Significant Outcomes
∙ Oral treatment with bupropion, desipramine or sodium butyrate failed to reduce immobility time of Swiss mice in the Porsolt test or repeated forced swim test.
∙ Oral or intraperitoneal treatment with fluoxetine failed to reduce immobility of Swiss mice in the Porsolt test.
∙ Oral treatment with fluoxetine failed to reduce immobility of Swiss mice housed in normal or reversed light/dark cycles in the Porsolt test.
Limitations
∙ The contribution of the administration route to the lack of the treatment effect was investigated only for fluoxetine.
∙ The contribution of the light/dark cycle to the lack of the treatment effect was investigated only for fluoxetine.
∙ Confirmatory experiments for the lack of effect of the desipramine was not performed.
∙ The study does not clarify the origin of the resistance to the treatment with antidepressants.
Introduction
The process to develop new antidepressants requires improvement of translational research (Reference Belzung1,Reference Millan, Goodwin, Meyer-Lindenberg and Ove Ogren2). In preclinical studies, animal models are standard approaches for the screening of putative antidepressants (Reference Berton, Hahn and Thase3). The accumulated knowledge on the current animal models should be taken advantage of and may help provide a strategy to find more predictive screening approaches (Reference Millan, Goodwin, Meyer-Lindenberg and Ove Ogren2,Reference Bourin4). The FST or Porsolt test (Reference Porsolt, Bertin and Jalfre5) is considered inexpensive, fast, sensitive, relatively selective for antidepressant drugs, and reproducible across laboratories (Reference Bourin, Chenu, Ripoll and David6–Reference Costa, Vieira and Bohner9). The original protocol of FST in mice (Reference Porsolt, Bertin and Jalfre5) consisted of one session of 6 min of forced swimming at 30 or 60 min after the pharmacological treatment. During the test, mice spent the first 2 min struggling and then became more immobile until the 6th minute (Reference Porsolt, Bertin and Jalfre5). Acute treatment with antidepressants before the test reduced the immobility time and increased its latency (Reference Porsolt, Bertin and Jalfre5,Reference Lucki, Dalvi and Mayorga7,Reference Castagne, Moser and Porsolt10). According to Castagne et al. (Reference Castagne, Moser and Porsolt10), the analysis of latency to immobility improved the predictive validity of the Porsolt test in mice. Additionally, Costa et al. (Reference Costa, Vieira and Bohner9) registered latency, time and frequency of the behaviours of Swiss mice to discriminate the anti-immobility effects of psychostimulants (caffeine or apomorphine) from those of monoaminergic antidepressants.
Due to the consistent response of outbred Swiss or Swiss-derived mice to the current antidepressants in the Porsolt test, the effects of putative antidepressants on these mice stocks were indicated as the first step in the screening process (Reference Bourin, Chenu, Ripoll and David6). The present work aimed to adapt the protocol of the repeated FST created for male rats (Reference Mezadri, Batista, Portes, Marino-Neto and Lino-de-Oliveira11) to male Swiss mice. For rats, repeated FST is a variation of the Porsolt test, allowing for the detection of acute and chronic antidepressant treatments in a single group of rats reducing the number of animals used for the screening of antidepressants (Reference Mezadri, Batista, Portes, Marino-Neto and Lino-de-Oliveira11,Reference Possamai, dos Santos, Walber, Marcon, dos Santos and Lino de Oliveira12). Therefore, the strategy selected to create a repeated FST in Swiss mice was similar to that employed for rats (Reference Mezadri, Batista, Portes, Marino-Neto and Lino-de-Oliveira11,Reference Possamai, dos Santos, Walber, Marcon, dos Santos and Lino de Oliveira12): three repetitions of the Porsolt test separated 7 days apart.
Aims of the study
The main hypothesis is that, as in male rats, repetition of the FST at regular intervals will increase immobility time of mice and enhance the sensitivity of the test. In addition, the expectation is that doses of antidepressants ineffective in the standard Porsolt test (<5 mg/kg) will reduce immobility time after repeated treatment. Conversely, doses of antidepressants higher than 10 mg/kg will reduce immobility time of mice in all sessions of the FST. [For effective and ineffective doses see (Reference Porsolt, Bertin and Jalfre5–Reference Castagne, Moser and Porsolt10).]
