Foetal supraventricular tachycardia is frequently an incidental diagnosis, with an estimated incidence of 14 in 100,000 pregnancies in our own practice. In some cases, the arrhythmia may remain undetected during the course of pregnancy, leading to hydrops, cardiac failure, dilated cardiomyopathy in the foetus, or neurological sequelae in the newborn. Furthermore, when complicated with hydrops, foetal tachycardia has been associated with a high mortality risk of up to 35%.Reference Simpson and Sharland 1
Despite the serious consequences of this rare arrhythmia, its prenatal management remains somewhat controversial.Reference Simpson and Sharland 1 – Reference Texter, Kertesz, Friedman and Fenrich 3 There are numerous confusing and complicated algorithms regarding anti-arrhythmic drug treatment of foetal supraventricular tachycardia.Reference Hansmann, Gembruch, Bald, Manz and Redel 4 – Reference Hornberger and Sahn 8 Although digoxin is advocated as the first-line anti-arrhythmic treatment in non-hydropic foetuses, its reported efficacy of approximately 50% is far from ideal.Reference Simpson and Sharland 1 , Reference Frohn-Mulder, Stewart, Witsenburg, Den Hollander, Wladimiroff and Hess 2 , Reference Ebenroth, Cordes and Darragh 9
Our aim is to emphasise the importance of rapid control of foetal tachycardia with flecainide and digoxin combination treatment in order to avoid foetal demise, which have been unacceptably high even in this current era.Reference Simpson and Sharland 1 , Reference Oudjik, Michon and Kleinman 10
Materials and methods
This retrospective study was carried out at a tertiary referral centre within a university hospital from January, 2001 to December, 2009. In all, 29 consecutive foetuses presenting with foetal supraventricular tachycardia were included in the analysis. Significant foetal tachycardia was defined as a foetal heart rate of more than 200 beats per minute.
In all foetuses, standard transabdominal foetal echocardiography was performed to confirm and determine the type of arrhythmia, to assess cardiac function, and to exclude any associated structural abnormality or hydrops. The cardiac dimensions were measured using M-mode echocardiography, the shortening fraction of a ventricle was calculated by established methods as described previously, and cardiac function was considered abnormal if the fractional shortening was under 26%.Reference DeVore 11 All studies were recorded on a hard drive or on videotapes for subsequent playback and analysis purposes.
Tachycardia that was interspersed with periods of sinus rhythm during ultrasound scan was called “intermittent”. Foetal hydrops was defined as the presence of fluid in at least two cavities. Every patient was also referred to the foetal medicine unit for a detailed scan to rule out any associated non-cardiac anomalies and for ongoing assessment of foetal well-being.
The first six foetuses out of 27 were initially treated with either digoxin – in five – or flecainide alone – in one – according to the protocols reported in the literature.Reference Oudijk, Visser and Meijboom 7 , Reference Hornberger and Sahn 8 , Reference Lulić Jurjević, Podnar and Vesel 12 Owing to the fact that there was no change in foetal heart rate after 4–14 days of monotherapy with digoxin or flecainide, in spite of high maternal drug levels, a second anti-arrhythmic medication, either flecainide or digoxin, was later initiated in all six patients. Hence, all 27 patients had received the combination treatment.
In 21 patients, the digoxin and flecainide combination treatment was started from the time of diagnosis. Digoxin was given at 250 micrograms three times a day, and flecainide at 100 milligrams three times a day. In case of hydrops or atrial flutter, the first digoxin dose was given at 500 micrograms and the subsequent two doses at 250 micrograms on the first day of treatment. The anti-arrhythmic dose was adjusted according to maternal tolerance, drug levels, electrocardiogram, and foetal clinical response to tachycardia. Anti-arrhythmic medication at lower doses was continued throughout the pregnancy even if the sinus rhythm was restored.
Maternal cardiac status, electrocardiogram, and renal and liver functions were monitored before the start of anti-arrhythmic therapy and weekly thereafter. Serum concentrations of digoxin and flecainide were checked on the fifth day of treatment, and weekly thereafter, or as deemed necessary. Anti-arrhythmic drug levels were maintained at the higher end of normal. On a 12-lead maternal electrocardiogram, QRS duration was not allowed to prolong more than 25% of the baseline, corrected QT interval was kept below 480 milliseconds, and PR interval was maintained below 200 milliseconds.
