American youth belonging to racial/ethnic minority groups (e.g., African Americans, Latinos, and Asian Americans; henceforth, minorities) are more vulnerable to mental and physical health problems (Alegria, Vallas, & Pumariega, Reference Alegria, Vallas and Pumariega2010; Braveman, Cubbin, Egerter, Williams, & Pamuk, Reference Braveman, Cubbin, Egerter, Williams and Pamuk2010; Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010), including mood and anxiety disorders (Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010), chronic fatigue (Dinos et al., Reference Dinos, Khoshaba, Ashby, White, Nazroo, Wessely and Bhui2009), and obesity (Ogden et al., Reference Ogden, Carroll, Curtin, McDowell, Tabak and Flegal2006). These health disparities are potentially the result of differential exposure to stressful life experiences, which in turn result in differences in the daily patterning of affective, cognitive, and biological processes that accumulate over time to foster psychopathology or physical illness (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Levy, Heissel, Richeson, & Adam, Reference Levy, Heissel, Richeson and Adam2016; Mays, Cochran, & Barnes, Reference Mays, Cochran and Barnes2007; Shields, Moons, & Slavich, Reference Shields, Moons and Slavich2017; Susman, Reference Susman2007). These physical and mental health disparities can be thought of as the products of developmental cascades, defined as the cumulative outcomes of interactions within and among developing systems that can spread effects across domains of functioning and across generations (Masten & Cicchetti, Reference Masten and Cicchetti2010). The field of cultural development and psychopathology has begun examining how cultural processes within the individual and within society can act within developmental cascades and set the stage for normal or abnormal behavior, risk, or resilience (for review, see Causadias, Reference Causadias2013). However, there is a scarcity of research testing the links between culturally bound daily psychological experiences and biological processes that may help explain racial inequalities in long-term health outcomes. The current study aimed to address this gap by examining associations between racial/ethnicFootnote 1a differences in daily affective states and diurnal variation in the functioning of the hypothalamic–pituitary–adrenocortical (HPA) axis, one of the body's primary stress-response systems. We focus on the HPA axis given evidence linking HPA dysregulation with concurrent and future symptoms of numerous mental and physical health conditions across the life span (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017; Shields & Slavich, Reference Shields and Slavich2017). In adolescence, dysregulated cortisol patterns have been associated with greater risk of current and future psychopathology such as depression and anxiety (Adam, Reference Adam2006; Adam et al., Reference Adam, Doane, Zinbarg, Mineka, Craske and Griffith2010; Carnegie et al., Reference Carnegie, Araya, Ben-Shlomo, Glover, O'Connor, O'Donnell and Lewis2014; Doane et al., Reference Doane, Mineka, Zinbarg, Craske, Griffith and Adam2013; Van den Bergh & Van Calster, Reference Van den Bergh and Van Calster2009), as well as higher odds of common physical health conditions such as obesity (Ruttle et al., Reference Ruttle, Javaras, Klein, Armstrong, Burk and Essex2013). We focused on affective states and their daily association with cortisol because there is increasing evidence that approximately 50% of variability in diurnal HPA functioning is state dependent, as opposed to showing traitlike continuity (Ross, Murphy, Adam, Chen, & Miller, Reference Ross, Murphy, Adam, Chen and Miller2013). Given the high responsivity of this system to daily fluctuations based on experience, it is important to understand its associations with daily subjective perceptions of affective states to better understand how the system may be calibrated by experience during development. Even though we do not examine health disparities directly in this study, our goal is to begin elucidating ethnic/racial differences in affective and biological processes that may set the stage for long-term health disparities.
Brief Overview of the HPA Axis
When confronted with physical or psychological challenges that tax or overwhelm the organism's capacity to cope, the body initiates a stress response consisting of physiological and behavioral responses mediated by the nervous, endocrine, and immune systems (Smith & Vale, Reference Smith and Vale2006). The HPA axis plays an integral role in these processes by mobilizing energy for coping with stressors and modifying the individual's responses to similar stressors in the future (Gunnar, Doom, & Esposito, Reference Gunnar, Doom, Esposito and Lerner2015; Sapolsky, Romero, & Munck, Reference Sapolsky, Romero and Munck2000). The activity of the HPA axis can be characterized along two basic dimensions, basal functioning and reactivity to stressors (Spencer & Deak, Reference Spencer and Deak2016). Basal HPA functioning follows a diurnal rhythm whereby cortisol, one of the main products of the HPA axis, is secreted in a pulsatile fashion across the day, reaching peak levels in the morning approximately 30 min after awakening, and declining gradually across the day to reach minimum levels at night (Gunnar et al., Reference Gunnar, Doom, Esposito and Lerner2015). Superimposed on this basal rhythm is the reactivity of the HPA axis to physical or psychological threats to well-being (i.e., stressors). The HPA axis is powerfully activated by social threats (Dickerson & Kemeny, Reference Dickerson and Kemeny2004; Gunnar & Adam, Reference Gunnar and Adam2012), as well as by physical threats more typically studied in nonhuman animals, such as immobilization (Smith & Vale, Reference Smith and Vale2006).
There are three indices of basal HPA functioning that are most commonly examined and that we focused on in this study. The diurnal slope is a negative slope from morning to evening cortisol levels, and deviations from this typical pattern such as flatter (i.e., less negative) slopes have been linked to deleterious emotional and physical health problems, particularly immune-related and metabolic outcomes (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017). The cortisol awakening response is the rise in cortisol production from wake-up to approximately 30 min later and is thought to reflect distinct processes related to anticipated demands for energy mobilization for the day (Fries, Dettenborn, & Kirschbaum, Reference Fries, Dettenborn and Kirschbaum2009). General life stress and depression have been associated with an elevated cortisol awakening response, whereas posttraumatic stress, fatigue, burnout, and exhaustion have been linked to an atypically low cortisol awakening response (Boggero, Hostinar, Haak, Murphy, & Segerstrom, Reference Boggero, Hostinar, Haak, Murphy and Segerstrom2017; Chida & Steptoe, Reference Chida and Steptoe2009). Finally, the area under the curve represents an integrated measure of total daily cortisol output, which is calculated based on repeated measurements throughout the day and the spacing between them (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). Chronic stress has been associated with greater total daily cortisol output, combined with a flatter slope; that is, this pattern results from lower than expected morning levels but higher afternoon and evening production (Miller, Chen, & Zhou, Reference Miller, Chen and Zhou2007).
Racial/Ethnic Differences in HPA Activity
The prior literature on racial/ethnic differences in HPA activity is somewhat limited, but these differences in physiology are important to explore as they may help us explain racial/ethnic health disparities. Some intriguing patterns have begun to emerge in studies with children (Bush, Obradovic, Adler, & Boyce, Reference Bush, Obradovic, Adler and Boyce2011; Martin, Bruce, & Fisher, Reference Martin, Bruce and Fisher2012), adolescents (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Hostinar, McQuillan, Mirous, Grant, & Adam, Reference Hostinar, McQuillan, Mirous, Grant and Adam2014; Zeiders, Causadias, & White, Reference Zeiders, Causadias and White2017), and adults (Bennett, Merritt, & Wolin, Reference Bennett, Merritt and Wolin2004; Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010; McCallum, Sorocco, & Fritsch, Reference McCallum, Sorocco and Fritsch2006; Suglia et al., Reference Suglia, Staudenmayer, Cohen, Enlow, Rich-Edwards and Wright2010). In particular, flatter cortisol slopes have been observed in African American compared to White youth (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Martin et al., Reference Martin, Bruce and Fisher2012). Results are more inconsistent when comparing other minorities (e.g., Latino or multiracial youth) with White youth, with one study finding that Latino adolescents exhibited flatter slopes than non-Latino White adolescents (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007), whereas another did not detect a statistically significant difference (Martin et al., Reference Martin, Bruce and Fisher2012). In adults, studies have consistently found flatter slopes in both African American and Latino compared to White adults (Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010; McCallum et al., Reference McCallum, Sorocco and Fritsch2006). In terms of daily cortisol output (area under the curve), ethnic minority kindergarten children tend to exhibit higher area under the curve compared to White children (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011), whereas Latino and African American adults show lower area under the curve compared to White adults (Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010). Finally, for the cortisol awakening response, African American adults exhibit a lower cortisol awakening response compared to White adults, an effect that is even more pronounced in low- socioeconomic status African Americans (Bennett et al., Reference Bennett, Merritt and Wolin2004). Overall, these patterns suggest that ethnic minorities tend to show flatter diurnal cortisol slopes and lower cortisol awakening response levels, with differences most consistently observed when comparing African Americans with Whites and results being more mixed when comparing Latino with non-Latino Whites. Racial/ethnic differences in total cortisol output (area under the curve) have been more rarely investigated, as only two studies to date, one examining children (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011) and one adults (Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010), have explored these differences.