Materials and methods
Animals, animal welfare and ethical statements
Male Swiss mice for experiments 1–5 and 7 (n=198) arrived in the laboratory on their 21st postnatal day (PND) from the central vivarium of Federal University of Santa Catarina. Male Swiss mice for experiment 6 (n=30) arrived in the laboratory on their 80th PND from the central vivarium of Federal University of Santa Catarina. All mice were housed in groups of eight per cage in white boxes (measuring 50×30×10 cm) with sawdust bedding and a grid lid. Food (Nuvital®) and water were available ad libitum. Animal house was maintained on an inverted light/dark cycle (lights on at 07:00 p.m.) except for experiment 7 (lights on at 07:00 a.m.). The room temperature was of 21±2°C. The younger and older mice remained in these housing conditions for 70 and 10 days before the experiments, respectively, to evaluate interferences of the time lodged in the laboratory animal house in the results. Although no signs of distress or disease were noticed during the experimental period, two mice randomly allocated to the fluoxetine 1 mg/kg group and one assigned to the SB 1 mg/kg group died of unknown causes in the days before antidepressant treatment. To minimise possible sources of stress and animal suffering, the following measures were applied: (1) all procedures were performed during the dark phase of the light cycle (except for one control and one experimental groups in experiment 7); (2) cleaning of cages was performed three times a week by the same experimenter; and (3) mice were allowed to dry out before returning to the home cage after the forced swim sessions by remaining individually in a clean cage, in a heated room with dim light. All procedures complied with International Guidelines and Brazilian laws for animal welfare and were approved by the Ethical Committee of our University (CEUA/UFSC-PP00764).
Drugs and treatments
The following monoaminergic antidepressants were employed in all pharmacological experiments in mice: fluoxetine (FLX, a selective serotonin reuptake inhibitor, F 3518; Sigma, St. Louis, MO, USA), desipramine (DMI, a selective noradrenaline reuptake inhibitor, SC200158A; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and bupropion (BUP, a norepinephrine-dopamine reuptake inhibitor, Eurofarma, São Paulo, Brazil). In addition, all experiments had a group treated with a potential new antidepressant, SB (a histone deacetylase inhibitor, B5887; Sigma). All drugs were administered at the following doses (mg/kg/day): 1 (experiment 2), 3 (experiment 3), 10 (experiment 4), and 30 (experiment 5). The effective doses (10 and 30 mg/kg) and ineffective doses (1 and 3 mg/kg) were selected according to previous studies (Reference Lucki, Dalvi and Mayorga7,Reference David, Renard, Jolliet, Hascoet and Bourin13–Reference Oh, Zupan, Gross and Toth21). In experiments 2–5 and 7, drugs and vehicle (VEH, sucrose 10%) were administered orally (gavage). In experiment 6, mice received acute FLX 20 mg/kg orally (gavage) or parenterally (intraperitoneal, IP) at 1 h before the Porsolt test (no repetitions were performed in these experiments). The FLX 20 mg/kg dose applied 1 h before the Porsolt test was considered the most effective treatment schedule for male Swiss mice after a systematic review of the literature (see Section S1, Supplementary Material). Ten per cent sucrose was the VEH for FLX administered through gavage, and 0.9% saline was the VEH for FLX IP. Although the gavage methods did not require palatable solutions, 10% sucrose was used as a VEH in order to make the present data comparable with those obtained in male Wistar rats (a positive control of the experimental conditions in the laboratory, see Section S2, Supplementary Material). In the experiments 2–5, the treatment with drugs occurred daily, from day 0 until day 14 (see experimental design), in the same time. In the days of behavioural testing treatments occurred 1 h before. In the experiments 6–7 the drug treatment occurred 1 h prior Porsolt test.