Treatment response was monitored by auscultation of foetal heart rate twice weekly by a midwife. A weekly ultrasound and Doppler scan surveillance was used to analyse foetal heart rate and rhythm, resolution of hydrops, cardiac function, foetal well-being, and amniotic fluid volume. The patients were often admitted to hospital in the first few days of treatment, or longer in the presence of hydrops.
All newborns had clinical examination by a neonatologist and a paediatric cardiologist separately. In addition, a 12-lead electrocardiogram and cardiac ultrasound examinations were carried out in all newborns after birth. Anti-arrhythmic treatment was not initiated automatically in all newborns, unless there was recurrence of arrhythmia after birth. All children were followed up at regular intervals by a paediatric cardiologist and by a paediatrician from a tachycardia and developmental point of view, respectively. If there were parental or medical concerns with developmental stage, learning, or gross motor functions of a child, a detailed neurological examination was performed by a paediatrician with expertise in childhood disabilities. The Ruth Griffiths mental developmental scale was used to assess the neurodevelopmental status of the affected children.Reference Huntley 13 Asymptomatic children were discharged from cardiac follow-up if they remained arrhythmia free for 12 months after discontinuation of anti-arrhythmic medication.
Statistical analysis was performed using a computer-based program (SPSS for Windows version 14.0). All data were expressed as mean plus or minus standard deviation. The quantitative values between the two groups were compared using the Mann–Whitney U test and the Wilcoxon signed-rank test for paired test. The Spearmen test was used to evaluate the correlation between maternal drug levels and maternal electrocardiographic parameters. A p-value less than 0.05 was considered statistically significant.
Results
Timing of presentation and diagnosis
Foetal supraventricular tachycardia was identified in 29 patients between 18 and 39 weeks of gestation, with a median of 29 weeks (Table 1). The maternal age at presentation was 17–40 years, with a median of 27 years. In 27 cases, foetal tachycardia was diagnosed incidentally; two patients self-presented because of reduced foetal movements in one and patient feeling unwell in the other.
Table 1 Maternal, foetal characteristics and clinical findings.

AFL = atrial flutter; D(n) = start of day of digoxin; FS = fractional shortening; F(n) = start of day of flecainide, ILVF = impaired left ventricular function; PE = pericardial effusion; SR = sinus rhythm; SVT = supraventricular tachycardia; VGA = vein of Galen aneurysm
Response time, n(n): first number indicates total days from the start of treatment, and the number in brackets denotes response time in days after initiation of a second medication
Associated anomalies
The foetal cardiac anatomy was considered to be normal in 28 cases, confirming a low association of congenital cardiac abnormalities with foetal tachycardia. There was one foetus with Ebstein's anomaly of the tricuspid valve. Postnatally, three more patients were found to have small muscular ventricular septal defects, in all of whom the defects closed spontaneously in the follow-up. The foramen ovale was noted to be prominent in two further cases, which were confirmed to be atrial septal defects postnatally. Initially, one foetus was diagnosed with vein of Galen aneurysm and later developed supraventricular tachycardia. In another foetus, severe hydrocephalus with aqueduct stenosis and hypoplastic cerebellum was detected at 21 weeks. One foetus had unexplained severe ascites in the presence of normal cardiac function. There were no chromosomal anomalies in any infants.
There was one case of maternal hypothyroidism that was adequately treated with thyroxine and remained euthyroid throughout the pregnancy. There were no other maternal risk factors.
