Adverse childhood experiences (ACEs), such as maltreatment (e.g., physical abuse), parent incarceration, and poverty, negatively impact development (e.g., Brown & Schillington, Reference Brown and Schillington2017; Cicchetti, Reference Cicchetti2013; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998) and tend to co-occur (Anda et al., Reference Anda, Croft, Felitti, Nordenberg, Giles, Williamson and Giovino1999; Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003). To best understand the impact of adversity in childhood, it is essential to consider this co-occurrence (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016). Empirical evidence supports an association between cumulative ACEs and maladaptive outcomes across the life span, including adolescence. For example, ACEs are associated with neurobiological impairments (e.g., Belsky & de Hann, Reference Belsky and de Haan2011; McEwen, Reference McEwen2017; Teicher, Samson, Anderson, & Ohashi, Reference Teicher, Samson, Anderson and Ohashi2016), externalizing behavior (Brown & Shillington, Reference Brown and Schillington2017), and substance use (SU; Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003). Adversity in childhood has also been shown to exert a negative and pervasive impact on brain development (e.g., Belsky & de Haan, Reference Belsky and de Haan2011; Lupien, McEwwn, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009; McEwen, Reference McEwen2017; Fox, Levitt, & Nelson, Reference Fox, Levitt and Nelson2010). Studies examining how ACEs “get under the skin” identify allostatic load as the mechanism driving the effects of early adversity on brain development. Allostatic load refers to “the strain on the body produced by repeated ups and downs of physiologic response, as well as by the elevated activity of physiologic systems under challenge, and changes in metabolism … that predispose the organism to disease” (McEwen & Stellar, Reference McEwen and Stellar1993, p. 2094). This includes the negative impact that adapting to these life stressors can have on the brain. For example, the continued influx of stress hormones due to repeated exposure to ACEs, such as cortisol, results in a toxic stress response (Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012). These negative responses can include epigenetic alterations involving DNA modifications in the brain (Meaney, Reference Meaney2010, for review; Fox, Levitt, & Nelson, Reference Fox, Levitt and Nelson2010), cellular death (Grassi-Olivera, Ashy, & Stein, Reference Grassi-Oliveira, Ashy and Stein2008), and inability to extinguish fear responses (Morgan & LeDoux, Reference Morgan and LeDoux1995; Morgan, Romanski, & LeDoux, Reference Morgan, Romanski and LeDoux1993). Epigenetic alterations in response to early stress exposure have been documented in brain regions associated with memory, emotion regulation, and executive function (McEwen, Reference McEwen2017), and an inability to extinguish fear responses may underlie the emergence of behavioral undercontrol and an inability to relate adaptively in social situations. Mechanisms linking ACEs and certain developmental outcomes, however, are not yet clear. By and large, developmental research supports cascade models of psychopathology, wherein externalizing behavior is a precursor to problematic SU (Chassin, Pitts, DeLucia, & Todd, Reference Chassin, Pitts, DeLucia and Todd1999; Dodge et al., Reference Dodge, Malone, Lansford, Miller, Pettit and Bates2009). Accordingly, we posit that altered brain function due to ACEs may be an important mediating mechanism linking ACEs and externalizing behavior that may lead to SU. Through two separate prospective models, the aim of this study was to examine neurobiological pathways through which ACEs lead to later problem behavior and SU.
ACEs and Substance Use
Research highlights the association between ACEs and substance-related behaviors in adolescence (Carliner, Gary, McLaughlin, & Keyes, Reference Carliner, Gary, McLaughlin and Keyes2017; Kalmakis & Chandler, Reference Kalmakis and Chandler2015), including early alcohol initiation (Dube et al., Reference Dube, Miller, Brown, Giles, Felitti, Dong and Anda2006), early smoking initiation (Anda et al., Reference Anda, Croft, Felitti, Nordenberg, Giles, Williamson and Giovino1999), and marijuana use (Khoury Tang, Bradley, Cubells, & Ressler, Reference Khoury, Tang, Bradley, Cubells and Ressler2010). For example, the likelihood of using drugs increased with each additional endorsement of an ACE; this graded relationship was strongest for drug initiation in early adolescence (Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003). Despite evidence linking ACEs and SU, longitudinal studies examining how risk associated with ACEs unfolds to predict later SU are lacking. Behavioral undercontrol, defined here as a collection of temperamental traits that are biologically based and comprise impulsivity, aggressiveness, and sensation seeking regardless of negative consequences (King & Chassin, Reference King and Chassin2004; Sher & Trull, Reference Sher and Trull1994), may represent a key risk factor. Poor inhibitory control is an underlying neurocognitive mechanism central to behavioral undercontrol (Zucker, Heitzeg, & Nigg, Reference Zucker, Heitzeg and Nigg2011) and an early precursor to later SU (Miller & Plant, Reference Miller and Plant2002). Accordingly, youth who have difficulty controlling their behavior may engage in more risk behaviors despite experiencing negative consequences.
ACEs and Externalizing Behavior
Behavioral undercontrol underlies externalizing behavior (e.g., aggression and delinquency), which is a strong predictor of early SU (Chassin et al., Reference Chassin, Pitts, DeLucia and Todd1999; Trucco et al., Reference Trucco, Hicks, Villafuerte, Nigg, Burmeister and Zucker2016). An association between ACEs and externalizing behavior is well documented. For example, youth with more ACEs were younger at first arrest and had more total arrests during adolescence (Baglivio, Wolff, Piquero, & Epps, Reference Baglivio, Wolff, Piquero and Epps2015) and more delinquent acts (Brown & Shillington, Reference Brown and Schillington2017). Similarly, adolescents with maltreatment histories were more likely to be aggressive, truant, and run away from home (Stouthamer-Loeber, Loeber, Homisch, & Wei, Reference Stouthamer-Loeber, Loeber, Homisch and Wei2001). Yet, research documenting whether the influence of cumulative ACEs on later SU operates via externalizing behaviors is not widely established. Furthermore, despite empirical evidence of the association between ACEs and externalizing behavior, the neurobiological mechanisms underlying this association are not well understood.