Porsolt test and repeated FST
Behavioural testing occurred 1 h after pharmacological treatment. The experimental room had a temperature of ~21±2°C, an illumination of 164 lx (measured with the Android app ‘Luximetro Altezza’) and a noise of ~45 dB (measured with the Android app ‘Decibelímetro’). Mice were placed in a plastic cylinder (24×14 cm) filled with water at 24°C in a way that mice were unable to touch the bottom with neither their paws nor tail. In the first session of the forced swimming (Porsolt test), mice were individually placed to swim for 6 min in the cylinder. The test session was then repeated on the 7th (retest 1) and 14th (retest 2) days after the first test. After every swimming session, mice were dried in a clean cage that was placed in a dark, heated room. All sessions were carried out in the afternoon (between 01:00 p.m. and 06:00 p.m.) and were video recorded by a camera positioned on the top of the cylinder. Videos were named with codes for subsequent blind scoring of the following behavioural categories: (1) immobility was scored when the animal was floating, with just enough paw movements to keep the head above water; (2) swimming was scored when the animal was moving the front paws horizontally, with or without body displacement; (3) climbing was scored when the animal was moving the front paws vertically, either near the wall or in the centre, and with or without body displacement. The three parameters that were extracted for each of the aforementioned categories during every behavioural session were as follows: (1) latency, which consists of the time elapsed between the beginning of the behavioural session and the first bout for a given category; (2) time or duration of all bouts for a given category; (3) frequency, which consists of the summary of all bouts scored for a given category. Behavioural scoring was performed with the aid of the software Ethowatcher (Reference Junior, Pederiva, Bose, Garcia, Lino-de-Oliveira and Marino-Neto22,Reference Crispim Junior, Pederiva, Bose, Garcia, Lino-de-Oliveira and Marino-Neto23), which can be freely downloaded (http://www.ethowatcher.ufsc.br/).
Experimental design and general procedures
Experiments 1–5 were carried out on the following schedule: (day −70) arrival of the mice (post-weaning, PND 21) in the laboratory vivarium until the age established for the experiment (PND 90); (day 0) test; (day 7) retest 1 and (day 14) retest 2 (Figs 1a and b). In experiment 1, an untreated group of mice (CT, n=9) was evaluated to observe the natural behaviour in the repeated FST. In experiments 2–5, each experimental group containing eight mice received a different type of treatments (VEH, BUP, DMI, FLX, and SB) by gavage in the following doses: 1 (experiment 2), 3 (experiment 3), 10 (experiment 4), or 30 (experiment 5) mg/kg/day from day 0 until day 14. Experiment 6 was carried out on the following schedule: (day −10) arrival of the mice (PND 80) in the laboratory vivarium until the age established for the experiment (PND 90); (day 0) drug treatment and test (Fig. 1c). Experiment 7 was carried out on the following schedule: (day −70) arrival of the mice (post-weaning, PND 21) in the laboratory vivarium with inverted (lights on at 07:00 p.m.) or standard (lights on at 07:00 a.m.) light/dark cycle until the age established for the experiment (PND 90); (day 0) drug treatment and test (Fig. 1d). Each experiment (1–7) was divided into 4 different days containing two mice from each treatment group per experimental day. Except for in experiments 5, 6, and 7, DMI was tested in all experiments. All procedures were carried out between 01:00 p.m. and 06:00 p.m. The experimenters (P.R.S experiments 1–6 and N.Z experiment 7) and behavioural scorers (L.C.T. experiments 1–6 and N.Z. experiment 7) were blind to treatments and outcome assessments, respectively. Only K.D., the person who prepared the drugs, knew the identity of the substances contained in the bottles used to treat the animals.

Fig. 1 Experimental design. (a) Experiment 1; (b) experiments 2–5; (c) experiment 7; (d) experiment 6; PND, postnatal day.
Actions to avoid bias
Experiments 1–7 were carried out once. However, doses supposedly effective of fluoxetine were tested in four independent experiments (experiments 4–7). In addition, confirmatory studies were performed to bupropion (Section S5, Supplementary Material) and SB (Sections S5 and S6, Supplementary Material). The number of animals per group was justified by previous work on male Swiss mice (Reference Costa, Vieira and Bohner9) without any sample size calculation or power analysis. The randomisation to allocate the pharmacological treatments in experiments 2–5 and 7 was carried out as follows: the experimenter arbitrarily housed the animals in cages, which were randomly allocated by lottery to pharmacological treatment. In experiment 6, mice were randomly assigned to their home cages by lottery before the random allocation of the cage to a pharmacological treatment and route of administration. The allocation was concealed only for experiments 6 and 7. In experiments 6 and 7, experimenters were blind to treatment, while blind outcome assessment was conducted in all experiments. Only three mice were excluded from the experiment for health reasons before the onset of the experiments (see Animals section). No exclusion criteria were established beforehand, and no data were excluded from the analysis.