Current theory suggests that these ethnic/racial differences are related to experiences of chronic stress in general and experiences of discrimination more specifically, which are more prevalent among African American and Latino groups (Lewis, Cogburn, & Williams, Reference Lewis, Cogburn and Williams2015). For instance, studies have found that both recent and long-term exposure to perceived discrimination is associated with flatter cortisol slopes (Adam et al., Reference Adam, Heissel, Zeiders, Richeson, Ross, Ehrlich and Eccles2015; Zeiders, Hoyt, & Adam, Reference Zeiders, Hoyt and Adam2014) or greater daily cortisol output (Zeiders, Doane, & Roosa, Reference Zeiders, Doane and Roosa2012). However, some studies have found that discrimination does not explain the associations between race/ethnicity and diurnal cortisol (Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; Martin et al., Reference Martin, Bruce and Fisher2012). A recent meta-analysis of associations between racial discrimination and cortisol parameters sheds some light on these discrepant findings (Korous, Causadias, & Casper, Reference Korous, Causadias and Casper2017). This meta-analysis reported that the average effect size for this association is small (r = .04, though effects are slightly larger when experimental protocols are used, such as exposing participants to instances of discrimination in the laboratory). The small effect size suggests that more research is needed to explore other factors that might further explain racial/ethnic disparities in cortisol output (Korous et al., Reference Korous, Causadias and Casper2017). Furthermore, researchers propose that specific experiences such as discrimination may not fully or consistently account for racial/ethnic differences in diurnal cortisol rhythms because they do not take into account individual differences in processing and coping with such experiences. Instead, considering participants’ affective states as aggregate measures of their responses to multiple streams of experiences might be a more proximal predictor of HPA patterns (Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006). For this reason, the current study aimed to examine the extent to which racial/ethnic differences in affect may explain racial/ethnic differences in diurnal cortisol patterns.
Another possible explanation for racial/ethnic differences in HPA activity is that they might be due to differences in socioeconomic status, which is closely intertwined with race/ethnicity in the United States. Previous studies have reported associations between socioeconomic status and cortisol parameters, with minorities and low-socioeconomic status participants exhibiting similar profiles. However, this research has indicated that racial/ethnic differences in cortisol parameters persist after accounting for socioeconomic status and independently predict cortisol outcomes (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011; Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010; Martin et al., Reference Martin, Bruce and Fisher2012). Moreover, ethnic minorities appear to benefit less from high socioeconomic status compared to Whites (Bennett et al., Reference Bennett, Merritt and Wolin2004). Overall, these patterns suggest that race/ethnicity and socioeconomic status may have unique impacts on cortisol output. As such, the current study will examine socioeconomic status as a covariate in the relationship between race/ethnicity and cortisol parameters.
Associations Between HPA Functioning and Affect
Because the HPA axis reacts powerfully to social and physical threats to well-being, most prior literature has focused on associations between HPA activity and negative affect such as fear, sadness, or worry. For instance, one naturalistic study of diurnal cortisol rhythms in adolescents found that momentary increases in state negative affect (worry, stress, anger, or frustration) were significantly associated with higher basal cortisol levels (Adam, Reference Adam2006). In another study of a diverse sample of adolescents, higher levels of negative affect were associated with flatter diurnal cortisol slopes (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007).
Research on associations between positive affect and HPA functioning has begun to emerge. For instance, exciting events can elevate cortisol, such as competitive events among adults (Bateup, Booth, Shirtcliff, & Granger, Reference Bateup, Booth, Shirtcliff and Granger2002; Carre, Muir, Belanger, & Putnam, Reference Carre, Muir, Belanger and Putnam2006) and Christmas Eve for children (Flinn, Reference Flinn2006). Furthermore, the “cortisol boost hypothesis” suggests that cortisol elevations provide a boost of energy and may promote positive, alert states as well as be protective against subsequent elevations in negative mood (Hoyt, Zeiders, Ehrlich, & Adam, Reference Hoyt, Zeiders, Ehrlich and Adam2016). Thus, the extent to which cortisol levels are differentially associated with negative versus positive affective states remains to be fully characterized. Furthermore, we know little about the extent to which differences in experiences of both positive and negative affect may explain racial/ethnic disparities in HPA activity. Nevertheless, we predicted that racial/ethnic minorities would report higher levels of negative affect and lower levels of positive affect compared to White youth based on prior evidence that they are at higher risk of developing mood and anxiety disorders (Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010), as well as their greater exposure to life stressors, perceived discrimination, stereotype threat, and sleep difficulties (Levy et al., Reference Levy, Heissel, Richeson and Adam2016), all of which can predispose toward negative affect and reduce positive affect.
The Present Study
Biological processes have historically been assumed to be hard-wired and universal, but recent research has challenged this view and begun exploring the interplay between biology and culture, including racial/ethnic differences in life experiences and psychopathology (Causadias, Reference Causadias2013; Causadias, Telzer, & Gonzales, Reference Causadias, Telzer, Gonzales, Causadias, Telzer and Gonzales2018; Causadias, Telzer, & Lee, Reference Causadias, Telzer and Lee2017). Many questions remain unexplored in this area of inquiry, particularly regarding the psychological interface between cultural and biological variation. The current study aims to address this gap by examining the role of affective processes as possible mediators of racial/ethnic differences in biological processes. Specifically, we aim to address the following questions:
Question 1. What is the magnitude of racial/ethnic differences in diurnal cortisol patterns in adolescence?
Hypothesis 1.1. We hypothesize that minority participants will exhibit flatter cortisol slopes than White participants.
Hypothesis 1.2. We hypothesize that minority participants will exhibit a lower cortisol awakening response than White participants.
Hypothesis 1.3. We hypothesize that minority participants will exhibit higher total cortisol output (area under the curve) than White participants.
Question 2. What is the magnitude of racial/ethnic differences in low-arousal and high-arousal positive and negative affect states in adolescence?
Hypothesis 2. We hypothesize that racial/ethnic minorities will report on average greater levels of negative affect and lower levels of positive affect.
Question 3. Do differences in affective patterns explain (i.e., statistically mediate) racial/ethnic differences observed in diurnal cortisol?
Hypothesis 3. We hypothesize that affective patterns will statistically mediate racial/ethnic differences observed in diurnal cortisol.