ACEs and Neural Correlates of Externalizing Behavior
A critical aspect of behavioral control is error monitoring. Error monitoring includes error detection and correction in support of improved task accuracy (Menon, Adleman, White, Glover, & Reiss, Reference Menon, Adleman, White, Glover and Reiss2001); it is an important aspect of cognitive control through its involvement in adaptive adjustment of performance (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, Reference Ridderinkhof, Ullsperger, Crone and Nieuwenhuis2004). The medial prefrontal cortex, including the anterior cingulate cortex (ACC), has been associated with error detection in numerous studies (e.g., Carter et al., Reference Carter, Braver, Barch, Botvinick, Noll and Cohen1998; Garavan, Ross, Murphy, Roche, & Stein, Reference Garavan, Ross, Murphy, Roche and Stein2002; Kiehl, Lidle, & Hopfinger, Reference Kiehl, Liddle and Hopfinger2000) and is believed to interact with the lateral prefrontal cortex to support performance adjustment (Ridderinkhof et al., Reference Ridderinkhof, Ullsperger, Crone and Nieuwenhuis2004; Stevens, Klehl, Pearlson, & Calhoun, Reference Stevens, Kiehl, Pearlson and Calhoun2009). Impairments in the neural mechanisms underlying error monitoring can impede an individual's ability to learn from mistakes, a process that is directly linked to behavioral regulation (Hall, Bernat, & Patrick, Reference Hall, Bernat and Patrick2007). Blunted activation of error monitoring circuitry during a go/no-go task in childhood has been shown to be prospectively associated with increased externalizing behaviors and early initiation of SU (Heitzeg et al., Reference Heitzeg, Nigg, Hardee, Soules, Steinberg, Zubieta and Zucker2014).
Furthermore, evidence suggests that error monitoring circuitry may be impacted by ACEs. Youth with maltreatment histories were found to have decreased structural connectivity of the ACC compared to controls (Teicher, Anderson, Ohashi, & Polcari, Reference Teicher, Anderson, Ohashi and Polcari2014). In addition, event-related potentials localized to the ACC have been shown to be indicators of error monitoring (e.g., van Veen & Carter, Reference van Veen and Carter2002) and may differ between individuals with and without early experiences of adversity. For example, McDermott, Westerlund, Zeanah, Nelson, and Fox (Reference McDermott, Westerlund, Zeanah, Nelson and Fox2012) found that children who were institutionalized had smaller amplitudes and slower latencies for event-related potential waveforms associated with response monitoring during a go/no-go task. Loman et al. (Reference Loman, Johnson, Westerlund, Pollak, Nelson and Gunnar2013) found children who had been previously institutionalized to demonstrate smaller amplitudes in brain activity related to cognitive control, including inhibitory control, stimulus discrimination, and categorization.
As a central hub between top-down cognitive control and bottom-up reward processing brain systems (Allman, Hakeem, Erwin, Nimchinsky, & Hof, Reference Allman, Hakeem, Erwin, Nimchinsky and Hof2001), ACC function may be an important mechanism linking ACEs and externalizing behavior that may lead to SU. Moreover, decreased functioning in the ACC has been identified in individuals with posttraumatic stress disorder, a potential outcome in the wake of trauma and adversity (see review by Sherin & Nemeroff, Reference Sherin and Nemeroff2011), and among those with a history of childhood maltreatment and current diagnosis of posttraumatic stress disorder (Stevens et al., Reference Stevens, Ely, Sawamura, Guzman, Bradley, Ressler and Jovanovic2016). To date, no studies have tested associations between ACEs, ACC function, and later SU. This knowledge could elucidate avenues for intervention by targeting brain areas and associated cognitive competencies needing additional support to prevent youth from developing SU problems.
Current Study
The current study will test two prospective models to examine associations between cumulative ACEs (before age 11), externalizing behavior (ages 12–14), brain functioning (ages 9–15), and adolescent SU (ages 15–17; see Figure 1). Model 1 examines the mediating role of externalizing behavior in early adolescence between ACEs occurring before age 11 and SU in late adolescence. We expected that high rates of ACEs would predict high levels of externalizing behavior in early adolescence, which in turn would predict high levels of SU in later adolescence. Model 2 examines the role of individual differences in brain activation during errors in an inhibitory control task as a potential mediator in the association between ACEs and early adolescent externalizing behavior among a smaller neuroimaging sample of youth from the larger overall study. Based on the extensive literature regarding ACC function and error monitoring, we expected that high rates of ACEs would be associated with reduced activation in the ACC during inhibitory errors. In turn, less activation in the ACC during inhibitory errors would predict more externalizing behavior in early adolescence. An integrated consideration of these two distinct models stands to increase understanding of potential mechanisms through which ACEs impact risk for later SU in adolescence.

Figure 1. Conceptual model.
Method
Participants
Participants included in Model 1 were 465 adolescents from 333 families taking part in the Michigan Longitudinal Study (MLS), an ongoing, prospective, high-risk study (Zucker, Ellis, Fitzgerald, Bingham, & Sanford, Reference Zucker, Ellis, Fitzgerald, Bingham and Sanford1996; Zucker et al., Reference Zucker, Fitzgerald, Refior, Puttler, Pallas, Ellis, Fitzgerald, Lester and Zuckerman2000). The MLS follows a community sample of high-risk families, which is composed of men convicted of drunk driving who met criteria of an alcohol use disorder (AUD) diagnosis, their son, and their son's biological mother. A comparison sample of low-risk families from the same neighborhoods who did not meet criteria for SU problems was also recruited. Community-identified AUD-diagnosed men and their families, also living in the same neighborhoods, were recruited as an intermediate-risk group. Full biological siblings were also included. Due to original recruitment strategies, the current sample was predominantly male (n = 344, 74.0%) and of European American ancestry (n = 432, 92.9%). To insure the families’ comfort, and to minimize no-show rates, all data collection in the early waves of the study was carried out in the families’ homes. See Zucker et al. (Reference Zucker, Ellis, Fitzgerald, Bingham and Sanford1996, Reference Zucker, Fitzgerald, Refior, Puttler, Pallas, Ellis, Fitzgerald, Lester and Zuckerman2000) for a full description of the sample and study procedures.
Participants included in Model 2 (N = 92; M = 12 years, SD = 1.59, age range 9–15 at time of scan; n = 60, 51.3% males, n = 57, 48.7% White) underwent functional magnetic resonance imaging (fMRI) during a go/no-go task.Footnote 1 The presence of most current, active primary Axis I disorders were exclusionary for entry into the fMRI substudy with the exception of unmedicated mood disorder or current or past history of conduct disorders or attention-deficit/hyperactivity disorder (ADHD). These were allowed because exclusion would preferentially eliminate part of the phenomena of interest. Axis I disorders were assessed by a clinical psychologist with version 4 of the Diagnostic Interview Schedule for Children (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone2000) using DSM-IV criteria. The parent-report version of the instrument (Diagnostic Interview Schedule for Children—Parent) was administered to the primary caregiver to supplement the child data. All participants were right-handed as determined by the Edinburgh Handedness questionnaire (Oldfield, Reference Oldfield1971). Psychostimulants used for treatment of ADHD were discontinued for 48 hr prior to the fMRI study. Additional exclusionary criteria for the fMRI study were history of psychosis or schizophrenia in first-degree relatives; neurological, acute, uncorrected, or chronic medical illness; treatment with other centrally active medications within the past 6 months; or MRI contraindications.