Statistical analysis
All data are represented as the mean±SEM. For analysis, categories scored in behavioural sessions are summarised in 6 min, or the first 2 min and the last 4 min (Reference Costa, Vieira and Bohner9). Normality and homoscedasticity were tested using the Kolmogorov–Smirnov test and Levene’s test, respectively. The results from experiments 1, 6, and 7 were analysed using a within-subjects analysis of variance (ANOVA). The results from experiment 2–5 were analysed using a 2-way ANOVA with repeated measures (factors: treatment and repetition). Post-hoc analyses were carried out using two-tailed Student’s t-tests (unpaired to treatment; paired to repetition) when an ANOVA delivered a significant result or when the difference between the VEH and treatment groups was higher than 20% (arbitrarily determined). Statistical significance was considered at p<0.05. The median of immobility time of the overall sample in the test, retest 1 or retest 2 was used as a cut-off value to classify mice into high immobility (HI) and low immobility (LI) categories in every behavioural session (adapted from (Reference Enriquez-Castillo, Alamilla and Barral25)). The number of animals analysed in each experimental group are in the legends of the figures and tables.
Results
Description and quantification of behaviours of the naïve Swiss mice submitted to the repeated FST:
In experiment 1, all naïve male Swiss mice performed swimming and displayed immobility abundantly, while climbing was seldom observed in the test (Table 1). Over retesting (Table 1) and the latencies to immobility (F(2,16)=8.24, p=0.0035) and swimming were decreased (F(2,16)=3.3, p=0.065). There was a four-fold increase in the frequencies of immobility (F(2,16)=18.6 p=0.00007) and swimming (F(2,16)=17.8, p=0.00009) over repetition. The immobility time increased significantly over the repetitions (F(2,16)=7.9, p=0.00412), while swimming time decreased (F(2,16)=8, p=0.00389).
Table 1 Latency, frequency, and duration of the male mice behaviour in the FST-r

Data expressed as mean±SEM of nine mice. Analysis of variance of repeated measurements with Duncan’s post hoc: *Significantly different from the test session. #Significantly different from retest 1. &Significantly different from retest 2.
Climbing was a rare event during the test, retest 1 and retest 2, as observed in a minute-by-minute analysis (Fig. 2). The minute-by-minute analysis revealed a predominance of swimming behaviour (~40 s) in the 1st minute of the test (Fig. 2). Immobility replaced swimming over the duration of the test, reaching a maximum of 50 s in the 5th minute (Fig. 2). In retest 1, the minute-by-minute analysis revealed low scores of swimming and a predominance of immobility in the first minute, reaching a maximum time in the 4th minute of retest 1 (Fig. 2). The behavioural pattern in retest 2 was similar to that in retest 1, that is, a predominance of immobility as the first minute of retest 2, reaching maximum at the 3rd minute (Fig. 2). The intersection point between the curves of the abundance of immobility and swimming in the test coincided with 2.5 min in the test, 1 min in retest 1 and with a negative time in retest 2 (Fig. 2). Because climbing was absent during the repeated FST in male Swiss mice, swimming mirrored immobility scores. Therefore, immobility was the main category scored in the following experiments.

Fig. 2 Behaviour of adult, male Swiss mice (n=9) recorded minute-by-minute in the Porsolt test (left), retest 1 (middle), and retest 2 (right). Graphs show duration (seconds in each minute) for immobility (circles), swimming (squares), and climbing (triangles) during 6 min of the test (upper graph), retest 1 (middle graph), and retest 2 (lower graph). Data expressed as mean±SEM.