We focused on adolescence because this is a period of massive changes in both the activity of the HPA axis and affective processes, which may explain the onset of psychopathology during this developmental window for many youth (Dahl & Gunnar, Reference Dahl and Gunnar2009). We used linear mixed modeling to capture daily within-person variations in cortisol slopes, cortisol awakening response, and area under the curve. We focused on multiple HPA indices given that they are influenced by different factors (Fries et al., Reference Fries, Dettenborn and Kirschbaum2009) and may point to different pathophysiological mechanisms toward later dysfunction. We studied a diverse group of adolescents, with sizable subsamples for multiple racial/ethnic backgrounds including African American, Latino, Asian American, White, and multiracial youth. This allowed us to capture racial/ethnic differences across multiple groups. In addition, we utilized a daily diary approach for assessing both positive and negative mood. Daily diary reports are the measurement method of choice for assessing affect because they are more accurate than methods that can be subject to recall biases (Bolger, Davis, & Rafaeli, Reference Bolger, Davis and Rafaeli2003).
Method
Participants
Participants included 370 adolescents (57.3% female) between the ages of 11.9 and 18 years (M age = 14.65 years, SD = 1.39 years; see Table 1 for detailed demographic information). Participants were recruited from the community using convenience sampling, including posting flyers at schools, posting on listservs serving ethnic minority families, recruiting participants from other studies who agreed to be contacted for other research studies, and word of mouth. Due to this method of recruitment, we do not have information on the percentage of the sample approached who participated. The sample was diverse in terms of race/ethnicity: 39.46% non-Latino White (from here on referred to as White, N = 146), 25.4% Asian American (N = 94), 17.8% Latino (N = 66), 10.8% African American (N = 40), and 6.5% mixed race or other race (N = 24). The sample covered a fairly broad range of the socioeconomic spectrum. When considering maternal education as an index of socioeconomic status, 9.73% of mothers had less than an eighth-grade education, 2.43% completed junior high school, 11.35% attended some high school, 24.05% completed high school, 4.59% attended trade or vocational school, 21.89% completed college, and 22.97% completed graduate school (2.97% declined to answer). Adolescents and parents completed written assent and consent in accordance with the institutional review board.
Table 1. Sample demographic information and descriptive statistics for primary constructs

Procedure
Participants received diary checklists for 14 days and a saliva collection kit to complete on days 2 through 5. They had the option to complete the diaries with paper and pencil or via a secure website. For those completing with paper/pencil, we monitored completion of the checklists by providing participants with 14 manila envelopes and an electronic time stamper. The time stamper is a small, handheld device that imprints the current date and time and is programmed with a security code so that the correct date and time cannot be altered. Participants were instructed to place their completed checklists into a sealed envelope each night and to stamp the seal of the envelope with the time stamper. For those completing the surveys on the secure website, an e-mail with the link to each daily survey was sent, and the time and date of completion were recorded via the website.
Measures
Diurnal cortisol
Adolescents provided saliva samples across days 2 through 5 of the daily diaries at four time points each day (i.e., 16 total samples): (a) wake-up time, (b) 30 min after waking up, (c) 5 p.m. (or before dinner), and (d) 8 p.m. (or before bed). Participants were instructed to take their samples before or at least 30 min after brushing their teeth, drinking, eating, or using tobacco. Participants were provided with a card to log the times of each sample using an electronic time stamper (Dymo Corporation, Stamford, CT), which imprinted the current date and time and was programmed with a security code such that adolescents could not alter the correct time and date. Adolescents were instructed to stamp the card beside the appropriate heading for each sample and to place the sample in their freezer. At the end of the saliva collection period, research staff returned to the home or participants brought the samples to the lab, which were immediately stored at −80 °C until shipment to the Laboratory of Biological Psychology at the Technical University of Dresden, Germany, where they were assayed using high-sensitivity chemiluminescence-immunoassays (IBL International, Hamburg, Germany). The interassay coefficient of variation was below 8%. A subsample of 251 participants provided information regarding medication they were taking, and among them, n = 6 reported using hormonal medications (corticosteroids, estradiol, etc.). Results were largely identical (within rounding error) when excluding these 6 participants, and thus we report our results on the full sample on which we conducted our analyses.
Participants received $10 for completing the diaries and $10 for completing the saliva samples. In addition, adolescents were told that they would receive a $20 bonus if inspection of the data indicated that they had completed all the diaries and saliva samples correctly and on time.
We computed cortisol slopes, the cortisol awakening response, and cortisol area under the curve using standard formulas. Cortisol slopes were computed as the difference between the fourth (bedtime) cortisol sample and the first morning sample, divided by the time elapsed between these two samples. We computed the cortisol awakening response as the increase in cortisol from wake to 30 min postwake. The total cortisol area under the curve was computed from the first, third, and fourth cortisol concentrations (i.e., excluding the second sample, which reflects the cortisol awakening response) using the standard trapezoid method (Pruessner et al., Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003).
Affect
Daily mood diary data from the 4 days when salivary cortisol was collected were used for our analyses in order to best capture potential associations between daily affect and cortisol. Daily mood was assessed with items taken from the Profile of Mood States (McNair, Lorr, & Droppelman, Reference McNair, Lorr and Droppelman1971). Adolescents used a 5-point scale ranging from 1 (not at all) to 5 (extremely) to indicate the extent to which they felt a number of affective states each day: enthusiastic, excited, interested, and joyful (these were the high-arousal positive valence states); calm, cheerful, and happy (the low-arousal positive valence states); angry, nervous, on edge, mad, uneasy, worried, embarrassed, and stressed (the high-arousal negative valence states); and discouraged, exhausted, fatigued, hopeless, sad, lonely, and bored (the low-arousal negative valence states). The scores for the affective states within each of these four superordinate categories were averaged together to create one score for the category. Confirming our grouping of these emotions in the four superordinate categories, the internal consistencies for these subscales were high for high-arousal positive affect (α = 0.88), low-arousal positive affect (α = 0.79), high-arousal negative affect (α = 0.89), and low-arousal negative affect (α = 0.82) states.
Data analysis plan
All analyses performed were linear mixed models that nested days (Level 1) within participants (Level 2). Fixed effects were tested at the level of participants (i.e., Level 2). This statistical approach accounts for dependency within participants and introduces less bias related to missing data compared to traditional statistical analyses, such as repeated-measures analysis of variance (Finch, Bolin, & Kelley, Reference Finch, Bolin and Kelley2014; Raudenbush & Bryk, Reference Raudenbush and Bryk2002). All analyses were conducted using the R statistical programming language, version 3.4.0 (R Core Team, 2017). Linear mixed models were estimated using the lmerTest package in R, version 2.0-33 (Kuznetsova, Brockhoff, & Christensen, Reference Kuznetsova, Brockhoff and Christensen2017); estimated marginal means and standard errors were derived using the lsmeans package in R, version 2.26-3 (Lenth, Reference Lenth2016). All degrees of freedom were estimated using the Satterthwaite approximation, which makes the analyses more robust to outliers and violations of normality but entails that the degrees of freedom contain numbers that are not integers (Keselman, Algina, Kowalchuk, & Wolfinger, Reference Keselman, Algina, Kowalchuk and Wolfinger1999; Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2017).
To address our first question, we conducted linear mixed models that included race/ethnicity as a Level 2 predictor of cortisol parameters (slope, cortisol awakening response, and area under the curve) examined independently in separate analyses. We first treated race/ethnicity as a binary variable (0 = “White,” 1 = “racial/ethnic minority”), which was further probed in follow-up analyses examining specific contrasts between five racial/ethnic groups: White, Asian American, Latino, African American, and mixed/other. For these follow-up contrasts, we report which findings remain significant after applying a Bonferroni correction (p < .005) to minimize risk of Type I error given the number of pairwise comparisons conducted. We report estimated marginal means and standard errors derived from our models for each racial/ethnic group, as these best represent our results. Age and sex were included as covariates in all analyses, though the results were essentially identical without these covariates included. For our second research question, we conducted linear mixed models that included race/ethnicity as a Level 2 predictor of four affect variables (low-arousal and high-arousal positive affect, and low-arousal and high-arousal negative affect). Similar to cortisol analyses, we first treated race/ethnicity as a binary variable, and then examined specific contrasts between all five racial/ethnic groups. For our third question, we examined whether racial/ethnic differences in affect explain cortisol differences. We entered the affect variables into linear mixed models as predictors of cortisol parameters to test cortisol–affect associations and to explore whether racial/ethnic differences in cortisol patterns persist after accounting for differences in affect. Finally, we conducted sensitivity analyses to test the extent to which the results of these analyses change when accounting for time of wake-up and maternal education (a proxy for socioeconomic status), both of which have been linked to cortisol functioning in past studies (e.g., Kudielka, Hellhammer, & Wüst, Reference Kudielka, Hellhammer and Wüst2009). We report confidence intervals and effect sizes (Hedge's g) for all primary results.