Procedure
MLS assessments were completed following initial recruitment (Wave 1, ages 3–5), with data collection occurring every 3 years (e.g., Wave 2, ages 6–8). Using these data, we examined the impact of ACEs on SU in late adolescence through externalizing behavior during early adolescence (Model 1). We focused on adverse experiences that occurred during childhood (Waves 1–3, ages 3–11), externalizing behavior during early adolescence (Wave 4, ages 12–14), and SU behavior in late adolescence (Wave 5, ages 15–17). Participants in the fMRI component of the MLS completed a go/no-go task while in the scanner. With these data, we examined the impact of ACEs on externalizing behavior via brain functioning in the ACC (Model 2). The same data on ACEs were used in this second model, in addition to externalizing behavior and fMRI data. Reports from multiple sources were used to minimize shared method variance. Informed consent and assent were obtained from the teachers, parents, and children after study procedures were reviewed. The institutional review board where this study was conducted approved the study.
Measures
ACEs
A measure of ACEs prior to age 11 was constructed by aggregating items across several questionnaires that are consistent with a recent operationalization of the ACE scale (Finkelhor, Shattuck, Turner, & Hamby, Reference Finkelhor, Shattuck, Turner and Hamby2015). This cumulative approach to measuring ACEs is common (e.g., Chapman et al., Reference Chapman, Liu, Presley-Cantrell, Edwards, Wheaton, Perry and Croft2013; Chartier, Walker, & Naimark, Reference Chartier, Walker and Naimark2010; Mersky, Topitzes, & Reynolds, Reference Mersky, Topitzes and Reynolds2013; Whitaker et al., Reference Whitaker, Dearth-Wesley, Gooze, Becker, Gallagher and McEwen2014) and has been deemed appropriate and even favorable due to its robust ability to predict developmental adversity, its insensitivity to collinearity of risk factors, parsimony, and its relatability to public policy (Evans, Li, & Sepanski Whipple, Reference Evans, Li and Sepanski Whipple2013). Five items were taken from the Oregon Social Learning Center Family Crisis List (Patterson, Reference Patterson1982). For this measure, caregivers are asked to mark crises the family experienced in the past 6 months. Of interest for the current study were items reflecting the following: not being able to pay bills, lacking clean clothes, family member seeing a mental health professional, something stolen from the house, and applying for welfare or unemployment. Eight items were taken from the Conflict Tactics Scale (CTS; Straus, Reference Straus1979; Straus, Gelles, & Steinmetz, Reference Straus, Gelles and Steinmetz1980). The CTS assesses family violence by asking caregivers ways in which family members resolved conflict in the past year. Of interest for the current study were items reflecting behaviors toward their child, such as insults, threatening to or actually hitting or throwing something at their child, and hitting their child. Items reflecting these same behaviors, but directed toward the parent and his or her spouse, were used to assess the child's exposure to domestic violence. In addition, an adolescent retrospective self-report measure of the CTS was used to assess whether the adolescent experienced sexual or physical abuse prior to age 11, complementing the information derived from the parent-reported CTS. Items were also taken from a modified version of the Coddington Family Events Questionnaire (Coddington, Reference Coddington1972a, Reference Coddington1972b). This measure is a parent-report measure focused on life events that occurred in the family during the past 3 years. Of interest for the current study were items relevant to the following: death of a sibling, parent incarceration, sibling involvement with SU, and bullying by classmates. Finally, 1 item was included that reflected presence of a parental AUD as assessed using the fourth version of the Diagnostic Interview Schedule (Robins, Helzer, Croughan, & Ratcliff, Reference Robins, Helzer, Croughan and Ratcliff1980). Accordingly, a total of 21 items were used to derive a measure of ACEs. Using each measure's original scoring scheme to determine if an event ever occurred, all items in our ACE composite scale were dichotomized to reflect whether the event occurred (1 = yes, 0 = no). If an event was endorsed during multiple time points, the event was still coded as 1 (i.e., max value = 21) rather than “double counting” an event, consistent with prior work (e.g., Finkelhor et al., Reference Finkelhor, Shattuck, Turner and Hamby2015; Hussong et al., Reference Hussong, Bauer, Huang, Chassin, Sher and Zucker2008).
Externalizing behavior
Externalizing behavior at Wave 4 (ages 12–14) was assessed using T scores from the aggressive (e.g., bullying) and delinquency subscales (e.g., steals) of the Teacher Report Form (TRF; Achenbach, Reference Achenbach1991).Footnote 2 For 24 participants, data from an earlier time point (Wave 3, ages 9–11) but following the scan, were used to maximize available data and minimize listwise deletion. In other cases (12 participants), a later time point (Wave 5, ages 15–17) was used to ensure temporal precedence of the go/no-go task data among older participants. On average, participants were 13 years old (SD = 1.21, range 9–17) when externalizing behavior was assessed. Items on the TRF are rated on a 3-point Likert scale (0 = not true, 2 = very true or often true). The TRF has been used extensively and has demonstrated strong reliability and validity. Internal consistency was good in the current sample (α = 0.95) and normally distributed (skewness = 0.84, kurtosis = 0.08).
SU
Problematic alcohol use at Wave 5 (ages 15–17; M = 16.55 years, SD = 0.94)Footnote 3 was assessed using a latent variable (α = 0.80) comprised of the following 3 items: number of maximum alcohol beverages consumed in 24 hr, number of binge (i.e., five or more alcoholic drinks in one sitting) drinking days, and a summed score reflecting number of problems associated with alcohol use (37 items) in the past year using the Drinking and Drug History form (Zucker, Fitzgerald, & Noll, Reference Zucker, Fitzgerald and Noll1990). An example of problems associated with alcohol use include being absent from school and experiencing physical or medical problems … because of your alcohol use. In order to test whether findings generalize to other substances of abuse, we also examined past-year cigarette and marijuana use. More specifically, youth were asked to report on their frequency of cigarette use in the past 12 months (skewness = 1.67, kurtosis = 1.32) and the number of occasions on which they used marijuana in the past 12 months (skewness = 1.68, kurtosis = 1.38).
