during July–February 1999, mainly from August to November. Ulva prolifera O.F. Müller, 1778 and Polysiphonia sp. were the most frequently found macroalgae during this period, while no seaweed species were collected from February to June 1998. The seaweed assemblage structure showed a circular variation from February–June to July 1998 and from July to January 1999. Fifteen macrozoobenthic taxa were collected in Station I1 during the sampling period. Seven macrozoobenthic taxa (V. maritima, C. orientale, A. segmentum, S. shrubsolii, H. diversicolor, G. aequicauda and Cumacea) were always present; five other taxa were less frequently found. The amphipod C. orientale and the gastropod V. maritima dominated the assemblage (mean monthly cumulative dominance of 77.7%). The variation in macrozoobenthic community structure showed a seasonal trend: it changed evenly from late winter–early spring to mid-spring, late spring–summer–early autumn and mid- and late autumn–winter. No decapod species were found in March and December 1998. The goby K. caucasica was the dominant fish; it was collected in early spring and from early summer onwards, showing its highest abundance in late summer. Sygnathus acus Linnaeus, 1758 was also collected during summer and autumn, while no small fish were found in late winter 1998 and in mid- and late spring of the same year.
When compared with each other, patterns deriving from macrophytes, from zoobenthos and from decapods were arranged along the angles of an almost equilateral triangle whereas the pattern deriving from fish was placed in the median position of the triangular surface.
STATION B2
Station B2 is located in the innermost part of Monolimni Lagoon and its benthic components experience a much larger environmental stress than those of Station I1 because it is isolated from the opening to the sea. The macrophyte composition in this part of Monolimni Lagoon consisted mainly of a perennial population of the submerged angiosperm Ruppia maritima Linnaeus, 1753. This population grew from April to October 1998; leaf and rhizome plus stem biomass increased progressively from April to October, root biomass peaked in June and October, while reproductive organs were observed in summer. In addition, five seaweed species occurred occasionally; the most abundant of them are the red macroalga Gelidium pusillum (Stackhouse) Le Jolis, 1863 collected during late autumn to early spring and the green alga U. rigida collected from late spring to early autumn were respectively the most frequent seaweed species. The monthly variation in macrophyte community structure showed a clear seasonal trend; it changed evenly from late autumn, winter and early spring to mid-spring and to late spring, summer and autumn. Fifteen macrozoobenthic taxa were collected in Station B2. Nine taxa (V. maritima, C. orientale, A. segmentum, S. shrubsolii, H. diversicolor, G. aequicauda, C. glaucum, Tubificidae and Chironomidae larvae) were always found, the two latter of fresh water origin. The gastropod V. maritima, the amphipod C. orientale and the bivalve A. segmentum were top dominants (mean monthly dominance of 78.3%).
Macrozoobenthic community structure showed a clear seasonal trend; it changed evenly from late winter to early–mid-spring, late spring–early summer and mid-summer–autumn–winter. Two epibenthic decapod species occurred in Station B2. The shrimp C. crangon was found during May–June and during August–December with its highest abundances in autumn; the crab C. aestuarii was collected during May to October with its highest abundances in late spring–early autumn, while no decapods were found during mid- and late winter and early and mid-spring. Three small fish species occurred in Station B2. The goby K. caucasica was found throughout the annual cycle, except for April, showing its highest abundances in late spring–early summer and in autumn. Sygnathus acus was collected mainly during summer and autumn showing its highest abundances in autumn. Aphanius fasciatus Nardo, 1927 was occasionally collected (August–September and December).
Epibenthic decapods were placed at the left-part of the second-stage MDS plot very close to macrophytes indicating a similar monthly variation in their assemblages' structure, while macrozoobenthos and small fish were placed at the right-part of the plot (Figure 2).
STATION C
Station C is located in Drana Lagoon. In September, the major part of the lagoon, including Station C, was drained for a period of three weeks hence it experienced the largest environmental stress. Vegetation was composed of an annual population of R. maritima and of the annual angiosperm Zannichellia palustris Linnaeus, 1753 to a lesser degree. Ruppia maritima was observed from March to August. Seedlings were observed in March and April. Biomass of most plant parts (leaves, rhizomes plus stems and reproductive organs) increased almost continuously from June to August; root biomass peaked in April and June. Zannichellia palustris was observed from May to August; leaf and rhizome plus stem biomass peaked in July, root biomass in May. Both angiosperms died leaving seeds as the habitat temporarily dried. Macrophyte community structure showed a clear seasonal trend: it changed evenly from early and mid-spring to late spring–summer and autumn–winter. Eleven macrozoobenthic taxa were collected in Station C. Brackish water taxa of freshwater ancestry (Chironomidae larvae and Tubificidae) were also included in the macrozoobenthic samples. The mudsnail V. maritima, Chironomidae larvae, the amphipod G. aequicauda and the polychaete H. diversicolor dominated the assemblage. No macrozoobenthic taxon was found in September when the habitat temporarily dried. Only one epibenthic decapod species (C. crangon) was collected in June and two demersal fish species (K. caucasica and A. fasciatus) occurred in Station C. No fish species were found in late winter–early spring 1998 as well as during and immediately after the drainage of Drana Lagoon (September–October). The goby K. caucasica was found throughout the remaining sampling period having its highest abundances during late spring and summer, while A. fasciatus was collected during summer, late autumn and early winter.