Results
Descriptive statistics
Table 1 displays sample characteristics on major constructs. Table 2 displays bivariate correlations among the major constructs of interest in the entire sample, whereas Table 3 shows the same correlations separately for the minority group and the White group. As can be seen in Table 2, cortisol indices showed expected correlations with each other, and affect variables also showed significant correlations with each other. The only cortisol measure showing significant associations with affect was the cortisol awakening response, which was positively correlated with high-arousal negative affect and inversely correlated with low-arousal positive affect. The slope and area under the curve did not show any strong or significant bivariate associations with affect variables.
Table 2. Bivariate correlations among major constructs in the entire sample

*p < .05. **p < .001.
Table 3. Bivariate correlations among major constructs within minority group (top panel) and White participants (bottom panel)

*p < .05. **p < .01. ***p < .001.
Question 1. What is the magnitude of racial/ethnic differences in diurnal cortisol patterns in adolescence?
Effects of minority status
A comparison of White and minority participants’ cortisol slopes controlling for age and sex indicated that there was a significant main effect of race/ethnicity, F (1, 344.7) = 5.26, p = .02, ω2partial = .012, such that minority participants had flatter (i.e., less negative) slopes (M = –1.19, SE = 0.05) compared to White participants (M = –1.36, SE = 0.06), 95% confidence interval difference (CIdiff) [0.02, 0.31], g = 0.25. This effect can be observed in Figure 1, which displays average cortisol diurnal slopes for minority and White youth. There was also a significant main effect of age, F (1, 350.0) = 28.67, p < .001, ω2partial = .073, indicating that slopes were flatter for older participants than younger (B = 0.14, SE = 0.03, β = 0.19). The effect of sex was not significant (p = .13; g = –0.16). Similar analyses of participants’ cortisol awakening response indicated that there was not a significant main effect of race (p = .055, ω2partial = .008), age (p = .06, ω2partial = .008), or sex (p = .23, g = 0.13).

Figure 1. Average diurnal cortisol patterns by race/ethnicity coded in a binary fashion as minority versus White.
Analyses of participants’ area under the curve cortisol output indicated that there was not a significant main effect of race (p = .21, ω2partial = .002). There were, however, significant effects for both covariates. The significant main effect of age, F (1, 351.2) = 33.64, p < .001, ω2partial = .085, indicated that cortisol area under the curve was lower for older participants compared to younger ones (B = –14.06, SE = 2.42, β = –0.20). The significant main effect of sex, F (1, 352.7) = 6.09, p = .01, ω2partial = .014, indicated that female participants exhibited larger cortisol areas under the curve (M = 168.02, SE = 5.23) than male participants (M = 151.30, SE = 4.40), 95% CIdiff [3.39, 30.04], g = 0.26.
Follow-up by specific racial/ethnic categories
To further probe the effects of minority status, we next considered race/ethnicity as a five-level category and examined pairwise contrasts between specific groups. For cortisol slopes, there was again a significant main effect of race, F (4, 348.5) = 2.91, p = .02, ω2partial = .021, and age, F (1, 344.8) = 16.65, p < .001, ω2partial = .043, with the effect of sex being nonsignificant (p = .12, ω2partial = .004). As illustrated in Figure 2, contrasts indicated that the cortisol slopes of African American participants (M = –1.02, SE = 0.11) were significantly flatter than the slopes of the White group (M = –1.36, SE = 0.06), t (339.8) = –2.76, p = .006, 95% CIdiff [0.10, 0.57], g = 0.30, and the Asian American group (M = –1.31, SE = 0.07), t (344.8) = 2.21, p = .03, 95% CIdiff [0.03, 0.54], g = 0.24. Furthermore, Latino youth also showed flatter slopes than the White group (M = –1.08, SE = 0.10), t (361.8) = –2.38, p = .02, 95% CIdiff [0.05, 0.51], g = 0.25. However, when the Bonferroni correction was applied using a cutoff of p < .005, these pairwise differences were no longer statistically significant. As before, the significant main effect of age indicated that older participants had flatter slopes than younger participants (B = 0.12, SE = 0.03, β = 0.16).

Figure 2. Average cortisol slopes by race/ethnicity (all five groups shown).
For the cortisol awakening response, analyses indicated that there was not a significant effect of race (p = .08, ω2partial = .013), age (p = .33, ω2partial < .001), or sex (p = .32, ω2partial < .001). Contrasts indicated that there was a significant difference between White and Latino participants, t (332.3) = 2.35, p = .02, 95% CIdiff [–6.34, –0.56], g = –0.26, such that Latino participants exhibited a lower cortisol awakening response (M = 2.11, SE = 1.23) compared to White participants (M = 5.56, SE = 0.74; see Figure 3). However, this was not a significant difference when applying the Bonferroni correction (p < .005). There were no other significant pairwise differences, ps > .05, |g|s < .17.
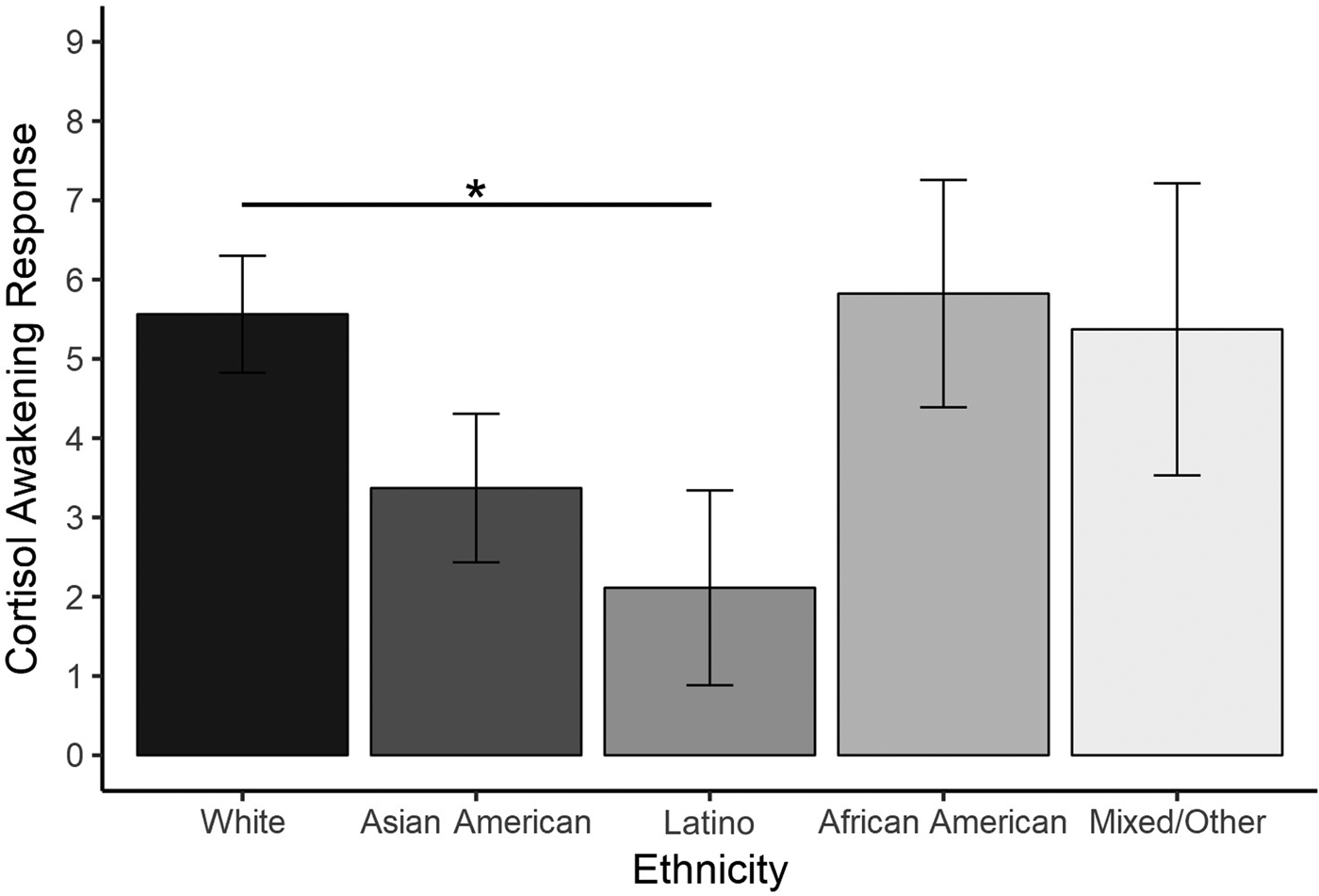
Figure 3. Average cortisol awakening response by specific racial/ethnic group. *p < .05.