Control variables
In both models, we controlled for participant biological sex (male = 0; female = 1) and race (non-White = 0; White = 1). In Model 1 we also included Wave 1 parent report of externalizing behavior using the aggressive (e.g., bullying) and delinquency subscales (e.g., steals) of the Child Behavior Checklist (Achenbach, Reference Achenbach1991), as well as Wave 5 age to account for potential differences in SU. Model 2 included scan age to account for potential differences in brain functioning. Selection of these variables is in line with current research related to neurodevelopment (Crane, Schuster, Fusar-Poli, & Gonzalez, Reference Crane, Schuster, Fusar-Poli and Gonzalez2013), externalizing behavior (Chung, Hill, Hawkins, Gilchrist, & Nagin, Reference Chung, Hill, Hawkins, Gilchrist and Nagin2002), and SU (Johnston, O'Malley, Miech, Bachman, & Schulenberg, Reference Johnston, O'Malley, Miech, Bachman and Schulenberg2017) outcomes in relation to these demographic variables.
fMRI task
An event-related go/no-go task (Durston, Thomas, Wordern, Yang, & Casey, Reference Durston, Thomas, Worden, Yang and Casey2002) was used to probe error monitoring. Participants were instructed to respond to target stimuli (letters other than X) by pressing a button (go trials) but to make no response to infrequent nontarget stimuli (letter X; no-go trials). Stimulus duration was 500 ms, followed by 3500 ms of fixation. There were 5 runs of 49 trials, each containing 11, 12, or 13 no-go trials. There was a total of 60 no-go trials out of 245 total trials. Reaction times, error rate (failure to inhibit a response to a no-go trial, i.e., “false alarm”), and hit accuracy (correct response to go trials) during the task were recorded. Before scanning, all participants practiced on a desktop computer. Brain activation during errors was analyzed to test whether it mediated the effect of ACEs on externalizing behavior.
fMRI data acquisition
Whole-brain blood oxygenated level-dependent images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using a T2*-weighted single-shot combined spiral in-out sequence (Glover & Law, Reference Glover and Law2001) with the following parameters: repetition time = 2000 ms; echo time = 30 ms; flip angle = 90°; field of view = 200 mm; 64 × 64 matrix; in-plane resolution = 3.12 × 3.12 mm; and slice thickness = 4 mm. The entire volume of 29 axial slices was acquired every 2 s. A high-resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient-recalled echo, repetition time = 25 ms; minimum echo time; field of view = 25 cm; 256 × 256 matrix, slice thickness = 1.4 mm).
Data analysis
Adolescents with missing data significantly differed by race. More specifically, non-White adolescents were more likely to have missing externalizing and SU data. This is mostly due to the design of the study, where non-White families were added in later waves. Otherwise, youth with missing data did not differ from those without missing data. Path models were estimated in Mplus version 7.4 (Muthén & Muthén, Reference Muthén and Muthén1998–2017) using full information maximum likelihood to maximize available data. As previously described, the current sample included siblings. Accordingly, multilevel analyses accounting for family clustering and controlling for biological sex, race, age, and prior rates of externalizing behavior (Model 1) were estimated. More specifically, we tested intercept and slope only models that accounted for the multilevel data structure due to sibling status.
Several options exist to assess mediated effects in Mplus. The first is the product-of-coefficients approach using the IND command. The second approach involves calculating bias-corrected bootstrap confidence intervals (BBCIs), which is more robust (Preacher & Hayes, Reference Preacher and Hayes2008). In Mplus 7.4 it is not possible to account for clustering while using resampling approaches. Therefore, indirect effects when controlling for family cluster effects were compared to BBCIs, a procedure used in prior work (e.g., Trucco, Villafuerte, Heitzeg, Burmeister, & Zucker, Reference Trucco, Villafuerte, Heitzeg, Burmeister and Zucker2014). Although the results were largely the same across procedures, we present path estimates calculated using 95% BBCIs (with 10,000 bootstrap samples) as these indirect effects are considered more robust. The following fit statistics were examined for model fit: the root mean square error of approximation (RMSEA), the comparative fit index (CFI), and the Tucker–Lewis index (TLI). Low RMSEA values (<.08) represent good model fit, whereas high CFI and TLI values (>.80) represent good model fit.
fMRI data processing
Functional images were reconstructed using an iterative algorithm (Fessler, Lee, Olafsson, Shi, & Noll, Reference Fessler, Lee, Olafsson, Shi and Noll2005), and head motion was corrected using FSL 5.0.2.2 (Analysis Group, FMRIB, Oxford, United Kingdom; Jenkinson, Bannister, Brady, & Smith, Reference Jenkinson, Bannister, Brady and Smith2002). Runs exceeding 3 mm translation or 3 degrees rotation in any direction were excluded. Remaining image processing was completed using Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging, UCL, London, UK). Functional images were spatially normalized to a standard stereotaxic space (Montreal Neurological Institute). A 6 mm full-width half-maximum Gaussian spatial smoothing kernel was applied to account for differences in anatomy and improve signal-to-noise ratio. Individual, subject-level analyses were performed using a general linear model. Three regressors of interest (correct no-go trails, failed no-go trials, and go trials) were convolved with the canonical hemodynamic response function. Motion parameters and white matter signal intensity were modeled as nuisance regressors to remove residual motion artifacts and capture non-task-related noise, respectively. Go trials were not included in the contrast due to their high frequency relative to other trial types (Devito et al., Reference Devito, Meda, Jiantonio, Potenza, Krystal and Pearlson2013). The main contrast of interest was failed no-go versus correct no-go trials. This contrast allows the investigation of the specific impact of errors on brain function involved in low-probability stimulus processing (Stevens et al., Reference Stevens, Kiehl, Pearlson and Calhoun2009). A one-sample t test in SPM8 was used to detect activation associated with inhibitory errors (i.e., failed no-go vs. correct no-go) at the group level at a family-wise error corrected threshold of p < .05 with an extent threshold of 10 voxels. Average beta values for each significant cluster were extracted using MarsBaR (Brett, Anton, Valabregue, & Poline, Reference Brett, Anton, Valabregue and Poline2002) and imported into Mplus 7.2 for further analysis.
