Introduction
Obesity and overweight are the main challenges to the health and well-being of most populations and kills more people than underweight.Reference Ogden, Carroll and Kit 1 – Reference Diniz, Beleigoli and Ribeiro 3 In the last three decades, obesity has more than doubled worldwide, and in 2014, 39% of adults aged 18 years and over were overweight and 13% were obese. Obesity is associated with diabetes mellitus and cardiovascular disease, which cause morbidity, mortality and high health-care expenditures.Reference Ng, Fleming, Robinson and Thomson 4 , 5 Different therapies have been investigated to minimize or prevent obesity and its comorbidities,Reference De Angel, Berrigan and Nez 6 – Reference Soares, Murhadi and Kurpad 8 for instance dietary calcium.
Mate tea, which is also known as toasted yerba mate (YM), is an infusion of herbs made from dried leaves of Ilex paraguariensis.Reference Bracesco, Sanchez and Contreras 9 Originating from the tropical regions of Asia and South America, this beverage is widely consumed in Brazil, Paraguay, Argentina and Uruguay.Reference Bastos, de Oliveirea and Matsumoto 10 Given the increasing reports of potential health benefits, YM is becoming popular in Europe and North America.Reference Bracesco, Sanchez and Contreras 9 YM is known to be rich in phenolic compounds, such as caffeoyl derivatives, flavonoids, methylxanthines, tannins, ursolic acid-derived saponins and vitamins.Reference Bastos, de Oliveirea and Matsumoto 10 Previous data described the beneficial effects of Ilex paraguariensis, which include antioxidant, hypocholesterolaemic and pro-thermogenic properties,Reference Bastos, de Oliveirea and Matsumoto 10 – Reference Arçari, Bartchewsky and dos Santos 12 the down-regulation of pro-adipogenic genes,Reference Arçari, Santos, Gambero and Ribeiro 13 protective effects against atherosclerosis,Reference Bracesco, Sanchez and Contreras 9 and improvements in the inflammatory responseReference Carmo, Rogero and Cortez 14 and insulin sensitivity.Reference Arçari, Santos and Gambero 15 In healthy women, Matsumoto et al.Reference Matsumoto, Bastos and Mendonça 16 showed that the acute consumption of roasted mate tea caused a decrease in plasma lipid oxidation, an increase in plasma antioxidant capacity and an increase in the gene expression of antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx). Due to all these beneficial effects, recent data in vitro, in animals and in humans have suggested that YM can be a good candidate against obesity.Reference Gamboa-Gómez, Rocha-Guzmán and Gallegos-Infante 17 – Reference Kim, Oh and Kim 20
Experimental, clinical and epidemiological studies have shown that nutritional, environmental and hormonal influences during early postnatal life may affect the physiology of several tissues during adulthood and are intimately associated with the ‘fetal origin’ hypothesis proposed by David Barker. This phenomenon is named metabolic programming or developmental plasticity. Programming is known to be based on epigenetic alterations that change the expression patterns of several genes involved in metabolism regulation.Reference Habbout, Li and Rochette 21 – Reference De Moura, Lisboa and Passos 23 Animals raised in small litters during lactation are considered to be a good experimental model for overweight research related to programming caused by postnatal early overfeeding (EO). These animals develop higher adiposity, liver dysfunction, which features increased oxidative stress and microsteatosis, and dysfunction of the hypothalamic system, which induces persisting central leptin and insulin resistance in adulthood.Reference Habbout, Li and Rochette 21 , Reference Li, Plagemann and Davidowa 24
Some studies have highlighted the beneficial effects of YM mate tea in experimental models of endocrine–metabolic dysfunctions. Hussein et al.Reference Hussein, Matsuda and Nakamura 25 demonstrated that the administration of YM to mice fed a high-fat diet (HFD) normalized hyperglycaemia and serum insulin levels and improved insulin sensitivity in a metabolic syndrome model. Furthermore, in mice with diet-induced obesity, reductions in body weight (BW) gain and serum cholesterol, triglycerides (TGs) and glucose were shown to occur irrespective of the dose ingested (0.5, 1 or 2 g/kg instant YM/kg BW).Reference Kang, Lee and Kim 26 YM also has effects on liver metabolism. Arçari et al.Reference Arçari, Santos and Gambero 15 showed that YM treatment improved the insulin signalling pathway after 8 weeks of feeding with a HFD. In addition, the YM treatment is able to protect the liver against lipid peroxidation;Reference Gao, Long and Jiang 27 however, liver antioxidant enzymatic protection has not yet been evaluated in an obesity model. A previous study from our group showed that 30 days of YM treatment was able to reverse abdominal obesity, leptin resistance and hypertriglyceridaemia in obese adult rats programmed by precocious undernutrition, which was caused by blocking the access of the pups to maternal milk during the last 3 days of lactation without mother deprivation, featuring a new early weaning model.Reference Lima, de Moura and Passos 28 This model has similarities with the EO model, such as obesity, dyslipidaemia and hypothalamic leptin resistance.Reference Rodrigues, de Moura and Passos 29 , Reference Rodrigues, de Moura and Passos 30 Thus, it is interesting to evaluate whether YM treatment could correct the metabolic dysfunctions programmed by EO.
