INTRODUCTION
During the last 10 yr, knowledge on climatic and environmental changes in terrestrial environments in the Basque area of the Iberian Peninsula has experienced a significant step forward. This has been possible on account of an increase on systematic investigations of different proxies, including vertebrate remains, palynology, sedimentology, malacology, and isotope geochemistry (Iriarte, Reference Iriarte2009; Martínez-García et al., Reference Martínez-García, Pascual, Rodríguez-Lázaro and Bodego2013; Castaños et al., Reference Castaños, Zuluaga, Ortega, Murelaga, Alonso-Olazabal, Rofes and Castaños2014; Aranburu et al., Reference Aranburu, Arriolabengoa, Iriarte, Giralt, Yusta, Martínez-Pillado, Del Val, Moreno and Jiménez-Sánchez2015). Among terrestrial vertebrates, small vertebrates constitute an important source of information on past environments, because of their good preservation, quantity, and relatively well-understood ecology. Therefore, they are becoming increasingly used in multidisciplinary studies of the late Pleistocene and Holocene (Murelaga et al, Reference Murelaga, Mujika, Bailon, Castaños and Sáez de Lafuente2008; Rofes et al., Reference Rofes, Zuluaga, Murelaga, Fernández Eraso, Bailon, Iriarte-Chiapusso, Ortega and Alonso-Olazabal2013; Suárez-Bilbao et al., Reference Suárez-Bilbao, Garcia-Ibaibarriaga, Arrizabalaga, Iriarte-Chiapusso and Murelaga2017; among others). They are a particularly important source of paleoenvironmental information in places like Basque Country, likely serving as a multidirectional corridor for animal species and prehistoric populations traveling between the Iberian Peninsula and the European continent (for more information about the idea of “Basque crossroads,” see Arrizabalaga, Reference Arrizabalaga2007).
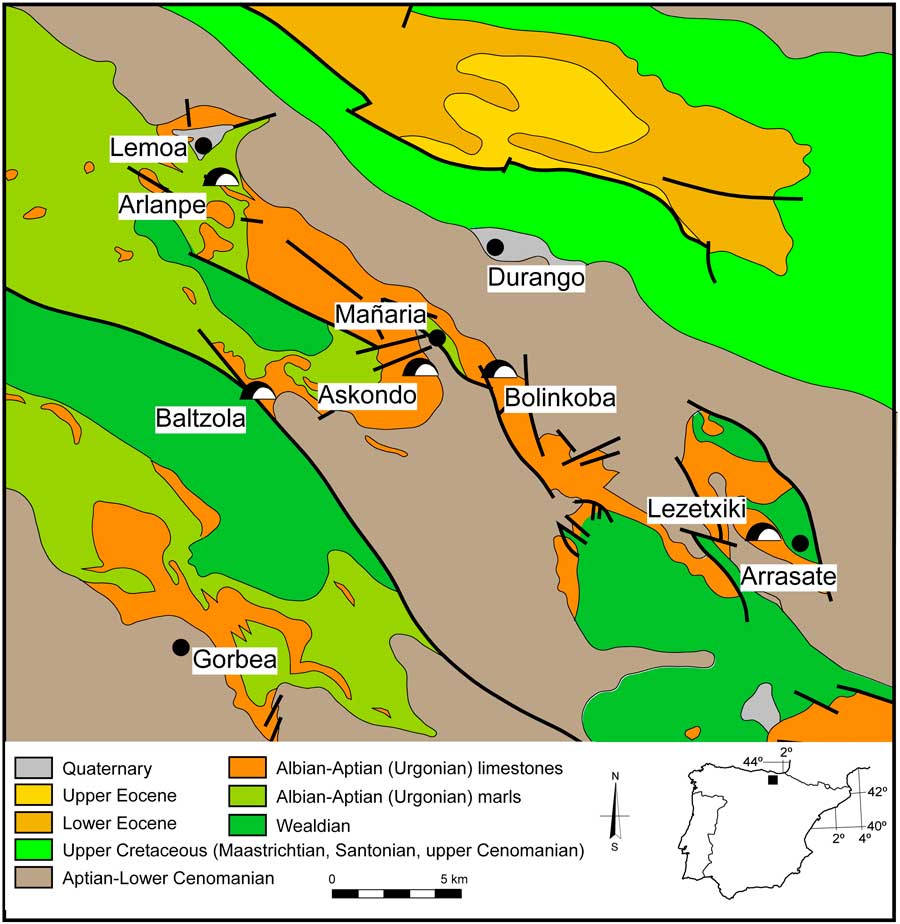
The Lezetxiki karst complex (Arrasate, Basque Country) is situated in the southwestern tip of the province of Gipuzkoa (Iberian Peninsula), in the central sector of northern Iberia (Fig. 1). The caves within this complex contain a rich paleoenvironmental record. Previous research on the cave deposits includes synthesis studies (Arrizabalaga, Reference Arrizabalaga2006; Álvarez-Alonso and Arrizabalaga, Reference Álvarez-Alonso and Arrizabalaga2012), macrofaunal investigations (Altuna, Reference Altuna1972; Castaños et al., Reference Castaños, Murelaga, Arrizabalaga and Iriarte-Chiapusso2011), descriptions of microfaunal assemblages (Chaline, Reference Chaline1970; Rofes et al., Reference Rofes, Garcia-Ibaibarriaga, Murelaga, Arrizabalaga, Iriarte-Chiapusso, Cuenca-Bescós and Villaluenga2012; Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Arrizabalaga, Iriarte-Chiapusso, Rofes and Murelaga2015a), and geoarchaeology (Arriolabengoa et al., Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015).
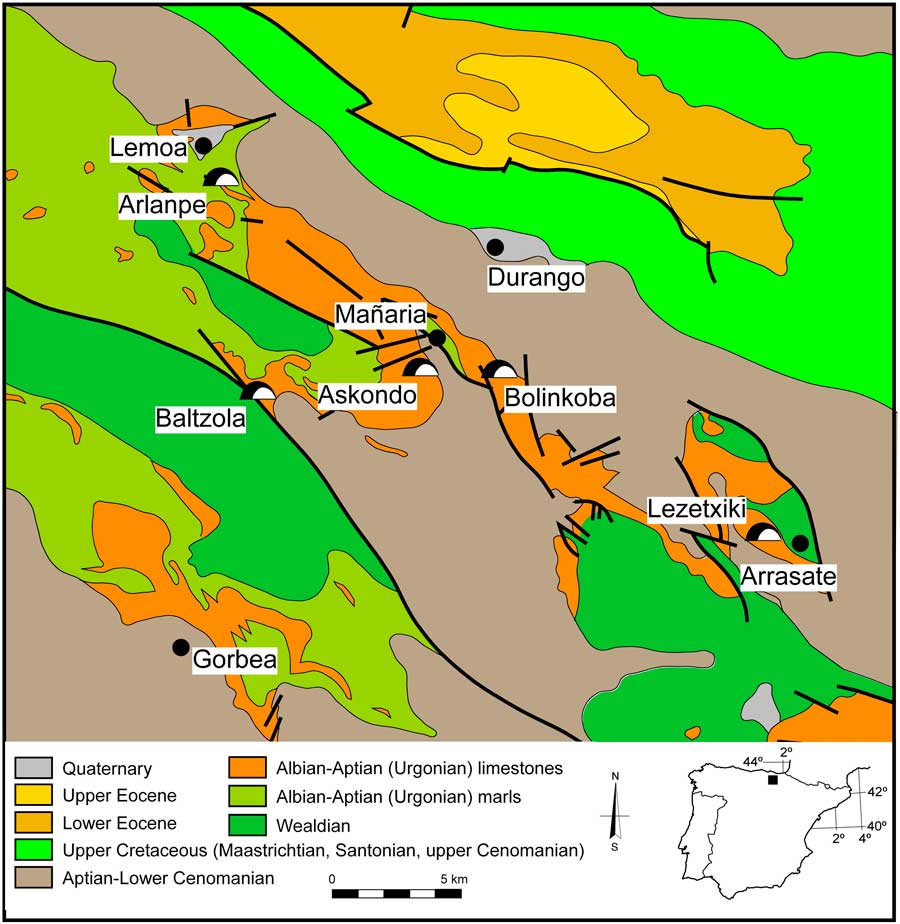
Figure 1 (color online) Geologic location of Lezetxiki Cave (Arrasate, Basque Country, Iberian Peninsula) and the location of some archaeological sites mentioned in the text. Modified from Rat (Reference Rat1959).