Results
Means, standard deviations, and correlations for study variables in the full sample are presented in Table 1. On average, adolescents had experienced approximately five ACEs, with parents hitting each other (88.8%), not having enough money for bills (62.0%), and being physically punished or abused (55.4%) being the top three endorsed types of ACEs. Females experienced lower rates of ACEs compared to males. White adolescents reported more problematic alcohol use and cigarette use but had lower teacher-reported rates of externalizing behavior compared to non-White adolescents. ACEs were associated with higher rates of externalizing behavior and SU. Externalizing behavior was associated with SU. Finally, all SU variables were significantly correlated. In the imaging sample, ACC activation was negatively correlated with ACEs (r = –.23, p < .05). ACC activation did not significantly differ across sex, race, or age. In addition, ACEs were not significantly correlated with performance measures of the go/no-go task (i.e., hit rate reaction time r = –.06, p = .57, false alarms r = .06, p=.57, false alarm reaction time r = –.11, p = .28). Descriptive statistics on the fMRI task performance measures are provided in Table 2.
Table 1. Means, standard deviations, and correlations of study variables

Note: SD, standard deviation. ACEs, adverse childhood experiences. Ext., externalizing. Bold values = p < .05 effects.
Table 2. fMRI task performance measures across all subjects (n = 92)

Note: ms, milliseconds. FA, false alarm. SD, standard deviation.
Model 1: Externalizing behavior mediates ACEs to problematic SU
Alcohol use
This model accounted for approximately 18% of the variance in problematic alcohol use (see Figure 2). Factor loadings for each of the problematic alcohol use indicators were above 0.3 and statistically significant. ACEs prior to age 11 predicted high levels of externalizing behavior in early adolescence. In turn, externalizing behavior predicted high levels of problematic alcohol use in late adolescence. ACEs prior to age 11 also had a direct effect on problematic alcohol use, whereby ACEs prior to age 11 predicted high levels of problematic alcohol use in late adolescence. Moreover, there was support for the role of externalizing behavior as a mediator in the association between ACEs and problematic alcohol use (estimate = 0.115, BBCI [0.047, 0.225]). Approximately 27% of the total effect of ACEs on problematic alcohol use operated through externalizing behavior.

Figure 2. Model for problematic alcohol use. Values represent standardized path coefficients. Only significant paths (*p < .05, **p < .01, ***p < .001) are depicted to ease presentation. The following covariates were included in the model but not depicted: biological sex, externalizing behavior in earlier childhood (3–5 years), age, and race. ACEs, adverse childhood experiences prior to age 11. Model fit: RMSEA = .046, CFI = .975, TLI = .953.
Cigarette use
This model accounted for approximately 19% of the variance in frequency of past-year cigarette use (see Figure 3). ACEs prior to age 11 predicted high levels of externalizing behavior in late adolescence. In turn, externalizing behavior predicted high rates of cigarette use in late adolescence. ACEs prior to age 11 also had a direct effect on cigarette use, whereby ACEs prior to age 11 predicted high levels of cigarette use in late adolescence. Moreover, there was support for the role of externalizing behavior as a mediator in the association between ACEs and cigarette use (estimate = 0.038, BBCI [0.020, 0.063]). Approximately 39% of the total effect of ACEs on cigarette use operated through externalizing behavior.

Figure 3. Model for cigarette use. Values represent standardized path coefficients. Only significant paths (**p < .01, ***p < .001) are depicted to ease presentation. The following covariates were included in the model but not depicted: biological sex, externalizing behavior in earlier childhood (3–5 years), age, and race. ACEs, adverse childhood experiences prior to age 11. Model fit: RMSEA = .044, CFI = .983, TLI = .905.
Marijuana use
This model accounted for approximately 15% of the variance in frequency of past-year marijuana use (see Figure 4). ACEs prior to age 11 predicted high levels of externalizing behavior in late adolescence. In turn, externalizing behavior predicted high rates of marijuana use in late adolescence. ACEs prior to age 11 also had a direct effect on marijuana use, whereby ACEs prior to age 11 predicted high levels of marijuana use in late adolescence. Moreover, there was support for the role of externalizing behavior as a mediator in the association between ACEs and marijuana use (estimate = 0.046, BBCI [0.020, 0.085]). Approximately 22% of the total effect of ACEs on marijuana use operated through externalizing behavior.

Figure 4. Model for marijuana use. Values represent standardized path coefficients accounting for family clustering. Only significant paths (**p < .01, ***p < .001) are depicted to ease presentation. The following covariates were included in the model but not depicted: biological sex, externalizing behavior in earlier childhood (3–5 years), age, and race. ACEs, adverse childhood experiences prior to age 11. Model fit: RMSEA = .012, CFI = .998, TLI = .991.
Model 2: ACC activation mediates ACEs to externalizing behavior association
A one-sample t test on the contrast of failed no-go versus correct no-go in the whole group found significant activation in three clusters (at family-wise error corrected p < .05 with an extent threshold of 10 voxels): ACC, mid cingulate cortex, and precuneus (Table 3 and Figure 5). As a first step, we examined bivariate correlations between extracted values from these three clusters and externalizing behavior. An examination of these bivariate correlations indicated that only the ACC (Cluster 1) was significantly associated with externalizing behavior (r = –.42, p < .001); neither of the other two clusters (i.e., mid cingulate or precuneus) were significantly associated with externalizing behavior. Given this, the remainder of our analyses focused exclusively on ACC activation during failed inhibitory control in relation to ACEs and externalizing behavior.