Considering that overweight rats raised in small litters display metabolic disturbances, such as higher visceral adiposity, oxidative stress and steatosis, and that YM acts as an antioxidant and anti-obesogenic tea, the present study evaluated the effect of YM treatment on liver morphology, oxidative stress and antioxidant enzymes in an obesity model involving programming by postnatal EO. Furthermore, we evaluated whether YM treatment is able to prevent hyperphagia through the normalization of central leptin and insulin signalling.
Materials and methods
Experimental model
The Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro approved our protocol (CEUA/006/2009). In total, 30 adult Wistar rats that were 120 days old were housed under controlled temperature (23±3°C) and light (12 h light/dark cycle), with free access to water and food. A total of 20 nulliparous female rats were placed with male rats at a 2:1 ratio for 5 days. After mating, 20 pregnant females were housed in individual cages until delivery. After the pups’ birth, all litters were adjusted to 10 male pups/dam. To induce EO group, on the 3rd day of life, the litter size was reduced to three male pups per dam. The control group (C group) was maintained with 10 male pups/dam until weaning, that is, postnatal (PN) day 21. After weaning, three C rats from each litter were randomly placed per cage as well as all three EO rats per litter per cage.
Oral treatment with mate tea
On PN150, two EO offspring from each litter were randomly subdivided into two groups: EO+mate tea (EO+mate, n=10), in which the rats received an instant YM solution at a dose of 1 g/kg BW in a total volume of 2 ml of water (n=4), and EO+water (EO, n=10), in which the rats received pure water (2 ml). The control offspring from each litter (n=10) received pure water (2 ml). At this age, two rats were placed per cage each group. All rats received mate tea or water once a day for 30 days via intragastric gavage. The prolonged administration of repeated doses (2 g/kg weight) of YM extract in rats and rabbits has been reported to cause no apparent symptoms or signs of toxicity.Reference Andrade, de Albuquerque and Maraschin 31 The mate tea was prepared from lyophilized instant roasted mate tea obtained from Matte Leão® (Leao Jr., Curitiba-PR, Brazil) from the same industrial batch. The total phenolic (8.35±0.5 g/l) content was estimated using the Folin–Ciocalteu method. A high-performance liquid chromatography analysis of soluble YM powder showed the presence of 41.20±8.0 mg/l of chlorogenic acid, 21.00±4.4 mg/l of caffeine, and 8.57±1.0 mg/l of teobromine; quercertin and rutin were not detected. The roasted mate tea was prepared fresh each day by dissolving the lyophilized instant roasted mate tea powder (Leao Jr.) in filtered water using a homogenizer.
The BW and food intake of the rats were monitored every 4 days from PN21 until the time of sacrifice on PN180. The food intake was calculated based on the difference between the weight of the food that remained in the food bin and the amount of food placed in the bin 4 days earlier, divided by the number of days (4) and the number of rats in the cage.
On PN180, the rats were fasted for 12 h before killing by decapitation using a rodent guillotine. The last mate tea gavage occurred 24 h before euthanasia. The visceral adipose tissue (epididymal, mesenteric and retroperitoneal depots) was dissected and weighed; the data were expressed as g/100 g BW. The blood samples were centrifuged (1000 g , 4°C, 20 min) to collect the plasma, which was stored (−20°C) until the time of analysis. The hypothalamus, liver, visceral adipose tissue were collected and kept at −80°C for subsequent analysis. The carcass was storage at −20°C.