Type of arrhythmia, cardiac failure, and hydrops
Atrioventricular re-entry tachycardia was diagnosed in 22 (75%) cases of which three were intermittent (Table 1). Hydrops occurred in five out of 22 cases (22%). In addition, decreased ventricular systolic function without hydrops was identified in five foetuses. The time to control of arrhythmia ranged from less than 24 hours to 24 days in 20 cases. In six patients, a single drug was used for an average of 11.8 plus or minus 4.4 days without any clinical response in spite of achieving therapeutic digoxin or flecainide levels, but following start of the combination treatment arrhythmia was controlled within 5.1 plus or minus 2.3 days On the contrary, in 14 patients, who had combination treatment from the onset, foetal tachycardia responded to medication within 4.7 plus or minus 3.8 days. The comparison of drug levels between the two groups who had initial monotherapy versus combination treatment did not show any statistical difference (p > 0.05; Table 2). There was one refractory case that neither responded to our proposed combination, nor to any other recommended anti-arrhythmic, including sotalol and amiodarone even when they were given directly to the foetus. This resistant case had severe hydrops with lung hypoplasia and was subsequently delivered at 29 weeks of gestation due to foetal distress. One foetus presented at 39 weeks was delivered without any treatment (Fig 1).
Table 3 Maternal ECG parameters before and after treatment.

ECG = electrocardiogram; HR = heart rate; PR = PR interval; QTc = corrected QT interval.

Figure 1 The outcome of all foetuses.
Atrial flutter was the cause of arrhythmia in seven cases and hydrops occurred in three (42%; Table 1). Digoxin and flecainide combination was started in six foetuses, but one foetus was delivered at 38 weeks of gestation at the time of presentation without any treatment. Despite the fact that rate control was achieved in all six foetuses with atrial flutter, sinus rhythm could be restored in only two (33%), one of which was hydropic. The time to restore sinus rhythm or to rate control arrhythmia ranged from 12 hours to 18 days, with a mean of 4.7 plus or minus 3.8 days.
Among all 29 foetuses, hydrops was present in eight cases (27%) at the time of diagnosis (Table 1). There was complete resolution of hydrops with treatment in four fetuses with re-entry supraventricular tachycardia. In one case with atrial flutter, hydrops resolved after 8 days of treatment (Fig 2).
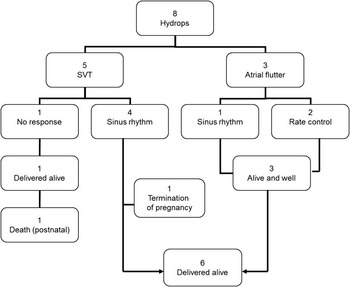
Figure 2 The outcome of foetuses with hydrops.
Maternal anti-arrhythmic tolerance
Nausea appeared to be the main side effect in five patients (18%), with headache, tiredness, and loss of appetite being mentioned in conjunction. One patient complained of sickness and visual symptoms related to digoxin despite the dose being in therapeutic range – 1.32 nanomol per litre. Nausea improved in three patients upon reduction of the doses of digoxin and flecainide. Maternal electrocardiograms were compared before and after treatment. PR interval, QRS and corrected QT durations were significantly prolonged with treatment (p < 0.05; Table 3), but a majority of these changes were within normal limits. PR prolongation beyond 200 milliseconds was observed in three patients, QRS widened beyond 25% of baseline in three patients, and corrected QT prolonged over 480 milliseconds in two patients. However, there was no statistically significant correlation between maternal drug levels and monitored electrocardiographic parameters (p > 0.05; Table 4). Only two patients who exhibited PR interval prolongation beyond 200 milliseconds also had digoxin levels above the normal range. Deranged liver function test in one patient was caused by obstetric cholestasis. Upon reduction of the offending drug dose, all of the above-mentioned side effects resolved rapidly.
Table 4 Correlation of maternal drug levels and monitored ECG parameters.

ECG = electrocardiogram; HR = heart rate; PR = PR interval; QTc = corrected QT interval.
Table 5 Postnatal characteristics and clinical findings.

A = atenolol; AD = amiodarone; AFL = atrial flutter; D = digoxin; DC = cardioversion; F = flecainide; NA = not applicable; P = propranolol; PAC = premature atrial contraction; PJRT = permanent junctional reciprocating tachycardia; PVC = premature ventricular contraction; S = sotalol; SR = sinus rhythm; SVT = supraventricular tachycardia; TOP = termination of pregnancy; VPS = ventriculoperitoneal shunt; WPW = Wolff–Parkinson–White syndrome
The postnatal outcome
A total of 28 babies were delivered alive. The gestational age at delivery was 38 weeks, with a range from 29 to 41 weeks; 20 foetuses (69%) had spontaneous delivery, although 10 were given induction of labour. There were nine (32%) Caesarean sections, with a majority, that is, seven of nine, being performed electively for obstetric reasons. A severe vein of Galen aneurysm complicated one case and the patient opted for termination of her pregnancy. There was one neonatal death due to severe pulmonary hypoplasia and gross foetal ascites (Table 5).