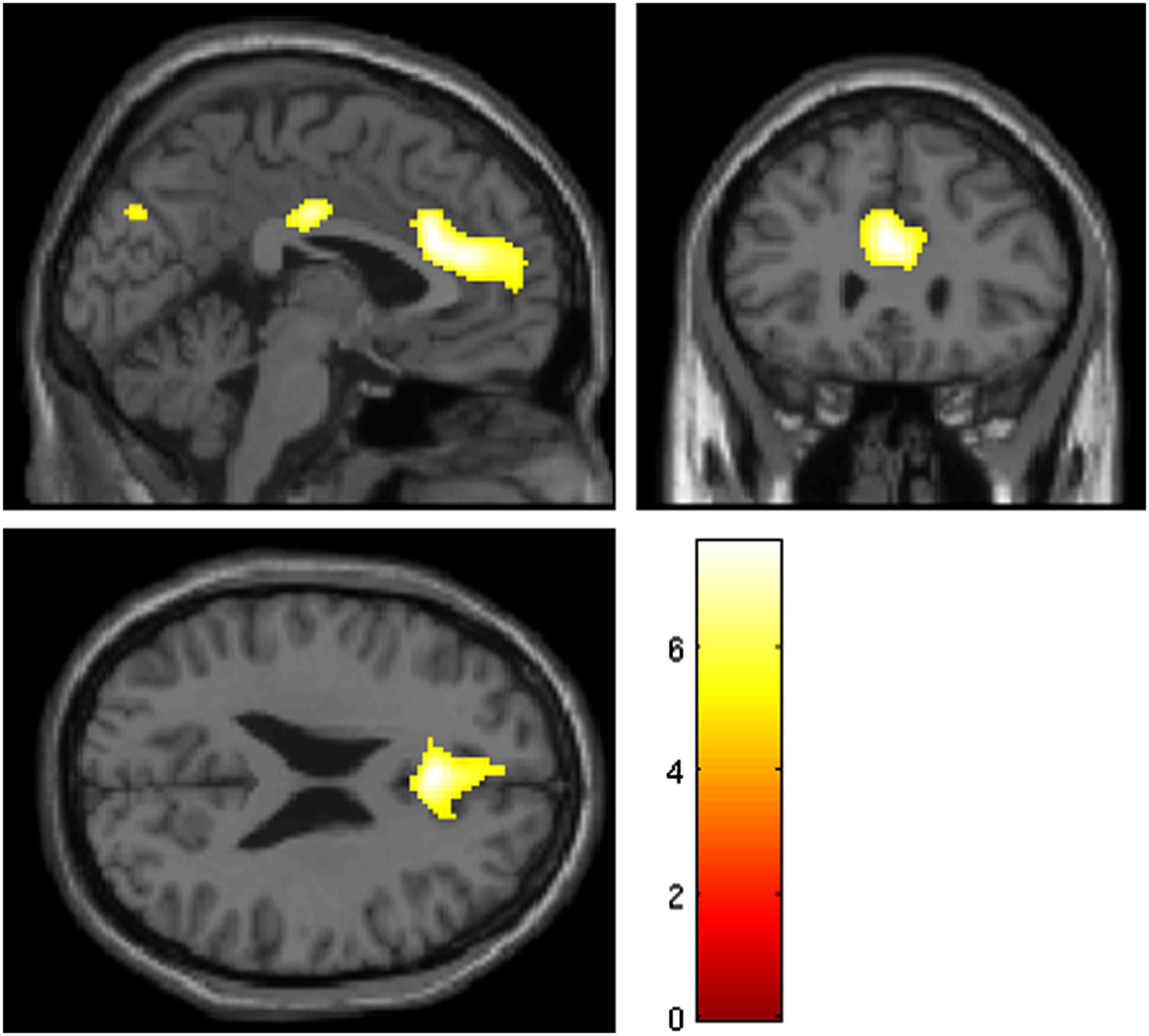
Figure 5. Failed no-go versus correct no-go, main effects. Whole-brain main effects analysis of inhibitory error processing (failed no-go vs. correct no-go) showed activation in anterior cingulate, medial frontal gyrus, mid cingulate gyrus, and precuneus. These regions are significant at a family-wise corrected threshold of p < .05 and voxel extent ≥ 10. The color bar represents t- = values and the Montreal Neurological Institute (MNI) coordinates are x = –2, y = 28, z = 24, corresponding to the global maximum.
Table 3. Areas of significant engagement (main effects; n = 92) during inhibitory errors (failed no-go vs. correct no-go)

Note: BA, Brodmann area. k, cluster size in voxels. FWE, family-wise error corrected.
Coordinates are in Montreal Neurological Institute (MNI) space. The contrast was thresholded at p < .05 (FWE corrected), k ≥ 10. All clusters are bilateral.
Model 2 accounted for approximately 37% of the variance in externalizing behavior during early adolescence (see Figure 6). ACEs prior to age 11 was associated with lower activation in the ACC to inhibitory errors during the go/go-no. In turn, less activation in the ACC to inhibitory errors during the go/no-go predicted high levels of externalizing behavior during early adolescence. There was also evidence for a direct effect of ACEs on externalizing behavior, such that more ACEs prior to age 11 were associated with high levels of externalizing behavior. Nevertheless, there was support for the role of differences in ACC activation to inhibitory errors during the go/no-go as a mediator in the association between ACEs and externalizing behavior (estimate = 0.235, BBCI [0.047, 0.532]). Approximately 63% of the total effect of ACEs on early adolescent externalizing behavior operated through ACC activation to inhibitory errors during the go/no-go.

Figure 6. Model for externalizing behavior. Values represent standardized path coefficients accounting for family clustering. Only significant paths (*p < .05, ***p < .001) are depicted to ease presentation. The following covariates were included in the model but not depicted: biological sex, age at scan, and race. ACEs, adverse childhood experiences prior to age 11. ACC, anterior cingulate cortex activation to inhibitory errors during the go/no-go task. Model fit statistics not available as this was a just-identified model.
Discussion
The current study supports and extends previous cross-sectional, brain imaging work by examining the prospective and developmentally relevant role of externalizing behavior and brain functioning as potential pathways through which childhood adversity influences problematic SU in adolescence. Given empirically supported associations between ACEs and each of this study's main constructs (i.e., problematic SU, externalizing behavior, and brain responsivity during error monitoring), our study stands to bridge previously disparate literatures. Furthermore, our findings highlight a potential neurobiological mediator of adolescent SU for a group of vulnerable youth, specifically those with a history of adversity. ACEs can “get under the skin” and exert negative and pervasive impact on brain development (e.g., Cicchetti, Reference Cicchetti2013; Danese & McEwen, Reference Danese and McEwen2012; Shonkoff et al., Reference Shonkoff, Garner, Siegel, Dobbins, Earls and Wood2012). Thus, early life experiences should be considered an integral component in the etiology and prevention of early and problematic SU.