Body composition
The body composition (total fat and protein mass) was determined via carcass analysis. C, EO and EO+mate offspring were eviscerated; the carcasses plus visceral adipose tissue were weighed, autoclaved for 1 h, and homogenized in distilled water (1:1).Reference Leshner, Litwin and Squibb 32 The homogenates were stored at 4°C for analysis. Briefly, 3 g of homogenates were used to determine the fat content gravimetrically. The samples were hydrolysed in a shaking water bath at 70°C for 2 h with 30% KOH and ethanol. Total fatty acids and no esterified cholesterol were removed with three successive washes with petroleum ether. After drying, all tubes were weighed, and the data were expressed as g fat/100 g carcass. The protein content in 1 g of homogenate was determined after centrifugation (2000 g for 10 min), and the protein concentrations of the supernatants were determined using the Lowry method.Reference Lowry, Rosebrough and Farr 33 The data were expressed as g protein/100 g carcass.
Lipid profile
The plasma levels of total cholesterol (TC), TGs and high-density lipoprotein (HDL) were analysed on PN180 using Biosystem commercial test kits (Biosystems S.A., Barcelona, Spain). Low-density lipoprotein (LDL-c) and very low-density lipoprotein (VLDL-c) levels were obtained using Friedewald calculations,Reference Friedewald, Levy and Fredrickson 34 as follows:
-
1. LDL-c (mg/dl)=TC−(TG/5) −HDL-c.
-
2. VLDL-c (mg/dl)=TG/5.
Glucose measurement
Glycaemia was determined in blood samples from the tail veins of fasting rats using a glucometer (ACCU CHEK-Active; F. Hoffmann-La Roche Ltd, Basel, CH, Switzerland).
Plasma hormone determination by radioimmunoassay (RIA)
Leptin was measured using a specific RIA kit (Linco Research Inc, St. Charles, MO, USA), with a range of detection from 0.5 to 50 ng/ml; the intra-assay variation was 4.9%. Insulin was measured using an Insulin RIA kit (Linco Research Inc). The limit of detection was 0.1 ng/ml. The intra-assay variation was 1.4%. Samples were measured in duplicate. All measurements were made in a single RIA assay for leptin as well as in a single RIA assay for insulin.
Western blotting analysis
The contents of the signalling proteins in the leptin pathway (JAK2, STAT3, phospho-STAT3 and SOCS3) and insulin pathway (insulin receptor β, IRS1, PI3K and Akt) were evaluated via Western blot analysis of the total hypothalamus. The tissues were processed as reported previously.Reference Rodrigues, de Moura and Passos 29 Briefly, whole tissue cell extracts were homogenized in ice-cold lysis buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1 mM MgCl2, 10 mM ethylenediamine tetraacetic acid (EDTA) and 1% Triton X-100, pH 6.4) with protease inhibitor (complete, EDTA-free; F. Hoffmann-La Roche Ltd). The homogenates were centrifuged at 1120 g for 5 min at 4°C. The protein concentrations in the supernatants were determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, San Jose, CA, USA). The homogenates were then analysed via sodium dodecyl sulfate -Polyacrylamide gel electrophoresis (SDS-PAGE) using 10 μg of total protein. The samples were electroblotted onto nitrocellulose membranes (Hybond ECL; Amersham Pharmacia Biotech, Amersham, London, UK). The membranes were incubated with Tris-buffered saline (TBS) containing 2% albumin for 90 min. Then, the membranes were washed with TBS and incubated with a specific primary antibodies against leptin and insulin signalling pathways [against JAK2, STAT3, phospho-STAT3, SOCS3, insulin receptor β, IRS1, PI3K (p85alpha), Akt and tubulin] (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at an adequate dilution overnight at 4°C. The membranes were washed and incubated with a secondary antibody (goat anti-mouse; Santa Cruz Biotechnology) conjugated with horseradish peroxidase (HRP) at an adequate dilution for 1 h at room temperature. Finally, the protein bands were visualized by chemiluminescence (Kit ECL plus; Amersham Biosciences, London, UK) followed by exposure to autoradiographic film (Hyperfilm ECL; Amersham Biosciences). The area and density of the bands were quantified using the Image J software (Wayne Rasband National Institute of Health, MA, USA). The results were expressed relative (%) to the control group.