Lezetxiki II has made a significant contribution to the paleontological and paleoenvironmental knowledge of the Pleistocene in the Iberian Peninsula, because it has yielded the first fossil record in the Cantabrian region of Allocricetus bursae and the southwesternmost record of Sicista betulina in Eurasia (Rofes et al., Reference Rofes, Garcia-Ibaibarriaga, Murelaga, Arrizabalaga, Iriarte-Chiapusso, Cuenca-Bescós and Villaluenga2012). The site also contains the earliest Quaternary fossil remains of Muscardinus avellanarius in the Iberian Peninsula (Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Arrizabalaga, Iriarte-Chiapusso, Rofes and Murelaga2015a) and the first fossil remains of Macaca sylvanus in the Cantabrian range (Castaños et al., Reference Castaños, Murelaga, Arrizabalaga and Iriarte-Chiapusso2011). Therefore, additional examinations of the cave deposits are being done to better constrain the timing of faunal history and environmental conditions at this important location.
THE CAVE OF LEZETXIKI II AND STUDY OBJECTIVES
The Lezetxiki II Cave, which is formed from Cretaceous limestones, is located on a steep hillside in an area of abrupt relief (Fig. 1), at an elevation of 380 m above sea level on the eastern flank of Bostate hill. The cave is part of a larger karst complex, which includes other cavities with Quaternary sediments and archaeo-paleontological infillings (Álvarez-Alonso and Arrizabalaga, Reference Álvarez-Alonso and Arrizabalaga2012). In addition, there are numerous archaeo-paleontological sites in the surrounding region, some of them with microvertebrate records (e.g., Artazu VII: Suárez-Bilbao et al., Reference Suárez-Bilbao, Garcia-Ibaibarriaga, Arrizabalaga, Iriarte-Chiapusso and Murelaga2017; Labeko Koba: Pemán, Reference Pemán2000; Askondo: Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Rofes, Bailon, Garate, Rios-Garaizar, Martínez-García and Murelaga2015b; Bolinkoba: Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Suárez-Bilbao, Ordiales and Murelaga2015c). Even though the natural systems of Arrasate have been subjected to heavy anthropogenic pressure, Pinus radiata plantations still share territory with the Cantabrian Holm oak (Quercus ilex ilex) in the region, with beech (Fagus sylvatica) forests at higher altitude.
The “classic” Lezetxiki deposit was first excavated during the 1956–1968 field seasons under the direction of José Miguel de Barandiarán, revealing an extensive Late Pleistocene sequence. In addition, three human fossil remains were recovered—namely, a humerus near the entrance of Leibar Cave (level VIII; Basabe, Reference Basabe1966) and provisionally dated to Marine Oxygen Isotope Stage (MIS) 6 (Arrizabalaga et al., Reference Arrizabalaga, Altuna, Areso, Falguerès, Iriarte-Chiapusso, Mariezkurrena and Pemán2005) and two Neanderthal teeth from level III (Basabe, Reference Basabe1970). A small adjacent cavity nowadays known as Lezetxiki II, and the focus of the present work, was also explored, but its excavation was halted as it was practically sediment-filled from the accumulation of the sieved material from Lezetxiki (Arrizabalaga, Reference Arrizabalaga2006). In 1996, excavations were restarted on the southern side of Lezetxiki by Alvaro Arrizabalaga and María José Iriarte using updated methodology (i.e., micromorphological analysis, palynological sampling, new dating, etc.; Arrizabalaga Reference Arrizabalaga2005, Reference Arrizabalaga2006), and since 1999 these efforts were extended to Lezetxiki II.
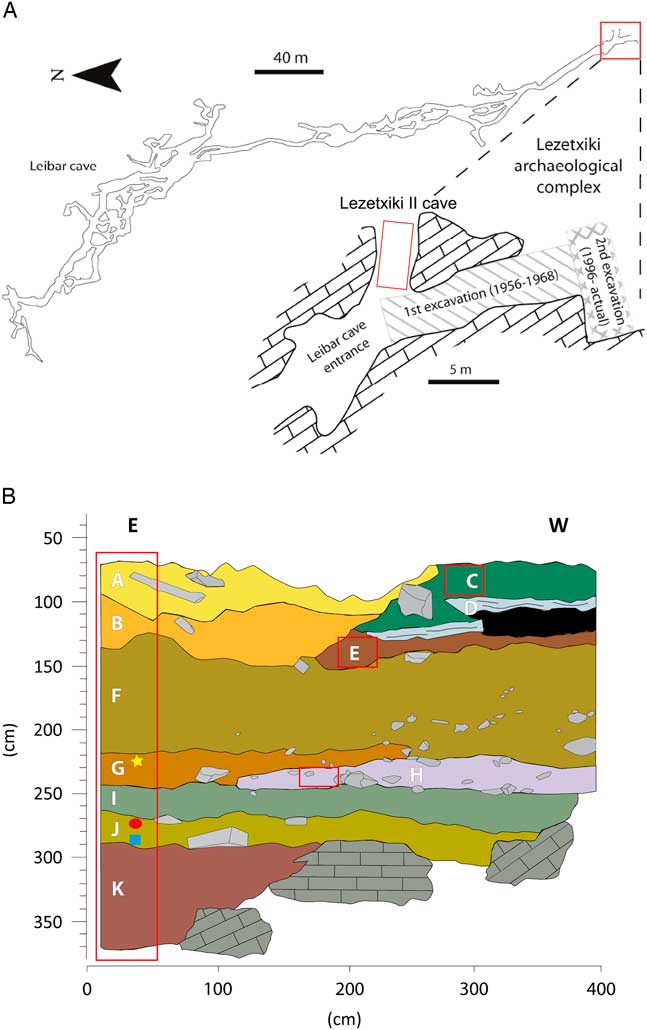
Lezetxiki II has a tunnel-type morphology, with an octagonal orientation to the “classic site” (north–south orientation) and is a minimum of 4 m in length (Fig. 2A). The entrance of the cave, located at the eastern end, is 1 m wide and 0.4 m high. The cavity was probably principally used as a hibernation and breeding den of Ursus spelaeus (Villaluenga, Reference Villaluenga2013), although the presence of other carnivores and ungulates may be related with sporadic occupation of the cave by carnivores and humans (anthropogenic activity is evidenced by scarce lithic tools). The origin and formation of endokarst fill in Lezetxiki II Cave was the product of multiple episodes of deposition and erosion (Arriolabengoa et al., Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015), so the stratigraphic sequence is not homogeneous in the excavated area (a test trench of 1×4).