In the 22 cases with re-entry tachycardia, 21 newborns were born in sinus rhythm; one foetus was terminated. One foetus who did not respond to any treatment antenatally showed sinus rhythm at birth. However, tachycardia recurred a few hours after birth, and adenosine effectively terminated the tachycardia. Sinus rhythm was maintained on amiodarone infusion, but the newborn died due to severe respiratory distress. In all, seven newborns developed atrioventricular re-entry tachycardia beyond 24 hours of age and needed anti-arrhythmic medication; however, direct current cardioversion was not required in any case.
Out of the seven cases of atrial flutter, only three were born in sinus rhythm; however, four neonates were noted to be in atrial flutter at birth, and therefore they were treated with direct current cardioversion to restore the sinus rhythm. There was no recurrence of atrial flutter in five cases in the long term. Anti-arrhythmic medication was needed in two newborns because of the emergence of atrioventricular re-entry tachycardia – in one patient a few hours after restoration of sinus rhythm by direct current cardioversion, and in the other a week after delivery. One term foetus with atrial flutter had not received any treatment, and had spontaneously reverted to sinus rhythm after birth.
A total of nine children – seven with re-entry supraventricular tachycardia and two with atrial flutter – required postnatal anti-arrhythmic treatment. In seven children, anti-arrhythmic medication was discontinued within a year. Only two children remained on anti-arrhythmic medication in the follow-up beyond the first year of age.
After birth, three newborns exhibited pre-excitation – Wolff–Parkinson–White Syndrome – two of whom had recurrence of atrioventricular re-entry tachycardia and required treatment for a few months. Both patients remained arrhythmia free after discontinuation of treatment (Table 5). A third patient has continued to show asymptomatic pre-excitation on a 12-lead electrocardiogram, but never developed arrhythmia following termination of atrial flutter with direct current cardioversion.
Table 2 Comparison of drug levels between the two groups who had initial monotherapy versus combination treatment.

Data are presented as mean ± standard deviation (minimum–maximum; median)
There were no concerns with regard to neurological development in any of the 26 patients by any primary care physicians, paediatricians, or parents. All children except one attend mainstream school. There were two children who had recurrent tachycardia in childhood; they were assessed using the Ruth Griffiths neurological developmental scale, and their development was considered to be entirely within the normal range. There is only one child with learning difficulties who also has a ventriculoperitoneal shunt for congenital hydrocephalus. It would be more likely that the neurological abnormalities were related to his hydrocephalus rather than foetal tachycardia.
Discussion
Foetal tachyarrhythmia most commonly relates to atrioventricular re-entry tachycardia and atrial flutter, and less often it may be due to focal atrial or ventricular dysrhythmias.Reference Simpson and Sharland 1 , Reference Van Engelen, Weijtens and Brenner 14 , Reference Jaeggi, Fouron and Drblik 15 Diagnosis of sustained and significant supraventricular tachycardia is relatively uncommon during the routine evaluation of foetal cardiac problems at a 20-week anomaly scan. It is commonly an incidental finding at a later stage in pregnancy.
In the foetuses who are delivered early, the outcome is often poor, with substantial mortality and morbidity due to inherent problems of prematurity.Reference Lulić Jurjević, Podnar and Vesel 12 Therefore, most clinicians would aim to treat the mothers with anti-arrhythmic medication in order to restore sinus rhythm in the foetus. If restoration of sinus rhythm is not achievable, reduction of foetal heart rate to 160 may be targeted to prevent or resolve signs of cardiac failure and to delay delivery of the foetus beyond 34 weeks of gestation or earlier if deemed necessary.Reference Simpson and Sharland 1 , Reference Huntley 13 We only needed to deliver one foetus prematurely at 29 weeks due to worsening hydrops.