Liver parameters
TG content
The total liver lipid content was solubilized in isopropanol (Vetec, Duque de Caxias, RJ, Brazil) and centrifuged (740 g , 10 min, 4°C). The supernatant TG content was measured using a commercial kit (Bioclin, BH, MG, Brazil). The absorbance was measured at 490–540 nm (Hidex, Turku, Finland).
Antioxidant enzyme activities
Liver samples (200 mg) were gently homogenized in potassium phosphate buffer with EDTA using a Potter homogenizer (Marconi Equipamentos, Piracicaba, SP, Brazil). After centrifugation, the homogenates were stored at −80°C until the time of subsequent analysis. The total protein content was determined using the Bradford method.Reference Bradford 35 Total SOD activity was assayed by measuring the inhibition of adrenaline auto-oxidation as the absorbance at 480 nm.Reference Bannister and Calabrese 36 CAT activity was measured based on the rate of decrease in H2O2 at 240 nm, according to the Aebi method.Reference Aebi 37 GPx activity was evaluated according to the method described by Flohé and GünzlerReference Flohé and Günzler 38 by measuring the oxidation of NADPH at 340 nm in the presence of H2O2.
Malondialdehyde (MDA) content
Lipid peroxidation was estimated by quantifying MDA using the thiobarbituric acid reactive substances method. To 200 μl of liver homogenate, 400 μl of 10% trichloroacetic acid were added. These samples were centrifuged (10 min, 1000 rpm, 4°C), and 500 μl of supernatant were incubated with 500 μl of 0.67% thiobarbituric acid (Sigma Chemical, St. Louis, MO, USA) at 100°C for 30 min. The absorbance was read using a spectrophotometer (532 nm).
Stereology
Liver samples obtained from different lobes were fixed in freshly prepared fixative (1.27 M-formaldehyde and 0.1 M-phosphate buffer, pH 7.2) for 48 h at room temperature, subjected to histological processing, embedded in Paraplast plus (Sigma-Aldrich, St. Louis, MO, USA), sectioned to 5 μm thick and stained with haematoxylin and eosin for visualization using light microscopy. The evaluation of hepatic steatosis was performed with the point counting method using a test system composed of 36 test points (PT). The volume density (Vv) was estimated as: Vv [steatosis, liver]=PP [steatosis]/PT [liver], where PP is the number of points counting fat droplets on hepatic tissue (steatosis) and PT the total test points. Three slices were performed with three cuts each slice (in a total of nine cuts) and 10 microscopic fields/animal (n=5/group) were analysed randomly (blind analysis). Digital images were acquired using an Olympus BX40 microscope with an Olympus DP71 camera (Olympus, Tokyo, Japan). The images were acquired in TIFF format and 36-bit colour, with a size of 1360×1024 pixels and magnifications of 60× and 100×.
Statistical analysis
The results are reported as the mean±s.e.m. The GraphPad Prism 4 software was used for statistical analyses and graphics (GraphPad Software Inc, CA, USA). The experimental data were analysed using one-way analysis of variance (ANOVA) and Newman–Keuls multiple comparison tests. The significance level was set at P<0.05.
Results
The effect of postnatal EO and YM treatment on food intake and biometric parameters
EO rats had higher BWs from the time of weaning (PN21) until PN150, when the YM treatment started (Fig. 1a). On PN180, the EO group was heavier than the C group (+15%); the BW of the EO+mate group was significantly lower than that of the EO group (−7%) (Fig. 1a). The EO group had higher food intake at PN180 (+17%), and the YM treatment was able to normalize the food intake (−18% v. EO group; Fig. 1b). At 180 days, the EO group exhibited higher total visceral fat (+104%), and YM treatment resulted in lower total visceral fat (−42% v. EO group) levels that were similar to those of the C group; these results were expressed as individual fat depots (Table 1). In addition, the total body fat was higher in the EO group (+40%) and normal in the EO+mate group (−35% v. EO group). The protein mass was higher in the EO group (+34%), but YM treatment did not change this parameter (Table 1).