Epibenthic decapods were placed in the left part of the second-stage MDS plot very close to macrophytes, fish in the upper and macrozoobenthos in the right part of the plot in Figure 2.
Associated environmental variables
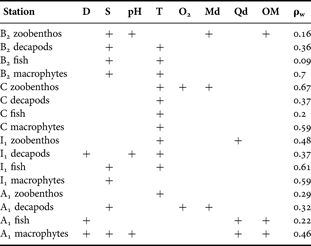
Salinity and temperature were the most frequently associated environmental variables with the patterns of the different benthic components in all four stations (Table 1) and those which reached the highest level of the values in the Spearman's coefficient (0.7). Depth and sediment characteristics were equally frequently correlated with the biotic patterns, in some cases with very low correlation values. However, oxygen availability, although less frequently associated with the biotic patterns, was better correlated with the pattern of zoobenthos in Station C.
Table 1. Results from the BIOENV analysis between patterns deriving from the benthic components and those deriving from the environmental variables.
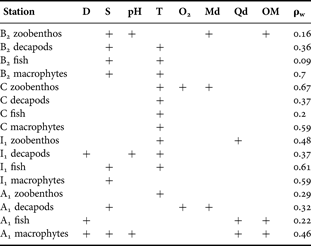
D, depth (m); S, salinity; T, water column temperature (°C); Md, median particle diameter; Qd, particle quartile deviation; OM, sediment organic matter (%); ρw, weighted Spearman's coefficient.
The higher stress status of the biotic components in Stations B2 and C is reflected in the relatively higher correlation of the macrophytes (Station B2) and of the zoobenthos (Station C) with the environmental variables measured (Spearman's coefficient of 0.7). However, strong correlation (e.g. values of 0.9 or higher) was not achieved by any of the above cases, implying that these results may be considered only as indicative. Finally, in the stations with a lower degree of stress (Stations A1 and I1) the biotic patterns were less correlated with the abiotic ones.
Second-stage analysis of the inter-component relations
The examined four benthic components of the innermost part of Monolimni Lagoon (Station B2) as well as those of the Drana Lagoon (Station C), which is the most isolated from the sea, were placed in the second-stage MDS plot (Figure 3) closer to each other than the components of Laki lagoon (Station A1) and of the outer station of Monolimni Lagoon (Station I1) which are characterized by the proximate contact with the sea. The monthly variations in the structure of the examined benthic assemblages in Station B2 were characterized by a clear seasonal periodicity mainly due to the fluctuations in the abundance of the same species, while Station C was noticeably affected by the temporary drainage of the habitat (Figure 3). In the remaining stations (A1 and I1) the distances between the benthic components were much larger.
The mutlivariate pattern of the four benthic components on the second-stage MDS plot was not the same for each of them. Macrophytes from the different stations were placed remote from each other. The macrophytes from Station B2 showed the smaller distance from the others. For the fish pattern, Stations A1 and B2 were placed closer together with Station C to show the larger distance from the others. In the pattern of the epibenthic decapods, Stations B2 and C which are characterized by higher isolation of the sea were placed close together, while Station A1 which had the highest contact with the sea had the larger distance from them. The pattern of the macrozoobenthos was similar to the one of the decapods, but Stations B2 and I1 were placed close to each other.
The ANOSIM test revealed no differences in the inter-component dissimilarities. ANOSIM resulted in a global R value of –0.09 (significance level: 13.8%). Consequently, the null hypothesis cannot be rejected, hence all stations were considered as homogeneous in terms of benthic inter-components mutlivariate patterns.
The Mann–Whitney test which compared only the selected distances between the benthic components at each lagoon, however, gave significant results: U = 11, P < 0.03. These results suggest rejection of the null hypothesis. Consequently, the stations experiencing more environmental stress are significantly different from those which are located near the sea, in terms of benthic inter-component multivariate patterns when only (dis)similarities in their relative hierarchy is taken into account.