For cortisol area under the curve, analyses revealed a significant main effect of race, F (4, 354.5) = 5.52, p < .001, ω2partial = .048, age, F (1, 351.9) = 11.41, p < .001, ω2partial = .029, and sex F (1, 353.6) = 5.38, p = .02, ω2partial = .012. The significant main effect of race was driven by Latino participants exhibiting significantly lower area under the curve values than each of the other groups and mixed/other participants showing significantly higher values than each of the other groups (see Figure 4). Latino participants (M = 125.20, SE = 9.13) showed lower area under the curve cortisol than White participants (M = 165.19, SE = 5.10), t (357.4) = 3.74, p < .001, 95% CIdiff [–61.03, –18.96], g = –0.39, African American participants (M = 152.83, SE = 10.13), t (362.4) = 1.99, p = .05, 95% CIdiff [0.35, 54.91], g = 0.21, Asian American participants (M = 164.55, SE = 6.58), t (364.0) = 3.36, p < .001, 95% CIdiff [16.29, 62.40], g = 0.35, and mixed/other participants (M = 192.73, SE = 12.74), t (347.7) = –4.26, p < .001, 95% CIdiff [–98.69, –36.38], g = –0.46. Mixed/other participants exhibited higher area under the curves than White participants, t (340.2) = –2.02, p = .04, 95% CIdiff [0.68, 54.40], g = 0.22, African American participants, t (349.5) = –2.47, p = .01, 95% CIdiff [–71.73, –8.08], g = –0.26, Asian American participants, t (344.8) = –1.98, p = .05, 95% CIdiff [–56.21, –0.16], g = –0.21, and, as noted above, Latino participants. When employing the Bonferroni correction (p < .005), the remaining significant contrasts were between Latino and White participants, Latino and Asian American participants, and Latino and mixed/other participants. As before, the significant main effect of age indicated that older participants had lower cortisol area under the curve than younger ones (B = –9.14, SE = 2.71, β = –0.13), and that female participants (M = 167.77, SE = 4.79) had larger cortisol area under the curve than males (M = 152.43, SE = 5.59), 95% CIdiff [2.33, 28.35], g = 0.25.

Figure 4. Average area under the curve (total daily cortisol output) by specific racial/ethnic group. Significant pairwise contrasts shown. *p < .05. **p < .01. ***p < .001.
Question 2. What is the magnitude of racial/ethnic differences in low-arousal and high-arousal positive and negative affect states in adolescence?
Effects of minority status
In analyses controlling for age and sex, minority participants reported lower levels of positive affect (both low-arousal and high-arousal positive affect) compared to White participants, with no significant differences in negative affect (see Figure 5). Specifically, a comparison of White and minority participants’ levels of high-arousal positive affect indicated a significant main effect of race, F (1, 387.9) = 6.43, p = .01, ω2partial = .014, such that minority youth reported lower levels of high-arousal positive affect (M = 2.94, SE = 0.06) compared to White participants (M = 3.17, SE = 0.07), 95% CIdiff [–0.40, –0.05], g = –0.26. Similarly, there was a significant main effect of race for low-arousal positive affect, F (1, 387.2) = 6.59, p = .01, ω2partial = .014, such that minority participants reported significantly lower levels of low-arousal positive affect (M = 3.26, SE = 0.05) compared to White participants (M = 3.45, SE = 0.06), 95% CIdiff [–0.35, –0.05], g = –0.26. The main effect of race was much weaker and did not reach statistical significance for either high-arousal or low-arousal negative affect, p = .50, 95% CIdiff [–0.17, 0.09], g = –0.07, and p = .31, 95% CIdiff [–0.19, 0.06], g = 0.10, respectively.

Figure 5. Self-reported affect by race/ethnicity (binary-coded as minority vs. White). *p < .05.
In terms of sex and age differences, for high-arousal negative affect there was a significant main effect of sex, F (1, 394.9) = 5.48, p = .02, such that female participants (M = 1.72, SE = 0.04) reported higher levels of high-arousal negative affect than male participants (M = 1.57, SE = 0.05), 95% CIdiff [0.02, 0.28], g = 0.24. In addition, there was a significant main effect of both age and sex for low-arousal negative affect. The significant main effect of age, F (1, 402.2) = 4.14, p = .04, indicated that older participants experienced higher levels of low-arousal negative affect than younger ones (B = 0.05, SE = 0.02, β = 0.08). The significant main effect of sex, F (1, 396.8) = 6.37, p = .01, indicated that female participants (M = 1.84, SE = 0.04) reported higher levels of low-arousal negative affect than male participants (M = 1.68, SE = 0.05), 95% CIdiff [0.03, 0.28], g = 0.25.
Follow-up by specific racial/ethnic categories
Next, we probed racial/ethnic differences in affect using the five-category race/ethnicity variable and each of the four affect variables as outcomes: high-arousal positive affect, low-arousal positive affect, high-arousal negative affect, and low-arousal negative affect. All models controlled for age and sex. Analyses predicting high-arousal positive affect revealed a significant main effect of race, F (4, 399.8) = 5.81, p < .001, ω2partial = .045. Specifically, White youth (M = 3.16, SE = 0.07) reported higher levels of high-arousal positive affect than African American youth (M = 2.71, SE = 0.13), t (398.3) = 3.07, p = .002, 95% CIdiff [–0.73, –0.16], g = –0.31, and Asian American youth (M = 2.81 SE = 0.08), t (381.8) = 3.25, p = .001, 95% CIdiff [–0.56, –0.41], g = –0.33. Furthermore, Latino youth (M = 3.37, SE = 0.12) also reported higher levels of high-arousal positive affect than both African American, t (415.8) = –3.63, p < .001, 95% CIdiff [–1.01, –0.30], g = –0.36, and Asian American youth, t (417.8) = –3.63, p < .001, 95% CIdiff [–0.86, –0.25], g = –0.35. All of these differences remained significant after Bonferroni correction (p < .005). White and Latino youth did not differ from each other, p = .15, 95% CIdiff [–0.49, 0.07], g = –0.14 (see Figure 6).