Fig. 1 The effect of postnatal (PN) overfeeding and mate treatment in biometric parameters. Body weight at PN21, PN150 and PN180 (a), and cumulative food intake from PN150 until PN180 (b). Values represent mean±s.e.m. of 10 rats/group. P<0.05, * v. C, # v. early overfeeding (EO).
Table 1 The effect of postnatal overfeeding and yerba mate treatment in body composition and liver parameters at 180 days old

C, control; EO, early overfeeding; EO+mate, early overfeeding treated with mate tea.
Values represent mean±s.e.m. of 10 rats/group.
P<0.05, * v. C, # v. EO.
The effect of postnatal EO and YM treatment on the lipid profile, glycaemia and plasma hormone levels
The EO group had higher VLDL-c and TG levels (+32.5 and 32%, respectively) than the control group, and these parameters were not improved by YM treatment. As already reported,Reference Patel and Srinivasan 22 glycaemia, insulinaemia and leptinaemia were not altered in the EO group on PN180. The YM treatment also failed to change these parameters (Table 2).
Table 2 The effect of postnatal overfeeding and yerba mate treatment in blood lipids, glucose and hormones at 180 days old
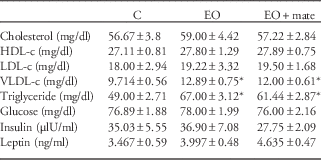
C, control; EO, early overfeeding; EO+mate, early overfeeding treated with mate tea; HDL-c, high-density lipoprotein; LDL-c, low-density lipoprotein; VLDL-c, very low-density lipoprotein.
Values represent mean±s.e.m. of 10 rats/group.
P<0.05, * v. C.
The effect of postnatal EO and YM treatment on the leptin signalling pathway in the hypothalamus
As expected, EO rats had lower JAK2 and phospho-STAT3 contents and increased SOCS3 content (Fig. 2). However, the STAT3 content and phospho-STAT3/STAT3 ratio (Fig. 2) exhibited no differences among the three groups. The YM treatment group was not different from the C or EO group.
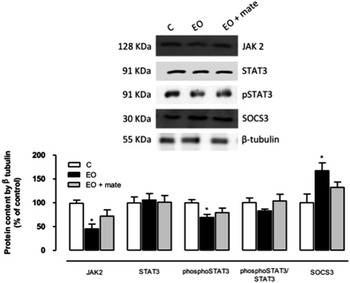
Fig. 2 The effect of postnatal (PN) overfeeding and mate treatment in hypothalamic leptin pathway. Hypothalamic protein levels of JAK2, STAT3, phospho-STAT3, phospho-STAT3/STAT3 and SOCS3 at PN180 and the representative Western blot bands. Protein contents were quantified by scanning densitometry of the bands, normalized through the β-tubulin loading control and expressed in % of control. Values represent mean±s.e.m. of eight rats per group. P<0.05, * v. C. EO, early overfeeding.
The effect of postnatal EO and YM treatment on the insulin signalling pathway in the hypothalamus
As depicted in Fig. 3, adult EO rats presented lower insulin receptor β (−26%), IRS1 (−16.5%) and Akt (−38%) protein contents in the hypothalamus on PN180, and the YM treatment normalized these parameters. In addition, the EO group had a lower PI3K protein content; however, the YM treatment did not affect this parameter.
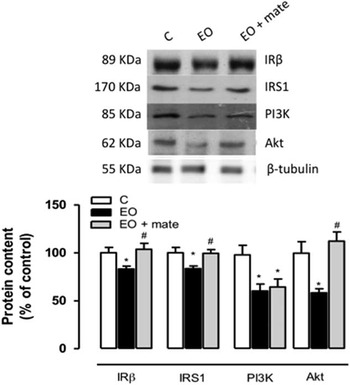
Fig. 3 The effect of postnatal (PN) overfeeding and mate treatment in hypothalamic insulin pathway. Hypothalamic protein levels of insulin receptor β, IRS1, PI3K and Akt at in PN180 and the representative Western blot bands. Protein contents were quantified by scanning densitometry of the bands, normalized through the β-tubulin loading control and expressed in % of control. Values represent mean±s.e.m. of eight rats per group. P<0.05, * v. C, # v. early overfeeding (EO).