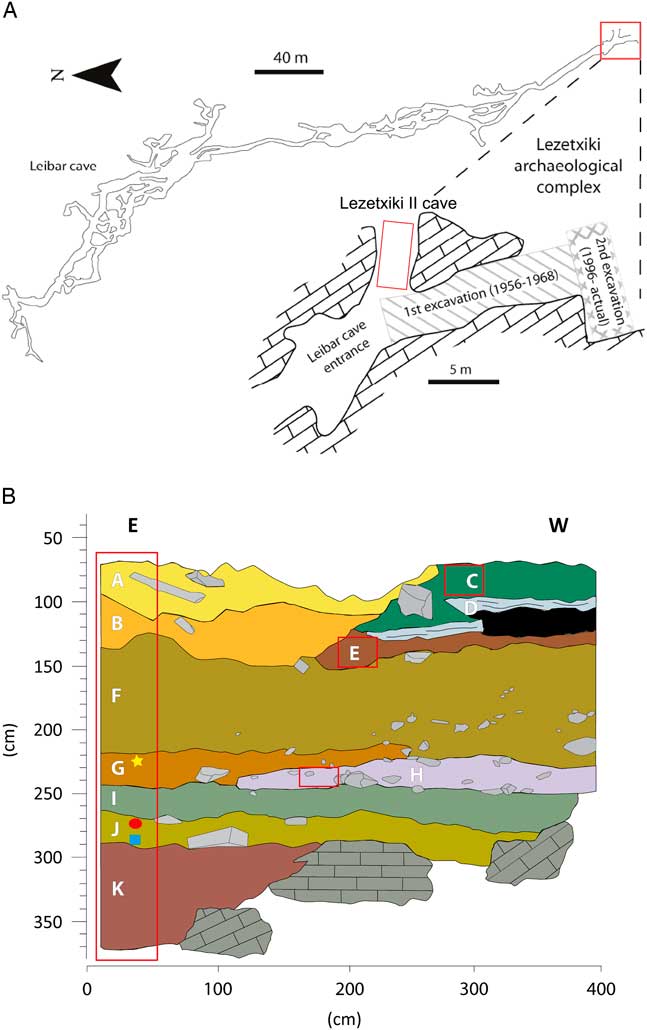
Figure 2 (A) Topography of Leibar Cave with the exact location of Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula) in red. (B) Stratigraphy of Lezetxiki II, indicating the location of micropaleontological samples (red boxes) and already published Muscardinus avellanarius (yellow star), Sicista betulina (red ellipse), and Macaca sylvanus (blue square) remains. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The absence of a systematic geochronology framework remains one of the most difficult methodological problems for interpreting the infilling history al Lezetxiki II. In this article, we (1) clarify and synthesize previously published and new chronological work at the cave and (2) describe newly examined small vertebrate assemblages and make paleoenvironmental inferences based on these data.
MATERIAL AND METHODS
Fieldwork and collecting techniques
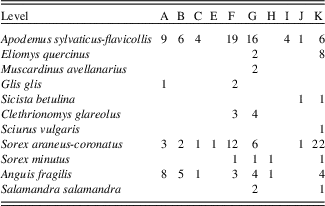
The sequence from Lezetxiki II (Fig. 2B) was divided into 11 lithostratigraphic levels (Arriolabengoa et al., Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015), named using letters in alphabetical order (from A to K; Table 1). In 2011, sampling for micropaleontological analysis was carried out at the entrance of the cave, where most of the stratigraphic units were present. In addition, samples were taken from levels identified in deeper parts of the gallery (levels C, E, and H; Fig. 2B), with the exception of level D (stalagmite flow). A total of 52 samples (279 L of sediment), of 0.33 m2 each, were taken through the 3-m-long stratigraphic sequence in subsequent sublevels 5 to 10 cm thick (Fig. 2B).
Table 1 Geologic description of the stratigraphic levels in Lezetxiki II Cave, modified from Arriolabengoa et al. (Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015).

The samples were processed with the water-screening method using a stack of sieves of decreasing mesh size (4 mm to 0.5 mm). In subsequent years, small-vertebrate bony remains were analyzed and quantified as part of a Ph.D. dissertation (Garcia-Ibaibarriaga, Reference Garcia-Ibaibarriaga2015). With the aid of a stereomicroscope, the disarticulated bone fragments and isolated teeth were separated from the concentrates, classified, quantified, and photographed at the University of the Basque Country UPV/EHU.
Systematic attribution and quantification
For the identification of bones, we followed the general criteria given by López-Martínez (Reference López Martínez1989) for lagomorphs; Cuenca-Bescós (Reference Cuenca-Bescós1988), Daams (Reference Daams1981), Heinrich (Reference Heinrich1982), Pasquier (Reference Pasquier1974), Pucek (Reference Pucek1982), and Van der Meulen (Reference Van der Meulen1973) for rodents; and Furió (Reference Furió2007), Niethammer and Krapp (Reference Niethammer and Krapp1982), and Reumer (Reference Reumer1984) for eulipotyphlans. The specific attribution of squamate and amphibians followed the criteria established previously by Bailon (Reference Bailon1991, Reference Bailon1999), Blain (Reference Blain2009), and Szyndlar (Reference Szyndlar1984). The taxonomic classification for small mammals was in accordance with Tesakov et al. (Reference Tesakov, Lebedev, Bannikova and Abramson2010), Waddell et al. (Reference Waddell, Okada and Hasegawa1999), and Wilson and Reeder (Reference Wilson and Reeder2005). Additionally, the systematical nomenclature used for amphibians and reptiles was based on Speybroeck et al. (Reference Speybroeck, Beukema and Crochet2010). Specific taxonomic attributions rest mainly on the best cranial and/or postcranial diagnostic element: isolated teeth for the lagomorphs; isolated teeth and mandibles for the rodents (first lower molars for the Arvicolinae); isolated teeth, mandibles, and postcranial skeleton for the insectivores; the vertebrae for newts, lacertids, and snakes; vertebrae, dental material, and osteoderms for Anguis fragilis; and the humerus, the ilium, and the scapula for the anurans.
The relative abundance of fossil species in Lezetxiki II was established using the minimal number of individuals (MNI), determined considering the best diagnostic element and its position in the skeleton. Laterality and sex (for amphibians) were taken into account, whenever possible.
Diversity
Diversity refers to the number of taxa present in a community or region and their relative evenness in proportions. It is likely related to environmental conditions and climate, as well as other variables (Fleming, Reference Fleming1973; Kerr and Packer, Reference Kerr and Packer1997; Andrews et al., Reference Andrews, Lord and Evans1979), so it is possible to use diversity as a general indicator of climatic changes (Montuire, Reference Montuire1995; Andrews and O’Brien, Reference Andrews and O’Brien2000; Blois et al., Reference Blois, Mcguire and Hadly2010). Warmer climatic conditions are often associated with increased diversity and ecosystem complexity (Margalef, Reference Margalef1974; Barbault, Reference Barbault1994). In this study, we used the Shannon index (sometimes referred to as Shannon-Weaver or Shannon-Wiener index) to quantify diversity, as the final result is independent of the size of the sample, and it is more suitable when it comes to highlight minority species. The indices were calculated with the Paleontological Statistics program (PAST; Hammer et al., Reference Hammer, Harper and Ryan2001).
Habitat types and climate categories
Paleoclimate and paleoenvironmental inferences from microvertebrate fossils were made based on knowledge of modern ecological preferences using two methods. Details of ecological preferences of the different small vertebrate species from Lezetxiki II Cave are extensively documented in Garcia-Ibaibarriaga (Reference Garcia-Ibaibarriaga2015). We used the taxonomic habitat index, a method developed by Nesbit Evans et al. (Reference Nesbit Evans, Van Couvering and Andrews1981) and Andrews (Reference Andrews1990, Reference Andrews2006), based on habitat weighting. According to this method, we organized the taxa identified at Lezetxiki II Cave by their ecological preferences in biotopes following Álvarez et al. (Reference Álvarez, Bea, Faus, Castién and Mendiola1985), Cuenca-Bescós et al. (Reference Cuenca-Bescós, Straus, González Morales and García Pimienta2008), Gosá and Bergerandi (Reference Gosá and Bergerandi1994), International Union for Conservation of Nature (2014), Palomo and Gisbert (Reference Palomo and Gisbert2005), Pleguezuelos et al. (Reference Pleguezuelos, Márquez and Lizana2002), Pokines (Reference Pokines1998), Salvador (Reference Salvador1998), and Sesé (Reference Sesé2016). In the case of fossil taxa, values were applied depending on the ecological affinities of related modern species, as established by Bartolomei et al. (Reference Bartolomei, Chaline, Fefjar, Jánossy, Jeannet, Koenigswald and von, Kowalski1975) and Sesé (Reference Sesé2005). However, we acknowledge that this approach induces considerable uncertainty, because it is not possible to establish the actual proportion represented by each species in different habitats. Consequently, we also examined the proportion of species that predominantly occur in wooded habitats (Eliomys quercinus, Muscardinus avellanarius, Glis glis, Sicista betulina, Clethrionomys glareolus, Apodemus sylvaticus-flavicollis, Sciurus vulgaris, Sorex araneus-coronatus, Sorex minutus, Anguis fragilis, and Salamandra salamandra). Numerous previous studies have shown that deciduous forests expanded in the Cantabrian region in response to warmer climates (Sese, Reference Sesé2005; Cuenca-Bescós et al., Reference Cuenca-Bescós, Straus, González Morales and García Pimienta2008; Iriarte-Chiapusso and Murelaga, Reference Iriarte-Chiapusso and Murelaga2012; Rofes et al., Reference Rofes, Zuluaga, Murelaga, Fernández Eraso, Bailon, Iriarte-Chiapusso, Ortega and Alonso-Olazabal2013).