Figure 6. Self-reported affect by specific racial/ethnic categories. *p < .05. **p < .01. ***p < .001.
Similarly, analyses of racial differences in low-arousal positive affect revealed a significant main effect of race, F (4, 398.7) = 2.99, p = .02, ω2partial = .019. Contrasts indicated that White participants (M = 3.45, SE = 0.06) reported higher levels of low-arousal positive affect than both African American (M = 3.12, SE = 0.11), t (396.5) = 2.59, p = .01, 95% CIdiff [0.08, 0.57], g = 0.26, and Asian American participants (M = 3.19, SE = 0.07), t (378.0) = 2.76, p = .006, 95% CIdiff [0.07, 0.44], g = 0.28, while Latino participants (M = 3.44, SE = 0.11) reported higher levels than African American participants, t (417.2) = –2.00, p = .05, 95% CIdiff [–0.00, 0.62], g = 0.20. However, none of these comparisons held up to the Bonferroni correction (p < .005).
There was not a significant main effect of race for high-arousal negative affect, p = .66, ω2partial < .001, but there were significant main effects of age, F (1, 390.2) = 3.95, p = .05, ω2partial = .007, and sex, F (1, 395.0) = 5.14, p = .02, ω2partial = .010. Older participants experienced more high-arousal negative affect than younger participants (B = 0.053, SE = 0.026, β = 0.09). Females (M = 1.70, SE = 0.05) experienced more high-arousal negative affect than males (M = 1.56, SE = 0.05), 95% CIdiff [0.02, 0.28], g = 0.23.
Finally, analyses of racial differences for low-arousal negative affect revealed significant main effects of race, F (4, 399.9) = 2.70, p = .03, ω2partial = .017, age, F (1, 392.1) = 7.33, p = .01, ω2partial = .016, and sex, F (1, 396.7) = 5.06, p = .02, ω2partial = .010. The main effect of race appeared to be driven by African American participants reporting higher levels of low-arousal negative affect (M = 1.94, SE = 0.09) than both Asian American (M = 1.70, SE = 0.06), t (395.8) = 2.24, p = .03, 95% CIdiff [0.03, 0.45], g = 0.22, and Latino participants (M = 1.57, SE = 0.09), t (414.7) = 2.94, p = .003, 95% CIdiff [0.12, 0.62], g = 0.29. However, only the difference between African American and Latino participants held up to the Bonferroni correction (p < .005). Older participants also experienced more low-arousal negative affect than younger participants (B = 0.067, SE = 0.024, β = 0.12), and females (M = 1.83, SE = 0.04) experienced more low-arousal negative affect than males (M = 1.70, SE = 0.05), 95% CIdiff [0.02, 0.26], g = 0.23.
Question 3. Do differences in affective patterns explain racial/ethnic differences in diurnal cortisol?
We next attempted to determine if racial/ethnic differences in affect explained differences in diurnal rhythms of cortisol. However, none of the affect variables we considered were significantly related to cortisol area under the curve or the cortisol awakening response (ps > .05) in models including race/ethnicity, age, and sex, entailing that racial/ethnic differences in affect did not mediate differences in diurnal rhythms of cortisol as measured by cortisol area under the curve or the cortisol awakening response. As for cortisol slopes, high-arousal positive affect was related to more positive (i.e., steeper) cortisol slopes, B = 0.09, t (1174.3) = 2.21, p = .03, as was low-arousal negative affect, B = 0.16, t (1103.4) = 2.28, p = .02, in models including race/ethnicity, age, and sex. However, these affect variables did not mediate racial differences in cortisol slopes (ps > .05), which remained largely identical after controlling for affect variables.
Sensitivity analyses
We conducted follow-up analyses to examine whether results were robust when controlling for time of wake-up, an important variable when considering diurnal variation in cortisol patterns, and when taking into account socioeconomic status. Results were unchanged when controlling for time of wake-up. When considering the binary race/ethnicity variable, controlling for time of wake-up did not change any of the results: in these analyses, race/ethnicity still did not significantly moderate the cortisol awakening response, F (1, 331.8) = 3.06, p = .081, ω2partial = .006, or area under the curve, F (1, 328.8) = 2.42, p = .121, ω2partial = .004, while still moderating cortisol slopes, F (1, 332.9) = 5.97, p = .015, ω2partial = .015. When the race/ethnicity variable including multiple categories was used, race/ethnicity was still not a significant moderator of the cortisol awakening response, F (4, 342.3) = 1.71, p = .146, ω2partial = .008, but consistent with prior analyses remained a significant moderator of cortisol slopes, F (4, 341.0) = 2.55, p = .039, ω2partial = .018, and area under the curve, F (4, 341.8) = 5.74, p < .001, ω2partial = .052.
We then added maternal education (a proxy for socioeconomic status) to models including race/ethnicity, age, and sex. After controlling for maternal education, the previous results regarding cortisol patterns, affect patterns, and mediation of cortisol differences by affect were unchanged, and many of the significant results identified above were strengthened (data available upon request). This is despite a significant role of maternal education in some of these models, which indicated that higher maternal education was associated with steeper diurnal cortisol slopes, F (1, 325.7) = 9.77, B = 0.072, SE = 0.023, β = 0.129, p = .002, and lower cortisol area under the curve, F (1, 333.5) = 4.63, B = –4.63, SE = 2.15, β = –0.089, p = .032. Maternal education was not significantly associated with the cortisol awakening response, F (1, 337.0) = 0.14, B = 0.11, SE = 0.31, β = 0.014, p = .71.
Discussion
The present study had three primary goals: (a) to examine racial/ethnic differences in diurnal cortisol patterns in adolescence, (b) to investigate how adolescents’ low-arousal and high-arousal positive and negative affect states differ by race/ethnicity, and (c) to assess whether differences in daily affective patterns explain racial/ethnic differences in diurnal cortisol.
Racial/ethnic differences in HPA activity
We first examined racial/ethnic differences in diurnal cortisol production. These analyses indicated that minority adolescents exhibited flatter cortisol slopes than White adolescents, with a small overall effect size for race/ethnicity (g = 0.25). Follow-up analyses indicated that these overall patterns were due to both African American and Latino youth exhibiting flatter slopes than White youth. The pairwise contrasts did not survive the Bonferroni correction, indicating that the effect sizes were small. Nevertheless, these results are consistent with prior research reporting flatter cortisol slopes in African American children and adolescents (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Martin et al., Reference Martin, Bruce and Fisher2012), adults (Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010), and older adults (McCallum et al., Reference McCallum, Sorocco and Fritsch2006) than in Whites. Replicating results from another study with adolescents, we also found flatter cortisol slopes among Latino youth compared to White youth (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007). The finding that Asian American youth had steeper slopes than African American youth and slopes that were similar to White youth is a novel finding. The cortisol slope patterns are noteworthy because flatter slopes have been linked to multiple emotional and physical health problems, including immune-related and metabolic conditions (Adam et al., Reference Adam, Quinn, Tavernier, McQuillan, Dahlke and Gilbert2017). Of note, these conditions (e.g., diabetes) are more prevalent among African American and Latino individuals compared to White and Asian American (Centers for Disease Control and Prevention, 2017). Together, these results suggest that flattened diurnal cortisol slopes may play a role in biological processes that contribute to racial/ethnic health disparities.
In contrast, the cortisol awakening response appeared to be a weaker candidate for explaining racial/ethnic health disparities, given only marginal differences in the cortisol awakening response by race/ethnicity irrespective of whether we considered it as a binary outcome (minority vs. White) or as a five-level categorical variable. The only significant pairwise difference we identified was that of a lower cortisol awakening response in Latino compared to White youth, which mirrors findings from another study with adults (Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010). However, the effect size was small and this finding did not survive the Bonferroni correction. It is possible that this pattern may signal a specific but small vulnerability of Latino groups to conditions linked in prior meta-analyses to a low cortisol awakening response, such as posttraumatic stress, fatigue, burnout, and exhaustion (Boggero et al., Reference Boggero, Hostinar, Haak, Murphy and Segerstrom2017; Chida & Steptoe, Reference Chida and Steptoe2009).