The effect of postnatal EO and YM treatment on liver parameters
The EO group had a higher TG (+39%) content in the liver, which was normalized in the EO+mate group (Table 1). Antioxidant enzyme activities in hepatic tissue, which were lower in the EO group (Table 3), were improved by YM treatment (SOD:+43%; GPx:+24%; and CAT:+72%). In addition, MDA, which was higher in the EO group (Table 3), was restored in the EO+mate group (−20%). The morphological analysis revealed hepatic dysfunction in the EO group (Fig. 4). This group presented microvesicular steatosis that was widely distributed in the liver tissue in comparison with the normal hepatic architecture of the C group (Fig. 4). The EO+mate group exhibited normalized hepatic histology (Fig. 4), with minimal lipid foci, highlighting the beneficial effects of the YM treatment.
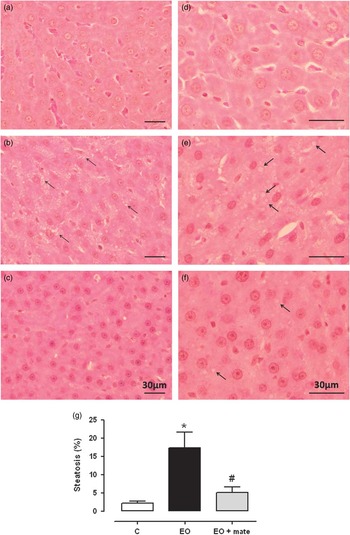
Fig. 4 The effect of postnatal (PN) overfeeding and mate treatment in liver morphology. Photomicrographs of liver tissue stained with haematoxylin and eosin in PN180 using a 60× objective (a–c) and using a 100× objective (d–f). C group (a,d); early overfeeding (EO) group (b,e) and EO+mate group (c,f). Percentage of steatosis performed by point counting method (g). The arrows represent the droplets of lipids in the hepatocytes characterizing a microsteatosis. Values represent mean±s.e.m. of five rats per group. P<0.05, * v. C, # v. EO.
Table 3 The effect of postnatal overfeeding and yerba mate treatment on the antioxidant enzymes activities and lipid peroxidation in the liver at 180 days old

C, control; EO, early overfeeding; EO+mate, early overfeeding treated with mate tea; SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde.
Values represent mean±s.e.m. of 10 rats/group.
P<0.05, * v. C, # v. EO.
Discussion
To our knowledge, for the first time in an EO model, we reported the effects of a 30-day treatment with Ilex paraguariensis (YM) on adiposity, lipid profiles, serum leptin and insulin levels and its hypothalamic signalling, liver microsteatosis and oxidative stress, which are directly related to obesity and its metabolic disorders. Here, we did not evaluate the possible effects in control rats, because our main aim was to evaluate the action of YM on obesity.
The literature shows that YM affects BW management in obese models,Reference Gamboa-Gómez, Rocha-Guzmán and Gallegos-Infante 17 – Reference Kim, Oh and Kim 20 which makes YM consumption a promising strategy for obesity prevention and/or reversion. Here, YM treatment for 1 month prevented weight gain in part due to a decrease in food intake. We previously reported the absence of positive correlation between fat depots and leptinaemia in the EO model, since small litter (SL) rats have visceral fat hypertrophy but normoleptinaemia, which were explained because they had decreased leptin content in visceral adipocyte at PN180, suggesting a lower leptin production.Reference Conceição, Trevenzoli and Oliveira 39 It is well established that adult EO rats present alterations in hypothalamic energy homoeostasis that are marked by resistance to the anorexigenic effects of both insulin and leptin.Reference Habbout, Li and Rochette 21 , Reference Rodrigues, de Moura and Passos 29 , Reference Plagemann 40 Both central leptin and insulin resistance contribute to the establishment of obesity, promoting hyperphagia and weight gain.Reference Rodrigues, de Moura and Passos 29 , Reference Plagemann 40 , Reference Conceição, Franco and Oliveira 41 The present study characterized the prevention of central insulin resistance in overweight animals treated with YM, as evidenced by the normalization of the observed reduced hypothalamic IRβ, IRS1 and Akt protein contents. However, the PI3K content remained lower in the EO+mate group. It is possible that PI3K activity is increased by YM treatment, which must be tested in a future study. A limitation of our study is that we did not evaluate the effect of the central injection of insulin on the food intake or on the neuropeptides involved in food intake control.