RESULTS
Chronology
Although the chronology of the cave deposits has been presented in several studies (Falguerès et al. Reference Falguerès, Yokoyama and Arrizabalaga2006; Maroto et al., Reference Maroto, Vaquero, Arrizabalaga, Baena, Baquedano, Jordá and Julià2012; Higham et al., Reference Higham, Douka, Wood, Ramsey, Brock, Basell and Camps2014), uncertainty still remains about the infilling history of Lezetxiki II Cave. In 2003, a U/Th age was obtained from a speleothem located at level D by CNRS specialists C. Falguères, H. Valladas, and N. Mercier (Arrizabalaga, Reference Arrizabalaga2004). This sample had been contaminated by thorium from the detrital part of the speleothem (intercalated clays), so it had to be corrected (Falguerès et al., Reference Falguerès, Yokoyama and Arrizabalaga2006). The sample provided an age of 74 ka (Table 2), suggesting that this level was deposited throughout the first phase of MIS 4. Additionally, in 2010, two Ursus teeth found in the level J were dated by amino acid racemization at the Polytechnic University of Madrid using aspartic acid d/l ratios (Castaños et al., Reference Castaños, Murelaga, Arrizabalaga and Iriarte-Chiapusso2011). The individual results are 70 and 86.8 ka, which give a mean value of 78.4±8.4 ka. These results most likely represent minimum age estimates, because they are not consistent with the sedimentologic and archaeological analyses.
Table 2 List of numeric ages from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula) including cultural periods, chronostratigraphic units, laboratory codes, and sample details.

Recently, level K has been dated by single-grain thermally transferred optically stimulated luminescence (TT-OSL), which we are presenting for the first time in this work. It provides an estimate of when quartz grains were last exposed to light prior to deposition and burial at the site, using a methodology presented in Arnold and Demuro (Reference Arnold and Demuro2015), Arnold et al. (Reference Arnold, Demuro, Parés, Pérez-González, Arsuaga, Bermúdez de Castro and Carbonell2015), and Demuro et al. (Reference Demuro, Arnold, Parés, Pérez-González, Ortega, Arsuaga, Bermúdez de Castro and Carbonell2014, Reference Demuro, Arnold, Parés and Sala2015). A single sample (LZ12-6) was collected from level K using a PVC tube, and dated using the TT-OSL methodological procedures and quality assurance criteria outlined in Arnold et al. (Reference Arnold, Demuro, Parés, Arsuaga, Aranburu, Bermúdez de Castro and Carbonell2014). Suitability of the single-grain equivalent dose (D e ) estimation procedures was confirmed with a dose-recovery test, and the natural D e distribution (n=84 grains) displayed a low overdispersion value of 25 ± 5%, which is indicative of sufficient optical resetting prior to burial (Fig. 3). The single-grain TT-OSL age for sample LZ12-6 (obtained using the central age model) is 215.7 ± 15.1 ka, with a 1-sigma error (Table 3).

Figure 3 Single-grain thermally transferred optically stimulated luminescence equivalent dose (D e ) distribution for sample LZ12-6, shown as a radial plot. The D e value for each grain is read by drawing a line from the origin of the y-axis (“Standardized Estimate”), through the data point of interest, to the radial axis (plotted on a log scale) on the right-hand side; the point of intersection is the D e (in Gy). The measurement error on this D e is obtained by extending a line vertically to the x-axis, where the point of intersection is the relative standard error (shown as a percentage of the D e value) and its reciprocal (precision). In radial plots, the most precise estimates fall farthest to the right, and the least precise estimates fall farthest to the left. The gray band is centered on the weighted mean D e value calculated using the central age model.
Table 3 Thermally transferred optically stimulated luminescence (TT-OSL) dose rate data, single-grain equivalent dose, and final age for sample LZ12-6. The final TT-OSL age has been derived by dividing the weighted mean equivalent dose (D e ) by the total dose rate.

a Field water content, expressed as percent of dry mass of mineral fraction, with an assigned relative uncertainty of ±10%.
b Calculated on dried and powdered sediment samples using a Risø GM-25-5 low-level beta counter.
c Specific activities and radionuclide concentrations have been converted to dose rates using the conversion factors given in Guérin et al. (Reference Guérin, Mercier and Adamiec2011), making allowance for beta-dose attenuation (Mejdahl, Reference Mejdahl1979; Brennan, Reference Brennan2003).
d Calculated from in situ measurements made at each sample position with a NaI:Tl detector, using the “energy windows” approach (e.g., Arnold et al., Reference Arnold, Duval, Falguères, Bahain and Demuro2012).
e Cosmic-ray dose rates were calculated using the approach of Prescott and Hutton (Reference Prescott and Hutton1994), and assigned a relative uncertainty of ±10%.
f Mean ± total uncertainty (68% confidence interval), calculated as the quadratic sum of the random and systematic uncertainties.
g Includes an internal dose rate of 0.03 Gy/ka with an assigned relative uncertainty of ±30%.
h Number of D e measurements that passed the single-aliquot regenerative-dose (SAR) rejection criteria of Arnold et al. (Reference Arnold, Demuro, Parés, Arsuaga, Aranburu, Bermúdez de Castro and Carbonell2014) and were used for D e determination/total number of grains analyzed.
i The relative spread in the D e data set beyond that associated with the measurement uncertainties for individual D e values, calculated using the central age model (CAM) of Galbraith et al. (Reference Galbraith, Roberts, Laslett, Yoshida and Olley1999).
j The CAM was used to calculate the final D e as this sample had a low overdispersion value, consistent with that observed in “ideal” well-bleached and unmixed sample from similar settings (Arnold and Roberts, Reference Arnold and Roberts2009; Arnold et al., Reference Arnold, Demuro, Parés, Arsuaga, Aranburu, Bermúdez de Castro and Carbonell2014, Reference Arnold, Demuro, Parés, Pérez-González, Arsuaga, Bermúdez de Castro and Carbonell2015; Demuro et al., Reference Demuro, Arnold, Parés, Pérez-González, Ortega, Arsuaga, Bermúdez de Castro and Carbonell2014).
k Total uncertainty includes a systematic component of ±2% associated with laboratory beta-source calibration.