Racial/ethnic comparisons regarding the cortisol area under the curve revealed that Latino youth exhibited the lowest area under the curve, whereas youth belonging to mixed/other groups showed the highest area under the curve of all groups. Even with the Bonferroni correction, Latino youth continued to show significantly lower area under the curve compared to White, Asian American, and mixed/other youth. However, the pattern showing higher area under the curve for the mixed/other group was less robust, did not survive Bonferroni correction, and should be replicated given lack of prior literature regarding this heterogeneous group. Again, we found that Asian American youth had area under the curve levels that were similar to White youth, which is a novel finding and suggests that similar biological processes may be in play for these two groups. Prior studies have revealed a potential developmental shift, such that racial/ethnic minority children display lower area under the curve compared to White children (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011), whereas in adults, Latino and African American groups show lower area under the curve compared to Whites (Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010). It is possible that early in development chronic stress associated with racial/ethnic minority status leads to increased daily cortisol output (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011), but over time this results in downregulation of the HPA axis and lower area under the curve, cortisol awakening response, and flatter slopes, the effects noted in Latino and African American participants in our sample. These complex patterns of hypocortisolism may be explained by a meta-analysis of studies on chronic stress and HPA activity (Miller et al., Reference Miller, Chen and Zhou2007), which revealed that HPA activity increases acutely after stressor onset but reduces over time as stressors become more chronic.
The psychological mechanisms that explain the HPA racial/ethnic differences we observed are not well understood. Experiences of discrimination in particular and life stress in general may influence patterns of cortisol production, as the HPA axis responds strongly to social stressors (Dickerson & Kemeny, Reference Dickerson and Kemeny2004; Gunnar & Adam, Reference Gunnar and Adam2012). However, some recent research has indicated that cortisol rhythms are not associated with or persist after accounting for exposure to discrimination (Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; Martin et al., Reference Martin, Bruce and Fisher2012). This indicates that other factors need to be explored in future research, as these may help to explain the racial/ethnic differences observed. We considered affect differences as a possible explanation.
Racial/ethnic differences in affect
The second goal of the study was to explore racial/ethnic differences in affect. These analyses indicated that racial/ethnic minority participants endorsed lower levels of positive affect (both high and low arousal) compared to White youth, whereas the differences between the two groups on negative affect (either high- or low-arousal negative affect) were small, close to zero. The most pronounced differences were that African American and Asian American youth reported lower levels of positive affect (both high arousal and low arousal) compared to White youth. Effect sizes were larger for high-arousal positive affect, where all the contrasts remained significant even after the Bonferroni correction. These results are consistent with the greater risk of mood disorders in ethnic minorities compared to White youth (Merikangas et al., Reference Merikangas, He, Burstein, Swanson, Avenevoli, Cui and Swendsen2010), and findings that indicate lower positive affect among minority samples are also consistent with some previous research that has noted differences in emotion expression and valence between Asian American and White children (Lewis, Takai-Kawakami, Kawakami, & Sullivan, Reference Lewis, Takai-Kawakami, Kawakami and Sullivan2009) and adults (Gross, Richards, & John, Reference Gross, Richards, John, Snyder, Simpson and Huges2006). Research on cultural differences in ideal affect, or affect that people would like to feel, has also indicated that Asian Americans are less likely to endorse high arousal positive states and more likely to endorse low arousal positive states than European Americans (for review, see Tsai, Reference Tsai2007).
Positive affect and greater neural responsivity to rewards protect against the development of adolescent depression (Forbes & Dahl, Reference Forbes and Dahl2005, Reference Forbes and Dahl2012). Furthermore, positive affect is associated with better health, lower morbidity, and greater longevity (Pressman & Cohen, Reference Pressman and Cohen2005). Thus, our findings suggest that African American and Asian American youth may be at increased risk of developing mental and physical health problems across their life span.
The group differences in measures of negative valence affect were small, suggesting either that the true difference in the population is zero or that the effect is too small to have sufficient power to detect it with a sample of 370. Previous research with adults has noted that affect may not be felt and expressed in the same manner across races (Jang, Kwag, & Chiriboga, Reference Jang, Kwag and Chiriboga2012), or that there may be differences in the ideal affect to be expressed among cultures (Tsai, Reference Tsai2007), and different associations with risk of psychopathology across races (Moazen-Zadeh & Assari, Reference Moazen-Zadeh and Assari2016). Thus, it could be that racial/ethnic minority youth were underreporting their negative affect, as some studies have found that African Americans tend to underreport negative affect (Bardwell & Dimsdale, Reference Bardwell and Dimsdale2001). However, taking the findings at face value could also suggest that this was a high-functioning, low-risk minority sample that experienced low levels of negative affect. While research is often biased toward finding deficits or signs of deprivation among minorities (for review, see García Coll et al., Reference García Coll, Lamberty, Jenkins, McAdoo, Crnic, Wasik and Garcia1996), we must remember that there is considerable diversity within and between minorities and that many minority youth are in good mental health despite facing race-based stressors.
Differences in affect did not explain cortisol differences
The third goal of the study was to assess whether affective patterns explain racial/ethnic differences in diurnal cortisol. We did not find any evidence of statistical mediation that would indicate this. This is consistent with another study of adolescents, which found that higher levels of negative affect were associated with flatter slopes, but negative affect did not explain racial/ethnic differences in cortisol slopes (DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007). There are several possible explanations for these findings. We may need to consider refining our models of relations between affect and HPA activity for a number of reasons. For instance, transactions between affect and HPA physiology occur on multiple time scales, from moments to days and years (Adam, Reference Adam2012), thus incorporating information on either affect or HPA physiology from other time points may allow us to better parse out momentary correlations between affect and physiology. Another possibility is that affect–physiology associations are moderated by various protective factors (e.g., coping strategies) that may differ by race/ethnicity. This would explain why some ethnic minorities differ from the majority group in levels of affect (e.g., Asian American youth compared to Whites), but this did not translate into physiological differences in HPA functioning, perhaps due to successful coping efforts. Furthermore, research indicates that the HPA system serves multiple biological functions (e.g., metabolic and immune) beyond its involvement in emotional processes (Gunnar & Adam, Reference Gunnar and Adam2012). Thus, it may be that affect–cortisol associations are difficult to capture without a large panel of covariates relevant to diet, physical activity, immune function, and so on. Furthermore, the HPA axis is only one of the body's stress-mediating systems and likely cannot fully explain racial/ethnic health disparities on its own. Considering multisystem indicators of allostatic load may capture stronger differences by race/ethnicity (Doan & Evans, Reference Doan, Evans, Causadias, Telzer and Gonzalez2017).
A second possibility is that racial/ethnic differences in physiology and affect may be small, making the detection of relations between them challenging. Deficit models of minority development often assume that racial/ethnic minorities should consistently show worse outcomes on any given measure (Causadias, Vitriol, & Atkin, Reference Causadias, Vitriol and Atkin2018), but our findings indicate that differences between minority and White youth are not always found, and even when differences exist, effect sizes tend to be small. As such, the current study adds to the important evidence base documenting both similarities and differences between minority and White youth. These findings suggest that, even in a society where there is structural inequality, we find evidence of equifinality (i.e., different developmental pathways can lead to similar outcomes on cortisol and affect) and multifinality (i.e., those with similar developmental pathways can have differing outcomes; Cicchetti & Rogosch, Reference Cicchetti and Rogosch1996). The heterogeneity within and between racial/ethnic groups should be further explored to understand the developmental processes underlying equifinality and multifinality.