It is now recognized that the chronic and low-grade inflammation that develops during obesity could connect overweight to the development of insulin resistance. SL rats presented higher tumour necrosis factor alpha (TNFα), TNF-receptor-1, interleukin-6 (IL6) and resistin messenger RNA expression.Reference Habbout, Li and Rochette 21 It is interesting that the inhibition of hypothalamic inflammation using the immunoneutralization of TNFα or its receptor reverses insulin resistance in an obesity model.Reference Könner and Brüning 42 Arçari et al.Reference Arçari, Bartchewsky and dos Santos 12 showed that mate tea treatment for 8 weeks decreased TNFα and IL6 expression in the adipose tissue in mice fed a HFD. Our study highlights the induction of a significant improvement in the insulin signalling pathway by YM treatment. Pimentel et al.Reference Pimentel, Lira and Rosa 43 reported that YM extract intake (0.01 and 0.02 g/day) for 2 months blunted pro-inflammatory effects in obese rats and consequently increased insulin sensitivity. Thus, in our study, treatment with YM probably normalized insulin signalling in the hypothalamus due to its anti-inflammatory properties, as reported previously by other authors.Reference Arçari, Bartchewsky and dos Santos 12 , Reference Pimentel, Lira and Rosa 43
Previously, our group reported the effects of YM treatment in early weaned rats. These rats present hyperphagia and central leptin resistance.Reference Lima, de Moura and Passos 28 Thirty days of YM treatment was able to normalize the food intake and prevent the development of obesity. The YM normalized the SOCS3 protein content in the hypothalamus, which was associated with the improvement of leptin anorexigenic effects in early weaned rats. In addition, this result was associated with a decrease of neuropeptide Y (NPY) in the hypothalamus.Reference Lima, Franco and Peixoto-Silva 44 Thus, maybe the same phenomenon is happening with EO+mate rats. The effect of YM on leptin signalling in the small litter size model seems to act through the same mechanism; however, despite a tendency, treatment did not result in a significant difference between C and EO untreated groups. Perhaps SL rats demand a longer YM treatment to achieve the same leptin signalling results.
The decrease in food intake contributes to the reduction of total body adiposity and visceral fat depots in the EO+mate group. This result is consistent with previous findings related to YM treatment in obese animals, which showed that YM acts directly upon adipocyte metabolism, decreasing adipocyte proliferation and lipid accumulation. Using YM treatment (1.0 mg/kg) in HFD-fed mice, Arçari et al.Reference Arçari, Santos, Gambero and Ribeiro 13 showed a reduction of pro-adipogenic genes and an up-regulation of genes related to adipogenesis inhibition. Moreover, this group used the same experimental protocol to show an increase in thermogenic capacity through the increase of uncoupling protein-1 expression in epididymal adipose tissue.Reference Arçari, Bartchewsky and dos Santos 12 Hussein et al.Reference Hussein, Matsuda and Nakamura 25 showed that obese diabetic mice treated with YM for 7 weeks exhibited reduced adipocyte proliferation. Another study demonstrated that YM treatment (0.5, 1.0 and 2.0 g/kg weight) for 4 weeks decreased the accumulation of lipids in adipocytes in mice fed with a HFD.Reference Kang, Lee and Kim 26
The YM treatment has well-known hypolipidaemic effects. Mate aqueous extract decreased serum lipid levels in a hyperlipidaemic hamster model due to the inhibition of SREBP-1c liver expression.Reference Gao, Long and Jiang 27 This transcription factor is responsible for inducing the expression of several genes involved in the uptake of lipoproteins and the synthesis of TC, TG and VLDL.Reference Begriche, Igoudjil and Pessayre 45 Despite this, in the current study, YM was not able to correct the lipid profile in the EO+mate group. Maybe, the differences observed between our study and previous reports are due to the shorter period of YM treatment as well as to the fact that rat, contrary to hamster or mouse, is less sensitive to the effects of YM on the parameters analysed.