Considering the new dating, and the data provided by the sedimentologic (Arriolabengoa et al., Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015) and microfaunal analysis, we propose the following chronological description. Level K contains the MIS 7–MIS 6 transition, TT-OSL dated to 215.7±15.1 ka. Therefore, we propose for levels J and I, an MIS 6 age (190–130 ka) based on stratigraphic relations with surrounding levels. Subsequent in the stratigraphic sequence are levels H–G, which do not have any direct age control. Both the sedimentologic evidence (i.e., the predominance of autochthonous material and speleothem formation) and the microfaunal analyses (the increase and diversity in woodland species) suggest that levels G–H represent a notorious breakup of the cave environment. Therefore, we propose an MIS 5e (ca. 128–110 ka) assignation for these levels. Consequently, the probable assignation for levels F–E, based on different lithic remains and their typological criteria, could be Middle Paleolithic, consistent with MIS 5d–b assignation (110–82 ka). Although there is a U/Th age for level D, suggesting a deposition during the MIS 4, we consider more consistent the sedimentologic assignation, which suggests the final phase of MIS 5a (ca. 82–74 ka; M.A., E.I., A.A., I.Y., A.A, personal communication). The chronological assignation for level C, based on archaeological material, is likely Aurignacian (MIS 3, ca. 60–30 ka), when the cave was apparently used as a secondary settlement (Arrizabalaga, Reference Arrizabalaga2001, Reference Arrizabalaga2003). Finally, the ceramic, faunal, and lithic fragments discovered in levels A and B suggest a Chalcolithic occupation (MIS 1: 14 ka–present; Arrizabalaga, Reference Arrizabalaga2000).
We take this opportunity to clarify some already published chronological assignations. In light of the new TT-OSL dating result, we propose that both Macaca sylvanus and Sicista betulina remains (Castaños et al., Reference Castaños, Murelaga, Arrizabalaga and Iriarte-Chiapusso2011 and Rofes et al., Reference Rofes, Garcia-Ibaibarriaga, Murelaga, Arrizabalaga, Iriarte-Chiapusso, Cuenca-Bescós and Villaluenga2012, respectively) have been found in levels that could be attributed to MIS 6. We support the previous assignation of the hazel dormouse (Muscardinus avellanarius) remains with an interstadial period of MIS 5 (Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Arrizabalaga, Iriarte-Chiapusso, Rofes and Murelaga2015a), probably with MIS 5e (taking into account the sedimentologic and paleontological composition of the level).
Taphonomic remarks
A preliminary study of the taphonomy of the small vertebrate assemblage from Lezetxiki II indicates that several predators likely contributed to the formation of the deposits (Andrews, Reference Andrews1990), and changes in preservation across the sequence indicate postdepositional processes varied through time. The mainly extensive alterations caused by digestion found in some remains, especially those of Gliridae and Murinae (Fig. 4A), suggest that the agent responsible for these small-vertebrate accumulations was likely to be a predator with great to extreme modification capacity. In contrast, other small mammal taxa digestion traces were practically absent or moderate. Among the herpetofauna, frog remains have evident signs of digestion, probably by a small carnivore (Fig. 4B). Regarding the causative agents that have altered the faunal composition, some of the remains could have surface modifications that may be related with weathering (Fig. 4C), whereas others show clear traces of chemical abrasion (Fig. 4D). An in-depth study is necessary to address these alterations in more detail, e.g., the lack of faunal remains in level H would be related with the sedimentologic composition of the level, as indicated by Arriolabengoa et al. (Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015).

Figure 4 Alteration traces identified in some small vertebrates remains from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula). (A) Glis glis left m2 with extreme digestion. (B) Rana temporaria-iberica premaxilla with evident signs of digestion. (C) Apodemus sylvaticus-flavicollis right M1 weathered. (D) Small mammal incisor abrased.
A main characteristic of the small vertebrate assemblage is the high fragmentation of the bones. Because of the high breakage, the number of identified skeletal elements is quite low, with 10,309 specimens out of more than 66,000 recovered remains. Among all identified fossils, 40% are metapodials and phalanges (4186), followed by incisors (10.66%, 1091 remains). Taking into account the raw frequency in which elements occur in a complete skeleton, the most represented elements are incisors (24.5%), followed by molars (17.5%).
The fragmentation and skeletal element representation changed over time (Fig. 5). The similar preservation stage of proximal limb bones compared with the conservation of femora and tibia-fibulae suggests that generally the predator consumed the whole carcasses in the cave. The identification of incisors in levels I, B, and A is quite low. Level K has noteworthy numbers of both radii and humeri, whereas in level F the identification of calcanei and astragali is about 40%. Another oddity is the conservation of ribs in level C, 15 times more than in the rest of the levels. Finally, the excellent preservation of the mandibles in levels K and J should be noted, with conservation of about 60%. Even if current natural accumulations tend to conserve 80–90%, this value indicates that these two levels suffered fewer postdepositional processes than the rest of the levels in this cave.
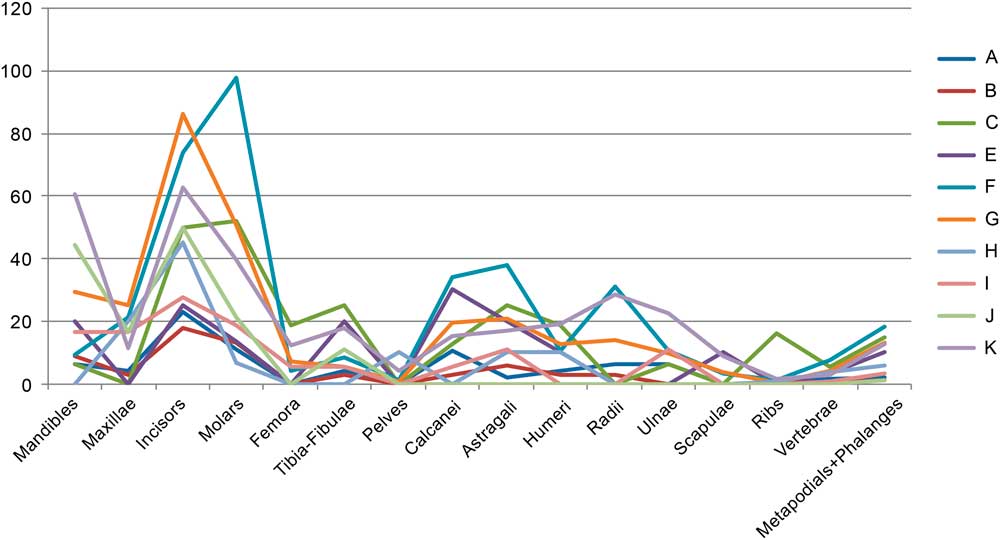
Figure 5 (color online) Relative abundance of small mammal skeletal elements from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula).
Small vertebrate assemblage from Lezetxiki II
The small vertebrate assemblage comprises 66,300 identified and unidentified disarticulated bone fragments (teeth, isolated mandibles, skull fragments, and postcranial bones). More than 2000 of them (2,211) have been identified at the genus and/or species level (number of identified specimens [NISP]), representing a total of 432 individuals (MNI). The small vertebrates comprise 32 taxa (Fig. 6A and B; Table 4): 1 lagomorph (cf. Oryctolagus cuniculus), 10 cricetids (Arvicola amphibius, Arvicola sapidus, Chionomys nivalis, Clethrionomys glareolus, Pliomys lenki, Microtus oeconomus, Microtus agrestis, Microtus arvalis, Microtus [Terricola] sp., and Allocricetus bursae), 1 murid (Apodemus sylvaticus-flavicollis), 3 glirids (Eliomys quercinus, Muscardinus avellanarius, and Glis glis), 2 sciurid (Marmota marmota and Sciurus vulgaris), 1 dipodid (Sicista betulina), 1 erinaceid (Erinaceus cf. europaeus), 4 soricids (Sorex araneus-coronatus, Sorex minutus, Neomys sp., and Crocidura russula), 1 talpid (Talpa cf. europaea), Chiroptera indet., 2 saurians (Lacertidae indet. and Anguis fragilis), 2 snakes (Coronella girondica and Vipera cf. seoanei), 1 salamandrid (Salamandra salamandra), 1 midwife toad (Alytes obstetricans), and 1 ranid (Rana temporaria-iberica).