Finally, another possible explanation is that the racial/ethnic groups we considered here may be heterogeneous and contain multiple meaningful subgroups once we consider other cultural aspects (e.g., immigration status, acculturation stress, family norms and values, and identity processes). Thus, a single aspect of racial/ethnic identity may not fully explain biological differences observed (for a more in-depth discussion of the complex nature of culture–biology interplay, see Causadias, Telzer, & Gonzalez, Reference Causadias, Telzer, Gonzales, Causadias, Telzer and Gonzales2018). For example, an individual's identification with his/her culture could act in concert with experiences they have in everyday life to influence their biology and affect (Causadias, Telzer, & Gonzalez, Reference Causadias, Telzer, Gonzales, Causadias, Telzer and Gonzales2018; Zeiders, Causadias, & White, Reference Zeiders, Causadias and White2017). We propose that, until we account for multiple aspects of cultural identity, the observed racial/ethnic differences in affect and physiology may remain difficult to explain.
The roles of age, sex, and maternal education
Our results indicate some noteworthy effects for age and sex in predicting both diurnal cortisol and daily affect. Age was a significant predictor of diurnal cortisol slopes and cortisol area under the curve, such that older participants exhibited flatter slopes and lower area under the curve than younger participants. These findings are somewhat consistent with previous research in adolescents, which has indicated that older adolescents exhibit flatter cortisol slopes (due to lower morning and higher evening cortisol output) and higher area under the curve cortisol than younger adolescents (Gunnar, Wewerka, Frenn, Long, & Griggs, Reference Gunnar, Wewerka, Frenn, Long and Griggs2009; Shirtcliff et al., Reference Shirtcliff, Allison, Armstrong, Slattery, Kalin and Essex2012). In previous work, however, the age of the participants ranged from age 9 to 15, while the participants in the current study ranged from age 11.9 to 18. Previous research has proposed that there is a possible “U-shaped” curve in the cortisol output of older children and adolescents, such that levels decrease in the preteen years, increase in early adolescence, and then decrease across adolescence (Shirtcliff et al., Reference Shirtcliff, Allison, Armstrong, Slattery, Kalin and Essex2012). Although this study did not examine children in the preteen stage, participants in the current study ranged from early through later adolescence so the results may have captured adolescents at the peak of the curve and on the way down. One hypothesis is that this curve signifies a time of increased environmental and neurobiological sensitivity early in adolescence (Shirtcliff et al., Reference Shirtcliff, Allison, Armstrong, Slattery, Kalin and Essex2012). Future research should examine the full extent of this developmental pattern from late childhood through early adulthood.
Sex was a significant predictor of cortisol area under the curve, such that girls had higher area under the curve values than boys. This is consistent with previous evidence indicating that girls tend to exhibit higher afternoon and evening cortisol levels than boys (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, Reference Klimes-Dougan, Hastings, Granger, Usher and Zahn-Waxler2001), although more research is needed to corroborate these patterns. When examining affect, there were no significant differences by age or sex for the positive affect indices. However, there were significant differences for both of the negative affect indices, such that older participants and girls reported higher levels of negative affect (both low arousal and high arousal). This result is consistent with previous research, which has indicated that there may be increases in negative affect as adolescents get older (Larson, Moneta, Richards, & Wilson, Reference Larson, Moneta, Richards and Wilson2002) and that girls tend to exhibit higher levels of negative affect than boys (Silk, Steinberg, & Morris, Reference Silk, Steinberg and Morris2003). Furthermore, girls have a higher incidence of depression during adolescence (Avenevoli, Swendsen, He, Burstein, & Merikangas, Reference Avenevoli, Swendsen, He, Burstein and Merikangas2015). These findings point to a need to consider the cumulative roles of race/ethnicity, age, and sex in predicting and mitigating future psychopathology and health problems.
When examining the role of socioeconomic status, as indexed by maternal education, we found that racial/ethnic differences persisted after accounting for maternal education statistically, consistent with prior studies with children, adolescents, and adults (Bush et al., Reference Bush, Obradovic, Adler and Boyce2011; Cohen et al., Reference Cohen, Schwartz, Epel, Kirschbaum, Sidney and Seeman2006; DeSantis et al., Reference DeSantis, Adam, Doane, Mineka, Zinbarg and Craske2007; Hajat et al., Reference Hajat, Diez-Roux, Franklin, Seeman, Shrager, Ranjit and Kirschbaum2010; Martin et al., Reference Martin, Bruce and Fisher2012). This suggests that maternal education does not explain these racial/ethnic differences, even though differences by maternal education mirrored the patterns observed in ethnic minority youth, for example, flatter cortisol slopes were noted for both minority youth and youth whose mothers had lower educational attainment. These patterns may be explained by similar processes, for example, a higher stress burden in both ethnic minority youth and low-socioeconomic status youth, which encompasses different types of stressors for each group, though this possibility will need to be confirmed empirically in future research.
Conclusions
This study has some noteworthy strengths. This is a large and diverse sample for adolescent research, which allows more fine-grained comparisons between the various racial/ethnic groups rather than a simple contrast between minorities versus Whites, which is common in prior literature. In addition, the frequent and rigorous sampling of cortisol (4 samples per day on 4 separate days) provides greater reliability of measurement than sampling participants on 1 or 2 days. However, this study is not without its limitations. As this was a cross-sectional design, we do not know how trait or long-term patterns of affect might relate to cortisol. Similarly, the measures of diurnal cortisol included here only capture momentary output, while a more chronic measure of output, such as hair cortisol (Meyer & Novak, Reference Meyer and Novak2012), may uncover a different set of associations. Moreover, analyses in this study modeled cortisol diurnal slopes by the difference between morning and evening levels. Therefore, we may have lost variability among individuals using this approach. Relatedly, a higher frequency sampling schedule would have added precision to our area under the curve estimates (Hoyt, Ehrlich, Cham, & Adam, Reference Hoyt, Ehrlich, Cham and Adam2016). The number of cortisol samples was chosen to minimize participant burden, and a recent study suggests that while adding more samples might have improved the accuracy of area under the curve estimates, it likely would not have increased accuracy of the cortisol awakening response and diurnal slope estimates though it would have imposed a much higher burden on participants (Hoyt, Ehrlich, et al., Reference Hoyt, Ehrlich, Cham and Adam2016). Finally, another limitation is that the African American and mixed/other groups had fewer than 50 participants in each group, potentially limiting our ability to detect significant differences between groups if these differences exist. Future studies should replicate our analyses with larger samples.
In sum, this study replicates a number of prior findings regarding racial/ethnic differences in cortisol and affect, but also raises novel questions regarding these patterns given that affective differences did not explain differences in hormonal output. Race/ethnicity is frequently treated as a simple demographic variable that is associated with specific experiences such as discrimination, but prior studies have shown that this factor does not fully account for the observed racial/ethnic differences in cortisol patterns. We hypothesize that conceptualizing race/ethnicity in a broader cultural framework that includes numerous cultural aspects such as norms, attitudes, media exposure, family and social networks, connections with a home country, and so on, may shed more light on racial/ethnic differences in affect and biology than our study and previous empirical investigations. In particular, understanding the complex influences on the socioemotional development of minority youth will require a comprehensive measurement of developmental competencies and challenges at multiple levels of analysis (García Coll et al., Reference García Coll, Lamberty, Jenkins, McAdoo, Crnic, Wasik and Garcia1996). Furthermore, more research is needed that will assess cultural aspects of development, affect, biology, and health outcomes within the same participants, in order to test key assumptions about the pathways to racial/ethnic health disparities. When we can better understand these pathways, we will be better equipped to design culturally sensitive interventions that can effectively combat existing health disparities.