The liver is an important organ for energetic management, which is responsible for glucose storage and release to ensure adequate levels of glucose during the day under hormonal and neuronal influence. In obesity, liver integrity is impaired due to the existence of a pro-inflammatory status and a high lipid influx from the adipose tissue that is insulin resistant.Reference Sahini and Borlak 46 In the present study, YM treatment resulted in a reduction of microsteatosis in the EO+mate group. Previous reports in a mouse obesity model showed that microvesicular hepatic steatosis is prevented by YM treatment (100 mg/kg/day) for 7 weeks, even with a HFD.Reference Hussein, Matsuda and Nakamura 25 Concerning this issue, Gao et al.Reference Gao, Long and Jiang 27 showed that YM treatment for 4 weeks increased peroxisome proliferator-activated receptor α (PPARα) expression and decrease SREBP-1c in the liver in association with increased β-oxidation and decreased de novo lipogenesis. Consequently, the animals became less prone to steatosis development.
It is well established that obesity is often characterized not only by chronic inflammation but also by increased oxidative stress. A recent study suggested that oxidative stress, which is the imbalance between the cellular production of reactive oxygen species and antioxidant defences, could be an early event in the development of obesity-related chronic diseases.Reference Gao, Long and Jiang 27 The antioxidant system involves several non-enzymatic compounds and antioxidant enzymes, such as SOD, CAT and GPx. Our recent data concerning the livers of EO rats revealed higher liver and plasma MDA contents and a decrease in the activities of these antioxidant enzymes, suggesting reduced antioxidant defence.Reference Conceição, Franco and Oliveira 41 The higher oxidative stress was associated with microsteatosis and insulin resistance in the livers of EO rats.Reference Conceição, Franco and Oliveira 41 Here, we demonstrated that YM treatment improved the liver antioxidant cellular enzymatic machinery and prevented lipid peroxidation and steatosis. Gao et al.Reference Gao, Long and Jiang 27 showed that mate tea increased plasma SOD and GPx activities and decreased MDA content in a hyperlipidaemic hamster model, corroborating our liver data. Another study showed that treatment with YM for 60 days increase the antioxidant defensive system and minimize peroxidative damage of mouse liver lipids.Reference Yun, Kang and Lee 47
We demonstrated that EO rats have central insulin and leptin resistance, hepatic steatosis and higher liver oxidative stress, which corroborate our previous data.Reference Rodrigues, de Moura and Passos 29 , Reference Rodrigues, de Moura and Passos 30 , Reference Conceição, Franco and Oliveira 41 Mate tea treatment prevents these changes, except for the leptin signalling pathway, which showed intermediate values. In addition, YM treatment significantly improved hypothalamic insulin signalling, as well as oxidative stress, microsteatosis and TG accumulation in the livers of EO+mate rats, suggesting an improvement in the peripheral action of insulin. These beneficial effects of YM on metabolic parameters in animals suggest that it can be useful as a therapeutic agent. Concerning YM use in human, it is important to point out that despite some association of YM ingestion in human populations with oesophageal squamous cell carcinoma, it seems that it is more related to the high temperature of the YMReference Lubin, De Stefani and Abnet 48 than any specific YM component, as showed by Rapozo et al.Reference Rapozo, Blanco and Reis 49 in an experimental model. Moreover, the increase in this type of carcinoma was associated with the higher consumption of barbecued meat that increases the ingestion of nitrosamine when associate with any hot beverage.
Conclusion
In summary, these findings suggest that mate aqueous extract may be useful in preventing and treating some endocrine–metabolic diseases related to obesity, such as liver steatosis, primarily due to its antioxidant and anti-inflammatory effects. Moreover, for the first time, we showed the possible hypothalamic mechanism of action of YM in a postnatal EO model and its improvement of oxidative stress and insulin sensitivity both centrally and peripherally. Therefore, our study suggests the importance of the future use of Ilex paraguariensis (YM) as a therapeutic strategy in the management of obesity, independent of its developmental origin.
Acknowledgements
All authors are grateful to Monica Moura, Ulisses Risso Siqueira and Nilton França for technical assistance.
Financial Support
This research was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq), and the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (Fundacão Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ).
Conflicts of Interest
None.
Ethical Standards
The Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro approved the protocol (CEUA/006/2009). The experiment was conducted to minimize the number of animals and any suffering, following the ethical doctrine of the three ‘Rs’ (reduction, refinement and replacement).