Figure 6 (color online) (A) Some small mammals from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula). cf. Oryctolagus cuniculus (1) right m2; Arvicola amphibius (2) left m1; Chionomys nivalis (3) left m1; Clethrionomys glareolus (4a–b) right molar; Pliomys lenki (5a–b) left m1; Microtus oeconomus (6) right m1; Microtus agrestis (7) left m1; Microtus arvalis (8) left m1; Microtus (Terricola) sp. (9) left m1; Allocricetus bursae (10) left m1-m2; Apodemus sylvaticus-flavicollis (11) right m1-m2; Eliomys quercinus (12) right m1; Glis glis (13) left m2; Muscardinus avellanarius (14) right M1; Sicista betulina (15) right P4; Erinaceus cf. europaeus (16) left m1; Sorex araneus-coronatus (17) left I; Neomys sp. (18) right P4; Crocidura russula (19) right M1/2; Talpa cf. europaea (20) left humerus. Scale bars: 1–14, 17–19=1 mm; 15=2 mm; 16, 20=0.5 mm. (B) Some amphibians and reptiles from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula). Salamandra salamandra (1a) trunk vertebra dorsal view, (1b) idem lateral view, (1c) idem ventral view; Rana temporaria-iberica (2) left premaxilla; Anguis fragilis (3) osteoderm; Vipera cf. seoanei (4a) trunk vertebra ventral view, (4b) idem lateral view. Scale bar=1 mm.
Table 4 Number of identified specimens (NISP) and minimum number of individuals (MNI) of small vertebrate species from Lezetxiki II (Arrasate, Basque Country, Iberian Peninsula), organized by cultural period and chronostratigraphic unit. Chal., Chalcolithic; Aurig., Aurignacian; MP, Middle Paleolithic; Ro, rocky; OH, open humid; OD, Open dry; Wa, water; Fo, forest.

Most of the taxa identified at Lezetxiki II Cave have extant representatives (País Vasco, Viceconsejería de Medio Ambiente, 1989), albeit some of them only inhabit other parts of the Iberian Peninsula. This is the case of the common shrew (S. araneus), as well as of A. amphibius, E. quercinus, and O. cuniculus, among others (Aranzadi, 1989). Some other species are absent even from the Iberian Peninsula, like M. oeconomus, which presently has a wide range extending from northwest Europe in the west to Alaska in the east (Linzey et al., Reference Linzey, Shar, Lkhagvasuren, Juškaitis, Sheftel, Meinig, Amori and Henttonen2008). The western limit (Denmark, Norway, and Austria) is similar to the birch mouse (Sicista betulina; Meinig et al., Reference Meinig, Zagorodnyuk, Henttonen, Zima and Coroiu2008), but not to M. avellanarius, which in Europe is only absent from Iberia, southwestern France, and northern Fennoscandia and Russia (Amori et al., Reference Amori, Hutterer, Kryštufek, Yigit, Mitsain, Meinig and Juškaitis2008). Finally, we have identified two extinct species at Lezetxiki II. One is P. lenki, whose last occurrence is recorded in the Upper Magdalenian levels from El Mirón Cave, in Cantabria (Cuenca-Bescós et al., Reference Cuenca-Bescós, Straus, García Pimienta, González Morales and López-García2010). The other is A. bursae, identified for the last time in the Iberian Peninsula in Ambrosio Cave (17,000 yr BP; Sesé and Soto, Reference Sesé and Soto1998).
Small vertebrate community changes over time
There are significant taxonomic differences in the small vertebrates between the base and the top of the Lezetxiki II Cave sequence (Fig. 7A and B, Table 4). The small-mammal distribution from the lower layers (levels K–E) is characterized by the predominance of M. agrestis with 88 MNI (Fig. 7A and B, Table 4), followed by A. sylvaticus-flavicollis (26 individuals). Few insectivorous and herpetofaunal remains occurred within these lower levels. Observing the internal variations among them, levels K, G, and F are the most numerous in both NISP and MNI, which turn out to comprise, in terms of overall coverage, the largest stratigraphic sequences. Level K (end of MIS 7) is the subunit with the most identified specimens (389) and determined individuals (205), mostly because of the abundance of M. agrestis and S. araneus-coronatus (Fig. 7A, Table 4). On the other hand, the small vertebrate distribution from levels G (MIS 5e?) and F (MIS 5d–b?) are characterized by the prevalence of A. sylvaticus-flavicollis, followed by the two species mentioned previously. Levels J, I, H, and E are the poorest subunits of the entire stratigraphic sequence (Fig. 7A and B) from a quantitative point of view, although it is true that only a single 5- to 10-cm-deep sample has been taken from each of them. Level C (MIS 3) is also poor in individuals (Fig. 7A and B, Table 4), although it has the highest NISP, related to the presence of numerous postcranial slowworm remains (notwithstanding that they correspond to a single individual). Finally, the last two levels (B and A) are similar to level C, with A. fragilis dominating the NISP and A. sylvaticus-flavicollis the MNI (Fig. 7A and B, Table 4).
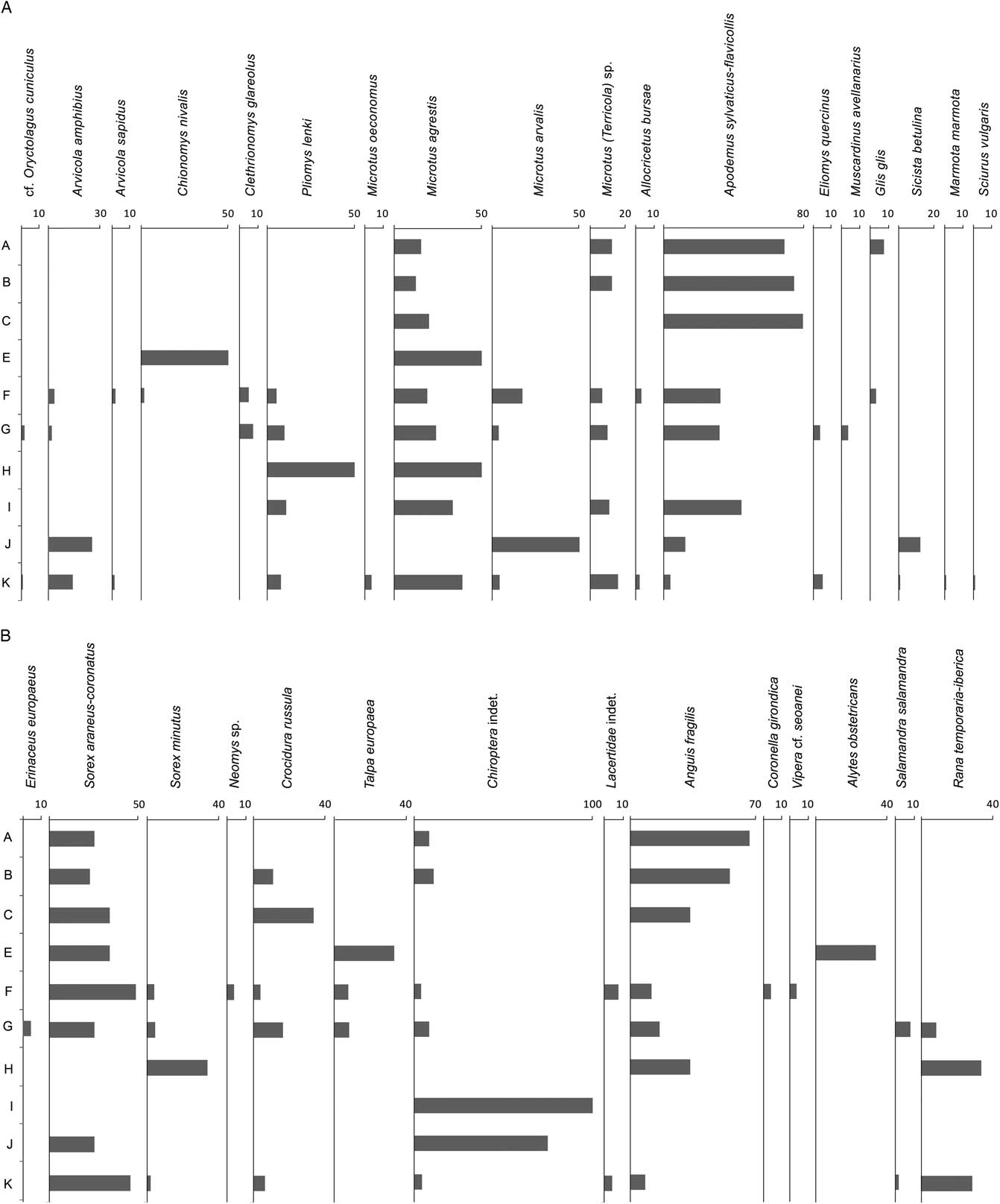
Figure 7 (A) Relative variations of abundance in a part of small mammals from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula), expressed as the percentage of the minimum number of individuals. (B) Relative variations of abundance in a part of small mammals and herpetofauna from Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula), expressed as the percentage of the minimum number of individuals.