Effects of oral treatment with antidepressants on the immobility time of Swiss mice in the repeated FST
In experiments 2–5 (Figs 1b, 3, and 4), there were no significant differences in immobility time between the VEH and antidepressant-treated groups in the test (experiment 2: F(4,30)=2.3, p=0.073; experiment 3: F(4,35)=0.46, p=0.76; experiment 4: F(4,32)=1.7, p=0.17; experiment 5: F(3,28)=2.9, p=0.48), in retest 1 (experiment 2: F(4,30)=1.9, p=0.13; experiment 3: F(4,35)=0.47, p=0.75; experiment 4: F(4,32)=1.4, p=0.23; experiment 5: F(3,28)=0.30, p=0.81) or in retest 2 (experiment 2: F(4,30)=1.2, p=0.29; experiment 3: F(4,35)=1.1, p=0.36, p=0.75; experiment 4: F(4,32)=1.5, p=0.20; experiment 5: F(4,36)=1.4, p=0.24). The absence of significant results was also observed when immobility time was scored during the initial 2 min or the total 6 min of the test, retest 1 and retest 2 (data not shown). In experiments 2, 3, and 5, the difference between the mean immobility time of the VEH and antidepressant-treated groups was smaller than 20% in all sessions of the repeated FST (Figs 3 and 4). Counts of swimming and climbing may be found in the Section S3 (Supplementary Material).

Fig. 3 Immobility time of adult, male Swiss mice, treated with antidepressants in the doses of (a) 1 mg/kg or (b) 3 mg/kg, in the last 4 min of the Porsolt test (left), retest 1 (middle), and retest 2 (right). Bars represent mean±SEM of 8 mice per group (except for FLX 1 mg/kg (n=06) and SB 1 mg/kg (n=07)). BUP, bupropion; DMI, desipramine; FLX, fluoxetine; SB, sodium butyrate; VEH, vehicle.

Fig. 4 Immobility time of adult, male Swiss mice, treated with antidepressants in the doses of (a) 10 mg/kg or (b) 30 mg/kg, in the last 4 min of the Porsolt test (left), retest 1 (middle), and retest 2 (right). Bars represent mean±SEM of 8 mice per group. BUP, bupropion; DMI, desipramine; FLX, fluoxetine; SB, sodium butyrate; VEH, vehicle.
Despite the lack of significance using ANOVAs, the difference between the mean immobility time of the VEH and antidepressant-treated groups was higher than 20% in the test and retest 1 of experiment 4 (Fig. 4). Therefore, unpaired two-tailed t-tests were used for comparison of the immobility time between the VEH group and each antidepressant-treated group. According to (Reference Bogdanova, Kanekar, D’Anci and Renshaw24), the t-test may be used to compare control and treated groups in the FST to avoid type II error. Differences between the VEH and BUP groups in the test and between the VEH and SB groups in retest 1 were statistically significant (t-test, p=0.02 and p=0.04, respectively). These significant differences were not seen in confirmatory studies (Sections S4 and S5, Supplementary Material).
Effects of oral and parenteral treatment with fluoxetine 20 mg/kg on the immobility time of Swiss mice in the Porsolt test
Oral (gavage) or parenteral (IP) treatment with FLX 20 mg/kg failed to reduce the immobility time of male Swiss mice in the FST compared with the VEH, that is, 10% sucrose or 0.9% saline, respectively (Fig. 5).
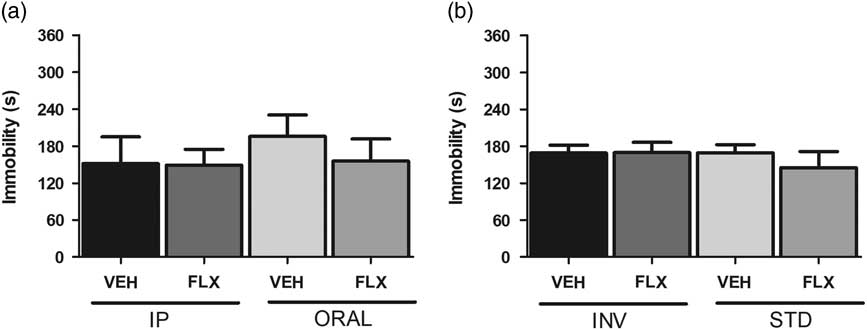
Fig. 5 (a) Immobility time of adult, male Swiss mice in the last 4 min of Porsolt test performed 1 h after the treatment through two different routes of administration (oral, gavage, VEH, n=8, FLX, n=7); Intraperitoneal (VEH, n=7; FLX, n=8). (b) Immobility time of male Swiss mice (PND 90), housed in STD or INV light/dark cycle, in the last 4 minutes of the Porsolt test performed 1 h after the oral treatment with VEH (n=8) or FLX (20 mg/kg/p.o., n=08). FLX, fluoxetine; FLX INV, fluoxetine inverted light/dark cycle; FLX STD, fluoxetine standard cycle; VEH, vehicle; VEH INV, vehicle inverted light/dark cycle; VEH STD, vehicle standard cycle. Bars represent mean±SEM.