Model 1: Externalizing behavior mediates ACEs to problematic SU
As hypothesized, ACEs were significantly and positively related to externalizing behavior and SU (i.e., alcohol, cigarette, and marijuana). It is well known that cumulative childhood adversity sets the stage for poor developmental outcomes (Cicchetti, Reference Cicchetti2013; Cook et al., Reference Cook, Spinazzola, Ford, Lanktree, Blaustein, Cloitre and van der Kolk2005; Evans et al., Reference Evans, Li and Sepanski Whipple2013; Mitchell, Tynes, Umaña-Taylor, & Williams, Reference Mitchell, Tynes, Umaña-Taylor and Williams2015; Parra et al., Reference Parra, Smith, Mason, Savolainen, Chmelka, Miettunen and Järvelin2017), such as externalizing behavior problems (Keiley, Howe, Dodge, Bates, & Petti, Reference Keiley, Howe, Dodge, Bates and Petti2001; Villodas, Litrownik, Newton, & Davis, Reference Villodas, Litrownik, Newton and Davis2015) and SU (Carliner et al., Reference Carliner, Gary, McLaughlin and Keyes2017; Dube et al., Reference Dube, Miller, Brown, Giles, Felitti, Dong and Anda2006; Kalmakis & Chandler, Reference Kalmakis and Chandler2015). Recently, Carliner et al. (Reference Carliner, Gary, McLaughlin and Keyes2017) found that cumulative trauma increased the risk of adolescents experimenting with drugs and developing substance use disorders. Adolescents in Carliner et al.’s (Reference Carliner, Gary, McLaughlin and Keyes2017) study were, on average, 15 years old, which is younger than the typical age of onset of substance use disorder, suggesting that youth who experience trauma may be especially vulnerable to negative substance-related outcomes. We found that ACEs were related to more externalizing behavior, which in turn was related to higher rates of SU for all measured substances when participants were, on average, 16.55 years old. Although previous research has also found externalizing behavior to mediate developmental pathways to SU (Chassin et al., Reference Chassin, Pitts, DeLucia and Todd1999; Trucco et al., Reference Trucco, Villafuerte, Heitzeg, Burmeister and Zucker2014), childhood adversity has not been widely examined. Some notable exceptions include the work of Rogosch, Oshri, and Cicchetti (Reference Rogosch, Oshri and Cicchetti2010) and Oshri, Rogosch, Burnett, and Cicchetti (Reference Oshri, Rogosch, Burnette and Cicchetti2011), which demonstrated that externalizing behavior in late childhood mediated the effect of childhood maltreatment on marijuana use in early adolescence. Our study recognizes the potential impact of ACEs on development and extends prior work by demonstrating a potential neurobiological mechanism through which cumulative childhood adversity may lead to adolescent SU.
Direct associations between ACEs and SU remained significant in the mediational models for all substances (i.e., alcohol, cigarettes, and marijuana), in addition to significant mediational pathways through externalizing behavior. By including both ACEs and externalizing behavior in the same model, our findings highlight the importance of looking beyond aggressive and delinquent behaviors and considering the cascading developmental influence of adversity in childhood on outcomes during adolescence.
Model 2: ACC activation mediates ACEs to externalizing behavior association
The current study also examines brain function during inhibitory errors as a mediator between ACEs and externalizing behavior. Whereas most research investigating the neurobiological impact of ACEs is limited to structural comparisons, our study extends this line of inquiry by assessing neural responsivity. Unlike structural comparisons, functional brain analysis examines subtle neurophysiological changes in the brain that occur in response to stimuli in the external environment (David et al., Reference David, Ware, Chu, Loftus, Fusar-Poli, Radua and Ioannidis2013); thus, they have the potential to provide greater insight into the neurobiological mechanisms underlying behavioral responses. This approach is especially important in order to understand how childhood adversity may affect future psychopathology and how youth with a history of adversity may process and view the world differently from their peers without these same negative experiences (McCrory, Gerin, & Viding, Reference McCrory, Gerin and Viding2017).
As hypothesized, our results and surrounding literature suggest that the path to externalizing behavior from childhood adversity operates at least in part through an effect on ACC responsivity during error monitoring. The ACC is considered an integrative hub between the limbic system and the prefrontal cortex (e.g., Botvinick, Cohen, & Carter, Reference Botvinick, Cohen and Carter2004) and is involved in executive functioning and self-regulation (Posner, Rothbart, Sheese, & Tang, Reference Posner, Rothbart, Sheese and Tang2007; Stadler et al., Reference Stadler, Sterzer, Schmeck, Krebs, Kleinschmidt and Poustka2007). Global deficits related to executive functioning (Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011) and diminished ACC activity (Teicher et al., Reference Teicher, Anderson, Ohashi and Polcari2014) have been reported among children exposed to adversity. For example, youth who had been maltreated and placed in foster care demonstrated less ACC activation associated with inhibitory control compared to healthy youth (Bruce et al., Reference Bruce, Fisher, Graham, Moore, Peake and Mannering2013). Moreover, a meta-analysis reported an inverse relationship between activity in the ACC and antisocial behavior in youth and adults (Yang & Raine, Reference Yang and Raine2009), which is congruent with our mediation findings of lower ACC activation predicting higher externalizing behavior in early adolescence.
Our findings are also in line with research demonstrating that the ACC is involved in determining appropriate responses and inhibiting inappropriate responses (Paus, Petrides, Evans, & Meyer, Reference Paus, Petrides, Evans and Meyer1993) as well as behavioral decision making (Gowin et al., Reference Gowin, Stewart, May, Ball, Wittmann, Tapert and Paulus2014). Failing to adapt one's behavior (e.g., SU) in response to errors (e.g., negative consequences) is a key component of poor self-regulation underlying externalizing behaviors and SU risk (Hall et al., Reference Hall, Bernat and Patrick2007). Moreover, neural activation in circuitry that responds to inhibitory errors in childhood is associated with SU problems later in development (Heitzeg et al., Reference Heitzeg, Nigg, Hardee, Soules, Steinberg, Zubieta and Zucker2014). Therefore, it is possible that if ACEs contribute to impaired error monitoring, adolescents with these negative childhood experiences may not be as adept at adjusting their behavior in the face of negative feedback or consequences. Thus, they may find themselves at heightened risk for externalizing behavior and later SU, a noted outcome among youth with histories of childhood adversity (Dube et al., Reference Dube, Felitti, Dong, Chapman, Giles and Anda2003; Kalmakis & Chandler, Reference Kalmakis and Chandler2015).