Paleoenvironmental and paleoclimatic evolution
The pattern of temporal changes in microfaunal assemblages from Lezetxiki II was used to reconstruct environmental change using both the method of habitat weighting (Table 4, Fig. 8) and the proportion of species that predominantly occur in wooded habitats (Table 5, Fig. 9). Our data suggest a paleoenvironment composed mainly of open humid habitats (53 vs. 77%), for the beginning of the sequence (level K), associated with species such as Microtus agrestis and Microtus (Terricola) sp. There are also thermophilous affinity species (like Eliomys quercinus or Sciurus vulgaris), indicating that the environmental conditions could not have been extremely cold. Gradually, the climate probably warmed in level J (Figs. 8 and 9), with an increase of temperature and the decrease of humidity, bringing an increase in forest-dwelling taxa (48% vs. 33%). Therefore, this level may be linked to warm and humid conditions associated with an interstadial period of MIS 6. The survival of taxa currently associated with continental steppes, such as Sicista betulina, reflect that the conditions were still quite different than today. This development of the forest is more noticeable from level I onward, although the two methodologies applied in this study differ in their inferred rates of change. Habitat-weighting indicates that the advance of forests slowed down at level H (Fig. 8); the methodology established based on the proportion of forest species suggests that the forest continued to increase during the deposition of level H (Fig. 9).
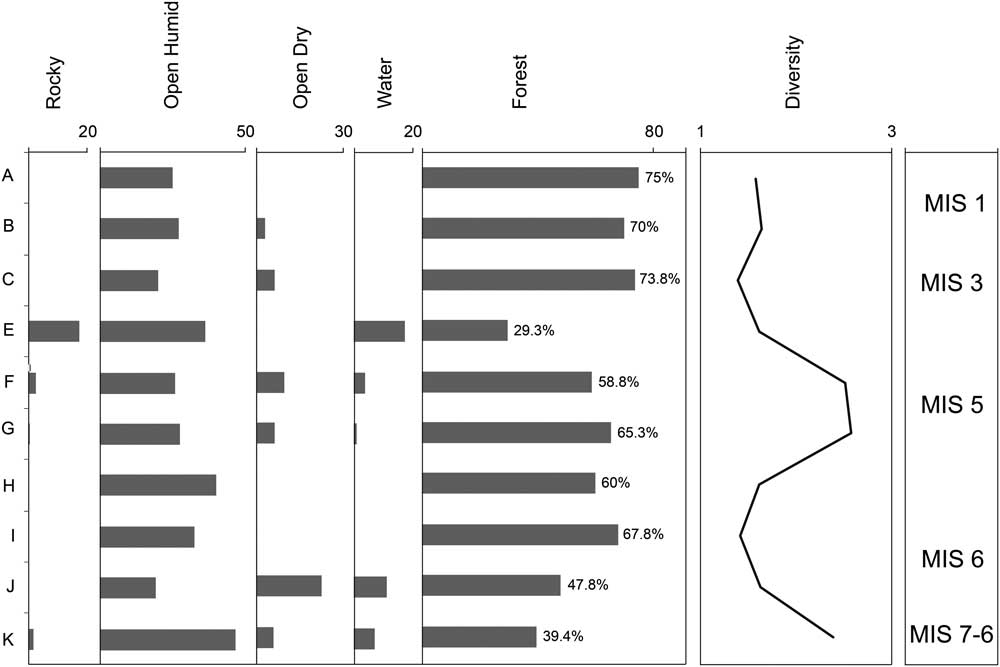
Figure 8 Habitat reconstruction of Lezetxiki II Cave (Arrasate, Basque Country, Iberian Peninsula) using the taxonomic habitat index method, and the diversity index, based on the small vertebrate assemblage. MIS, Marine Oxygen Isotope Stage.
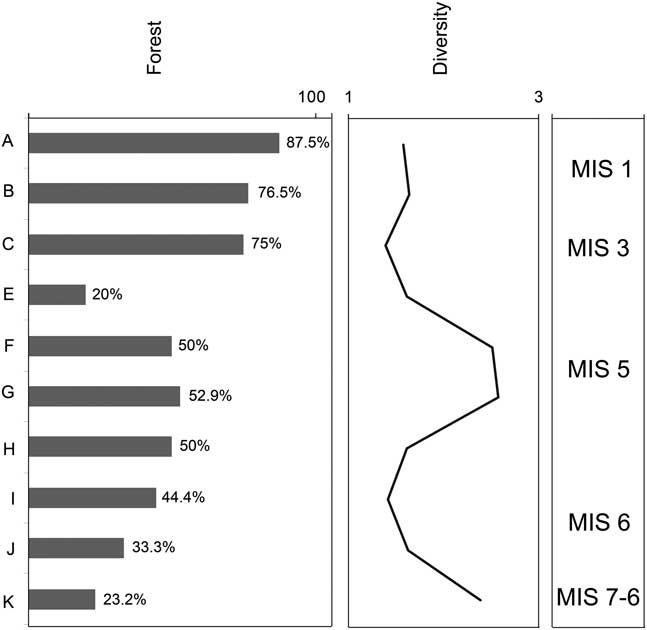
Figure 9 Paleoenvironmental and paleoclimatic reconstruction of Lezetxiki II Cave (Arrasate, Gipuzkoa) using the relative proportion of species predominantly of wooded habitats, and the diversity index, based on the small vertebrate assemblage. MIS, Marine Oxygen Isotope Stage.
Table 5 Minimum number of individuals (MNI) of small vertebrate species from Lezetxiki II (Arrasate, Basque Country, Iberian Peninsula) used to establish the paleoclimatic and paleoenvironmental evolution.
Throughout level G there were likely increased temperatures indicated by the higher diversity (H’=2.583) and the increase in woodlands habitat taxa (Figs. 8 and 9). These changes may have been brought on by warm and humid conditions associated with an interstadial period of MIS 5, most likely MIS 5e (Eemian interglacial period). Although the percentage of woodland species in level F is somewhat lower, the highest thermophilous taxa diversity is recorded, characterized by the presence of E. quercinus, M. avellanarius, C. glareolus, P. lenki, and A. sylvaticus-flavicollis. In addition, the Shannon index shows great diversity (H’=2.521). Toward the end of the MIS 5 (level E), temperatures may have slightly cooled (the presence of woodland taxa is 30% or 20% for each methods; Figs. 8 and 9). The progressive climatic warming continued during MIS 3 (level C, Aurignacian period), with a patchy landscape surrounding the cave dominated by forests. This is determined by the great representation of A. sylvaticus-flavicollis (50%) and A. fragilis. Finally, warm conditions characterize the Chalcolithic period in Lezetxiki II, with woodland dominating the landscape (Figs. 8 and 9), consistent with the climate of the present interglacial period. However, these interpretations should be treated with caution because of the limited size of the samples.