DISCUSSION
Intra-lagoonal comparisons
The macrophyte community pattern was closer to the one deriving from the decapod one and farther from those coming from benthos and fish in the Laki sampling station (A1). Closely related trends in the former two benthic components could clearly be attributed to their congruent monthly distribution patterns: no seaweed species occurred during March whereas February and April were without Ulva rigida, a trend which is well associated with the absence of decapods in February 1998 and March samples. This finding could be attributed to the fact that the presence of algal cover may offer decapods a refuge from fish and bird predation and/or a high food availability, namely high numbers of prey populating seaweed communities (e.g. gammarids) (Cottiglia et al., Reference Cottiglia, Tagliasacchi, Masala and Serra1983; Möller et al., Reference Möller, Pihl and Rosenberg1985; Arias & Drake, Reference Arias and Drake1994). In Stations B2 and C, the most isolated ones, the inter-component relationships showed a similar configuration with zoobenthos and demersal fish located at varying distances from the other two components. This common trend is mostly owed to the patterns deriving from the occurrence and abundance of the decapods and macrophytes in these stations. However, vegetation was present in Station C over a much shorter period than in the other three stations because of its temporal drainage in September which, nevertheless, appears to have consequences for much longer periods. The absence of angiosperms in Station C and of macroalgae in Station B2 was not synchronized. Taking into account the fact that the above stations are located in the innermost parts of the lagoons, then the similar functioning which may be concluded from their relative closer placement in the MDS plots may derive from the high degree of disturbance they encounter. The latter simply means that the high disturbance level has a selective power in favouring only species with a high degree of tolerance to be present or even abundant during the same time periods.
In the case of Station I1, inter-component distances were more even, indicating a rather specific pattern in each of them. This result may well be explained by the proximity of the station to the sea which plays a major role in providing the lagoonal environment with species larvae and most importantly of labile organic resources.
Therefore, for these lagoonal systems it seems that their communication with the open sea is a very important factor affecting the functioning of the benthic components. The environmental stress resulting from the low connectivity with the sea can act as an ecological filter reducing the species diversity and affecting the functioning of the benthic components.
Inter-lagoonal comparisons
The trends observed during this study show that the similarity in the monthly variation in the structure of the assemblages of the main benthic components may be directly linked to the degree of communication with the sea. In systems with a limited communication or no communication at all with the sea, the monthly variations in the structure of the assemblage of main components show higher degrees of similarity. The latter is determined by a clear seasonal periodicity which is mainly derived from the life cycle of the constant brackish-water species. This trend has been visualized on the second-stage MDS plots, as shorter inter-component distances whereas the corresponding distances in the less disturbed stations were much larger, indicating a rather component-specific multivariate pattern. Inter-component patterns detected in each of the second-stage plots for each station were also kept in the overall second-stage MDS plot.
The ANOSIM, which was applied to test for differences in these multivariate patterns, showed that the inter-component relationships do not differ significantly among all stations. This test, however, cannot concentrate on the dissimilarities between the hierarchical levels to which the benthic components compared belong to. ANOSIM, by nature, compares all dissimilarities between the components taken into account.
The Mann–Whitney test, on the other hand, rejected the null hypothesis and indicated that the inter-component relationships were significantly different in the stations with greater environmental stress from those with direct communication with the open sea. These differences, however, were only significant when dissimilarities were compared between the hierarchal levels of the components taken into account, reflecting the ‘hierarchal-response-to-stress’ hypothesis (Olsgard et al., Reference Olsgard, Somerfield and Carr1998).
The results from the BIOENV analysis offer an additional indication that the degree of disturbance is, indeed, reflected in the Spearman's values since species assemblage multivariate patterns deriving from fish and macrophytes in Station I1 (outermost) are less correlated with the environmental variables than those from the macrophytes in Station B2 (innermost). This finding, although of purely indicative value (no strong correlation found), is in agreement with the general expected trend according to the theory of Olsgard et al. (Reference Olsgard, Somerfield and Carr1998) who state that in the most impacted environments species and environmental variable patterns appear to be much closer to each other than in less impacted or totally undisturbed environments.
However, highly fluctuating factors such as salinity and temperature are responsible for this homogeneity in multivariate patterns among the benthic components in all lagoonal stations encountered in this study. This is in accordance with the environmental structuring mechanism as suggested by Schimper (Reference Schimper1903) and Tofts & Silvertown (Reference Tofts and Silvertown2000). Therefore, although the latter may hold true for the benthic components used in this study, it is probably not the case for all ecosystem components in the lagoonal systems.
ACKNOWLEDGEMENTS
This study forms part of the synergy in the core scientific biodiversity programmes of the Institute of Marine Biology and Genetics (Hellenic Centre for Marine Research), the Laboratory of Evironmental Research and Education (Democritus University of Thrace) and the Institute of Botany (Aristotle University of Thessaloniki). The authors are much indebted to Professor A Eleftheriou and Mrs M. Eleftheriou for the critical reading of the manuscript. Many thanks go to Ms T. Boubonari and Ms V. Kalpia for sampling and laboratory assistance. The authors acknowledge support by the MarBEF EU funded Network of Excellence (contract No. GOCE-CT-2003-505446). Support was also received from the Greek National Project on Marine Biodiversity (GSRT) (contract No. 64667) and from the Research Bureau of the Democritus University of Thrace.