At the same time, there is more than one way to view an adolescent's response to adversity. What can be viewed as aggressive and/or delinquent behavior may also be a strategy for survival in response to an unsafe and/or negative environment, albeit a strategy that may not be adaptive outside of this negative environment. Latent vulnerability theory (McCrory & Viding, Reference McCrory and Viding2015; Puetz & McCrory, Reference Puetz and McCrory2017) provides a framework for understanding neurobiological changes as adaptations to aide survival in a difficult environment that may have provided benefit in the short term, but confer risk in the long term (McCrory et al., Reference McCrory, Gerin and Viding2017). Research on self-protection mechanisms can help to explain the current findings from a latent vulnerability and resilience perspective, rather than one that sees changes after adversity as damage. When a person is confronted with negative or threatening information (e.g., trauma/adversity, negative feedback regarding academic competence, or emotional/verbal abuse), his or her self-view or self-concept is threatened (e.g., Cicchetti, Reference Cicchetti2013; Hoefler, Athenstaedt, Corcoran, Ebner, & Ischenbeck, Reference Hoefler, Athenstaedt, Corcoran, Ebner and Ischebeck2015). Decreased functioning in the ACC may, in part, protect one's self-concept, because it diminishes the ability to integrate negative feedback into one's view of himself or herself (Hoefler et al., Reference Hoefler, Athenstaedt, Corcoran, Ebner and Ischebeck2015). With the proper support and intervention, preserving a positive sense of self could contribute to one's ability to overcome adversity.
Clinical implications
In light of these findings and the surrounding literature, intervention and prevention efforts aimed at adolescent externalizing behavior problems and SU should account for both the impact of prior ACEs and altered brain functioning when addressing poor decision making and risk taking. That is, this research supports a direct association between ACEs and neural function in the ACC, externalizing behavior, and SU. Despite this, healing from trauma as a method of SU prevention is not widely adopted. Results from our study suggest that knowing about youths’ ACEs can inform the course of treatment and prevention. For example, when youth report ACEs, interventions focusing on the neurobiological components involved in improving self-control and behavioral regulation may help prevent vulnerable youth from engaging in externalizing behavior and progressing to problematic SU. Research suggests that instead of intervening once problems arise, early interventions that have a remediating effect on the brain following early traumatic events can help to reduce the likelihood of negative outcomes (Bick & Nelson, Reference Bick and Nelson2016). This would include interventions that focus on self-regulation training (Schibli, Wong, Hedayati, & D'Angiulli, Reference Schibli, Wong, Hedayati and D'Angiulli2017) such as mindfulness (Marchand, Reference Marchand2014) and neurofeedback (van der Kolk, Reference van der Kolk2014; van der Kolk et al., Reference van der Kolk, Hodgon, Gapen, Musicaro, Suvak, Hamlin and Spinazzola2016), in addition to the interventions that have already demonstrated success related to childhood adversity (e.g., trauma-focused cognitive behavioral therapy; Cohen et al., Reference Cohen, Mannarino, Jankowski, Rosenberg, Kodya and Woldford II2016). As an example, nascent research on mindfulness-based programs has begun to address potential neural mechanisms at play through this practice. Mindfulness has been shown to enhance attention, emotion regulation, and behavioral control (Marchand, Reference Marchand2014), and research has demonstrated an association between mindfulness and activation in medial frontal regions of the brain, including the ACC (Marchand, Reference Marchand2014).
Strengths and limitations
The current study builds upon previous research in several ways, including capturing data on childhood adversity and integrating it into adolescent SU research, utilizing a longitudinal study design involving prospective data from a high-risk sample, focusing on a critical period of development; a mediation explanatory framework; and multiple reporters to minimize shared method variance. There are, however, limitations to note. First, although reports from multiple sources minimizes shared method variance, having parents report on their child's ACEs may be problematic given a desirability bias. Unfortunately, child reports of ACEs prior to age 11 were not available. Future work could couple existing data with administrative-level data (e.g., Child Protective Services data and community poverty-level indicators) to validate accounts of childhood adversity. Second, we considered cumulative ACEs in this study. Although the cumulative impact of childhood adversity is informative, understanding how specific types of adversity differentially impact development is also important (e.g., child maltreatment vs. having an incarcerated parent). An alternative conceptual model of childhood adversity has been proposed by McLaughlin, Sheridan, and Lambert (Reference McLaughlin, Sheridan and Lambert2014) that differentiates between dimensions of adversity (i.e., deprivation and threat) that impact neural development and developmental mechanisms. Yet, while a cumulative approach may be limited in differentiating between specific mechanistic pathways (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016), it is still a robust predictor of developmental outcomes (Evans et al., Reference Evans, Li and Sepanski Whipple2013) and a starting point for the consideration of associations that have not otherwise been tested, as is the case with this study. Third, based on supporting literature, we limited our focus to inhibitory deficits. However, it is likely that additional areas of brain functioning are also impacted by childhood adversity (e.g., emotion regulation and working memory). Future work should also examine these as potential mediators of childhood adversity and adolescent SU. Fourth and finally, the sample size is also a potential limitation. Although we had a robust sample for Model 1 (n = 814), the sample size was significantly smaller for Model 2 (n = 92). This is due primarily to the fact that only a subset of participants in the MLS participate in the neuroimaging component. While neuroimaging studies are typically based on sample sizes that are comparable to, and often smaller than, those in the current study (see David et al., Reference David, Ware, Chu, Loftus, Fusar-Poli, Radua and Ioannidis2013; Yang & Raine, Reference Yang and Raine2009), a larger sample size would allow researchers to distinguish between different types of childhood adversity, systematically control for more potential confounding variables (McCrory et al., Reference McCrory, Gerin and Viding2017), and provide enough power to test one multiple mediator model that encompasses ACEs, brain responsivity, externalizing behavior, and SU.
Conclusions
In sum, our findings bridge multiple areas of research that are typically disparate. Examining adolescent SU in the context of childhood adversity, we highlight a reduction in brain responsivity related to error monitoring as a pathway to externalizing behavior, and externalizing behavior as a pathway to SU. Taking this step is critical if we are to better understand adolescent psychopathology on a mechanistic level, and thus become more effective in our prevention efforts. Latent vulnerability theory provides a framework for understanding neurobiological changes as adaptations to aide survival in a difficult environment and “clues” for points of intervention as these adaptations may provide benefit in the short term, but confer risk in the long term (McCrory et al., Reference McCrory, Gerin and Viding2017; McCrory & Viding, Reference McCrory and Viding2015; Puetz & McCrory, Reference Puetz and McCrory2017). These findings also point to the importance of understanding potential neurobiological mechanisms that promote SU risk among a vulnerable group of youth experiencing early life adversity.
Financial support
This research was supported in part by National Institute on Alcohol Abuse and Alcoholism Grants K08 AA023290 (to E.M.T.), R01 AA007065 (to R.A.Z. and M.H.), T32 AA007477 (to F.B.); National Institute on Drug Abuse Grant R01 DA027261 (to M.H. and R.A.Z.); and National Institute on Minority Health and Health Disparities Grant U54 MD012393; Sub-Project ID: 5378 (to E.M.T.).