Owing to the fact that only a handful of foetal tachycardia cases are seen every year, the management of these cases varies greatly on individual preference and experience. There is also no consensus regarding how aggressively foetal tachycardia should be treated. Uncommonly, some algorithms suggest the wait-and-see approach as long as 1 or 2 weeks before considering a change in the treatment.Reference Simpson and Sharland 1 , Reference Frohn-Mulder, Stewart, Witsenburg, Den Hollander, Wladimiroff and Hess 2 , Reference Lulić Jurjević, Podnar and Vesel 12 Digoxin has been widely used in the treatment of foetal tachycardia as a first-line agent, but results are rather conflicting with varying success rates of 46–62%.Reference Simpson and Sharland 1 , Reference Frohn-Mulder, Stewart, Witsenburg, Den Hollander, Wladimiroff and Hess 2 , Reference Ebenroth, Cordes and Darragh 9 , Reference Lulić Jurjević, Podnar and Vesel 12 , Reference Lisowski, Verheijen and Benatar 16 In case of hydrops, digoxin becomes rather ineffective, with lower success rates of 0–10%.Reference Simpson and Sharland 1 , Reference Frohn-Mulder, Stewart, Witsenburg, Den Hollander, Wladimiroff and Hess 2 , Reference Ebenroth, Cordes and Darragh 9 , Reference Lulić Jurjević, Podnar and Vesel 12 , Reference Maxwell, Crawford, Curry, Tynan and Allan 17 Several investigators have also used sotalol and flecainide. Oudijk et alReference Oudjik, Michon and Kleinman 10 reported low conversion rates of 40–60% and higher foetal mortality rates of 19% with Sotalol in foetal supraventricular tachycardia. It is rather controversial to leave a developing foetus to the harmful effects of fast heart rate and ineffective circulation. In contrast, our approach was proactive as we had applied the same principles of treating supraventricular tachycardia in foetus as we would have in treating a vulnerable neonate. This particular approach in our practice had resulted in survival of all foetuses, with high conversion rates in atrioventricular re-entry tachycardia – 21 of 22 foetuses (95%) – and successful rate control of foetal atrial flutter (100%). These results are more effective and far superior than digoxin monotherapy regimen.
Digoxin exhibits its anti-arrhythmic effect primarily by increasing refractoriness of the atrioventricular node, and also has positive inotropic properties. Digoxin monotherapy in atrial flutter has no electrophysiological basis due to arrhythmia being confined to a macro re-entry circuit within the right atrium. Digoxin as a single agent is not recommended to be used in supraventricular tachycardia with accessory pathway, because digoxin blocks atrioventricular nodal conduction, and in the presence of an accessory pathway with atrial flutter or fibrillation this might facilitate rapid antegrade conduction over the accessory pathway. This particular feature is known to cause sudden collapse and death in older children and adults. In one study, 21% of patients with foetal tachycardia were found to have a delta wave after birth.Reference Hahurij, Blom and Lopriore 18 Even in our study, we identified a delta wave in three newborns. In support of this information, in one study three intrauterine deaths were encountered in foetuses – two non-hydropic and one hydropic – treated with digoxin alone.Reference Simpson and Sharland 1 Although speculative, a similar arrhythmia might have occurred in those foetuses as well that had been treated with digoxin alone in the series by Simpson et al.Reference Simpson and Sharland 1 We would therefore advocate that digoxin should only be used in combination with flecainide in foetal tachycardia. Flecainide significantly depresses both antegrade and retrograde conduction of the accessory pathway and prevents such rapid conduction to the ventricles even in the presence of digoxin. One might postulate that if those foetuses had been treated with digoxin and flecainide combination from the onset, these deaths could have been prevented. This hypothesis was indeed supported in our own experience as all patients except one had responded to digoxin and flecainide combinations with no intrauterine death.