DISCUSSION
Comparison with other paleoenvironmental proxies
In addition to small vertebrates, certain other paleoenvironmental proxies have provided useful information to compare with our data. These include sediment and pollen data, which combined with our results, provide a more general picture of the climatic and environmental changes that occurred in the region. The mineralogical and sedimentologic data (Arriolabengoa et al., Reference Arriolabengoa, Iriarte, Aranburu, Yusta and Arrizabalaga2015) display the characteristics of fluviokarst sedimentation for level K, indicating the concurrence of humid conditions and scarce vegetation cover. For level J, this proxy indicates the start of warmer conditions, which is in accordance with the representation of woodlands inferred by the palynological data (Castaños et al., Reference Castaños, Murelaga, Arrizabalaga and Iriarte-Chiapusso2011). From level J onward, the endokarst sediment reflects the infiltration of autochthonous soils, denoting lesser fluviokarst activity and an increase in water infiltration, as well as the precipitation of speleothems in levels H and D. The presence of finer allochthonous sediments in level C suggests greater fluviokarst activity and erosion as a consequence of cooler conditions. Finally, the autochthonous sediments and slight calcite precipitation of levels B and A are related with warmer and wetter conditions.
Comparing our results with those obtained from the mineralogical and sedimentologic study (and the single pollen reference) of Lezetxiki II, we generally find more agreement among the various environmental proxies; however, some differences occur. For example, the small vertebrates suggest that any warming within level J was likely not long-lasting, as meadow-inhabiting taxa still dominate this interval. Additionally, the cooler conditions suggested by mineralogical and sedimentologic data are not indicated by the small vertebrate record, which suggests abundant woodlands and likely warmer conditions.
Other paleoenvironmental reconstructions with small vertebrates from northern Iberia
In the Iberian Peninsula, there are several sites where Middle–Late Pleistocene and Holocene sequences with small vertebrates have been studied from a paleoenvironmental and paleoclimatic point of view, comparable with our data. In this sense, the first site is Arlanpe Cave, a small cave situated in the Arratia Valley (Lemoa, Bizkaia), where a speleothem has been dated at 184,271 +34,258/−26,576 yr BP (Rios-Garaizar et al., Reference Rios-Garaizar, Garate and Gómez-Olivencia2013). Comparing the paleoenvironmental reconstruction of level K from Lezetxiki II with the corresponding time span at Arlanpe, we find a very similar pattern of habitat, with woodland landscape albeit with considerable meadows, especially ones with wet soil (Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Murelaga, Bailon, Rofes and Ordiales2013).
For the MIS 6, we focus again on Arlanpe, where both level VI of the entrance and level 4 of the central sector correspond to this period. As in Lezetxiki II, the microvertebrate species associated with open habitats are in the majority, ranging from 53% (level 4) to 47% (level IV). Additionally, the herpetofaunal assemblage from the archaeological site Estanque de Tormentas de Butarque H-02 (Manzanares Valley, central Iberian Peninsula) pointed out that during MIS 6, the climate was colder (−3.0°C) and slightly wetter (+122.8 mm) than at present, with a large representation of dry environments (Blain et al., Reference Blain, Rubio-Jara, Panera, Uribelarrea, Laplana, Herráez and Pérez-González2017).
The microfauna of MIS 5 has been studied mainly on the coast of Catalonia sites, for example in Teixoneres (López-García et al., Reference López-García, Blain, Burjachs, Ballesteros, Allué, Cuevas-Ruiz and Rivals2012a) and Cova Gegant (López-García et al., Reference López-García, Blain, Sanz and Daura2012b). Any perspective that these Mediterranean sites might supply therefore likely diverges significantly from the Cantabrian sites. Nevertheless, the microvertebrates of the last interglacial period (late MIS 5) identified at the archaeological site of Cueva del Camino (Pinilla del Valle, Madrid) are typically thermophilous species (Arsuaga et al., Reference Arsuaga, Baquedano, Pérez-González, Sala, Quam, Rodríguez and García2012), similarly to the level F of Lezetxiki II.
The woodland domains inferred by the small vertebrate assemblage during the MIS 3 at Lezetxiki II (level C), is similar to the interpretations for level 8 (ca. 29,600 cal yr BP) at Askondo, where transitory warming seems to correspond with the warmer tendency detected after the Heinrich Event 3 in the NGRIP-GICC05 (North Greenland Ice Core Project-Greenland Ice Core Chronology 2005) record that culminated in the warm Greenland Interstadial 4 (Garcia-Ibaibarriaga et al., Reference Garcia-Ibaibarriaga, Rofes, Bailon, Garate, Rios-Garaizar, Martínez-García and Murelaga2015b). One of the few sites from this period with a microfaunal study is the now disappeared neighboring site of Labeko Koba (Arrasate, Basque Country), which records a complete lack of cold-related taxa and a relatively high abundance of Glis glis for the Aurignacian period (levels VII to IV; Pemán, Reference Pemán2000). It is important to highlight that in both cases, the microfaunal data are in contradiction with the data obtained from other environmental proxies.
Finally, microvertebrate fossil remains from the uppermost levels (B and A) of Lezetxiki II Cave have been assigned to the Chalcolithic period. The results coincide with a Holocene climate, with a warm, wet climate similar to the current one. Woodlands were the dominant landscape as in many archaeological sites of similar chronology (El Mirón: Cuenca-Bescós et al., Reference Cuenca-Bescós, Straus, González Morales and García Pimienta2009; Santimamiñe: Rofes et al., Reference Rofes, Murelaga, Martínez-García, Bailon, López-Quintana, Guenaga-Lizasu and Ortega2014; El Mirador: Bañuls-Cardona et al., Reference Bañuls-Cardona, López-García and Vergès2013).
CONCLUSION
The small vertebrate assemblage from Lezetxiki II Cave, the oldest record of the Cantabrian Range, provides a rare opportunity to articulate the paleonvironmental and paleoclimatic scenario of the “Basque crossroad” during the late middle Pleistocene and late Pleistocene, improving additionally our knowledge of the northern third of the Peninsula. In addition, the significance of the basal levels from Lezetxiki II should be noted as they represent the possible context of the human humerus found in 1964 in Leibar Cave (Arrizabalaga et al., Reference Arrizabalaga, Altuna, Areso, Falguerès, Iriarte-Chiapusso, Mariezkurrena and Pemán2005), which represents the oldest human fossil of the Basque Country.
The 3-m-deep deposit began with a cold period, at the end of MIS 7 or beginning of MIS 6 (level K), while levels J–I could correspond to MIS 6. The small vertebrate assemblage from Lezetxiki II Cave could provide useful information about the internal variations of MIS 5, because the changes observed in levels H–E could correspond to these fluctuations. Likewise, during MIS 3 (level C) warming is suggested by the small vertebrate remains. Finally, the woodland reconstruction of the uppermost two levels (B and A) is consistent with their middle Holocene age.
ACKNOWLEDGMENTS
We would like to thank J. Rofes for his assistance with eulipotyphlans and to Christine Laurin for editing the English text. The technical and human support provided by SGIker (UPV/EHU) is also gratefully acknowledged. AS-B has a predoctoral fellowship from the Basque government. The archaeological work in Lezetxiki was funded by Aranzadi Science Society, Gipuzkoako Foru Aldundia, the Municipality of Arrasate, and Kobate Quarry. We also received financial support from the Research team GIU15/34 of the University of the Basque Country, the Consolidated Research Group (IT-622-13) in Prehistory of the University of the Basque Country, and the PALEOGATE project of the Spanish Science Ministry (HAR2014-53536-P). Additional financial support for this research was provided by Australian Research Council (ARC) Future Fellowship project FT130100195, ARC Discovery Early Career Researcher Award DE160100743. LA and MD thank Carlos Pérez Garrido for his assistance with preparing and measuring the luminescence dating samples at the CENIEH luminescence dating laboratory, Burgos, Spain. We thank also the reviewers of the manuscript Dr. H. A. Blain and Dr. C. Sesé for their valuable comments.