Flecainide exhibits its anti-arrhythmic effect by blocking sodium channels in cardiac tissues. As a single agent, or in combination with digoxin, flecainide has been shown to have up to 95% efficacy and no mortality in a series.Reference Krapp, Baschat, Gembruch, Geipel and Germer 19 On the contrary, Simpson and SharlandReference Simpson and Sharland 1 had encountered a mortality rate of 14.8% – 4 of 27 foetuses – in their earlier work when flecainide was used in hydropic foetuses as a single agent. Nevertheless, flecainide has been used in children and foetuses with excellent safety record in our practice and in others, with no mortality rates.Reference Krapp, Baschat, Gembruch, Geipel and Germer 19 However, some investigators may have reservations for the use of flecainide due to its proarrhythmic effect derived from the cardiac arrhythmia suppression trial in adults.Reference Echt, Liebson and Mitchell 20 In our series, we used close monitoring of maternal electrocardiogram and serum drug levels, in addition to frequent assessment of foetal heart rate and foetal well-being, to avoid any anti-arrhythmic drug-related complications. As a result of these policies, we have not encountered any intrauterine death. Our high success rate of 96% may have provided further support for the use of flecainide and digoxin combination in all foetuses with or without hydrops in atrioventricular re-entry tachycardia and in atrial flutter. In a large series, flecainide was reported to have controlled the foetal arrhythmia rapidly with resolution of hydrops more frequently than other medications.Reference Simpson and Sharland 1 , Reference Krapp, Baschat, Gembruch, Geipel and Germer 19 Disappointingly, in the same series three intrauterine deaths had occurred in hydropic foetuses with supraventricular tachycardia within 24 hours of starting flecainide. The authors argued that this complication might have occurred because of the known negative inotropic effects of flecainide, or might have been a random occurrence in foetuses that were at risk of intrauterine death, regardless of the drug used. On the other hand, flecainide is known to slow atrial conduction in atrial flutter, thereby allowing the atrioventricular node to conduct faster in a one-to-one manner, which may result in serious haemodynamic compromise and even death. Although this is speculative, it may well explain the high unexpected foetal demise due to flecainide in the series by Simpson et al.Reference Simpson and Sharland 1
We have shown in our experience that digoxin and flecainide combination therapy is safe in hydropic foetuses as well. Resolution of hydrops may take as long as 2 weeks after normalisation or reduction of foetal heart rate below 160 beats per minute. We can postulate that digoxin might have provided a positive inotropic effect in hydropic foetuses, and that its atrioventricular nodal blocking effect might have negated the potential side effects of flecainide in atrial flutter.
We preferred to continue combination treatment until delivery, as there is a small chance of tachyarrhythmia recurring. Recurrence of foetal tachycardia was noted in one of our cases when the mother had stopped the medications because of intolerance. However, the tachyarrhythmia resolved upon restarting the proposed combination treatment at a lower dose.
Neurological development may be affected in foetuses with tachycardia and hydrops.Reference Oudijk, Gooskens, Stoutenbeek, De Vries, Visser and Meijboom 21 Our combination treatment appeared to have been successful in reducing the occurrence of such serious morbidity as well. There is only one child with learning difficulties at age 4, who also has a ventriculoperitoneal shunt for hydrocephalus. His neurodevelopmental delay was not considered to be related to foetal tachycardia.
In conclusion, digoxin and flecainide combination offers a safe and effective treatment in foetal supraventricular tachycardia and atrial flutter. The beneficial effect of this combination has also been shown in hydropic foetuses. Both medications are well tolerated by foetuses as well as pregnant women, with no foetal demise or maternal proarrhythmia or serious side effects.
We acknowledge the limitations of this study due to its retrospective nature. In addition, foetal supraventricular arrhythmia is rare and, due to the limited number of foetuses treated with the proposed combination, it is difficult to conduct comparative studies in one centre. Thus, there is a need to seek multicentre collaboration and comparative trials to address these issues in the future.
Acknowledgements
We would like to thank all the midwives, nurses, ultrasonographers, radiologists, obstetricians, adult cardiologists, neonatologists and paediatricians involved in the detection, management and follow up of these patients. KB and SM were self funded overseas clinical fellows at the time of manuscript preparation and returned to their respective homelands; we greatly acknowledge their invaluable contribution to this review.