Effects of the antidepressants in light/dark cycle
Oral (gavage) treatment with FLX 20 mg/kg failed to reduce the immobility time of male Swiss mice in the FST compared with the VEH, independent of the type of light/dark cycle in the animal house (Cycle, F(1,28)=0.37, p=0.54), (Treatment, F(1,28)=0.11, p=0.74) (Cycle/treatment F(1,28)=1.25, p=0.27) (Fig. 5c).
Effects of antidepressant treatment on the immobility time of LI and HI Swiss mice in the repeated FST
After statistical analysis, we divided all mice into two distinct groups based on the values of immobility time, independent of the treatment received (Section S6, Supplementary Material). Therefore, it is important to acknowledge that drug treatment may influence the levels of immobility time of the mice in the present analysis. This post-hoc decision was based on the work by (Reference Enriquez-Castillo, Alamilla and Barral25) and (Reference Flores-Serrano, Vila-Luna, Alvarez-Cervera, Heredia-Lopez, Gongora-Alfaro and Pineda26), which observed subgroups of rats with HI and LI in their experiments. The presence of HI and LI mice in our samples could explain the variability of the data leading to the lack of significant effects of the treaments. In the test (see statistical data in Supplementary Material Table S3), the median value of immobility time for all mice, independent of treatment, was 258.93 s. From 86 mice classified as HI in the test, that is, with an immobility time above the median, 21 were controls, 40 were from sub-effective dose groups and 25 were from effective dose groups (Fig. 6a). From 93 mice classified as LI in the test, that is, with an immobility time below the median, 26 were controls, 21 were from sub-effective dose groups and 46 were from effective dose groups (Fig. 6a).

Fig. 6 Percentage of adult, male Swiss mice, treated with vehicle or different doses of antidepressants (1–30 mg/kg) segregated in the subgroups low (LI) or high (HI) immobility time in the Porsolt test (a), retest 1 (b), and retest 2 (c).
In retest 1 (see statistical data in Supplementary Material Table S4), the median value of immobility time for all mice was 305.78 s. From 75 mice classified as HI, that is, with immobility times above the median, 19 were from control groups, 39 were from sub-effective dose groups and 19 were from effective dose groups (Fig. 6b). In retest 2 (see statistical data in Supplementary Material Table S5), the median value of the immobility time for all mice was 321.38 s. From 75 mice classified as HI, that is, with immobility times above the median, 16 were from control groups, 45 were from sub-effective dose groups and 14 were from effective dose groups (Fig. 6c).
Discussion
The main finding of the present study is that acute or chronic oral treatment with antidepressants failed to reduce the immobility time of the male Swiss mice in doses considered effective in the Porsolt test. The expected outcomes of BUP, DMI, and FLX treatments were absent even after post-hoc analysis using more robust statistics or in confirmatory studies. Furthermore, the expected effects of FLX in Porsolt test were absent even after the intraperitoneal injection or the housing of the male Swiss mice in standard or inverted light/dark cycle. Present data were surprising because previous studies have shown that oral treatment with antidepressants reduced the immobility time of Swiss mice in the Porsolt test in a variety of experimental conditions (Reference Jesse, Wilhelm and Nogueira19,Reference Lenzi, Rodrigues and Ros Ade20,Reference Nascimento, Macedo-Junior and Borges27–Reference Ren, Luo, Li, Zuo and Wu31). A paper reporting conditions similar to those here (Swiss mice, oral treatment, behavioural testing in the afternoon, blinding, and randomisation) found an effect size above 60% for FLX treatment on immobility time (Reference Pawar, Agrawal, Phadnis, Paliwal, Vyas and Solanki29). The possibility that drugs were not working properly seems unlikely once that they were obtained from different commercial sources. In the case of FLX, for example, the same solution of FLX used in the Swiss mice reduced the immobility time of the Wistar rats in the FST that occurred simultaneously in the laboratory (unpublished data) and the same salt was employed in an experiment performed afterwards (Supplementary Material). Therefore, it seems that the type of antidepressant, the dose and route of administration, and the housing and experimental conditions could not entirely explain the failure of the treatment in the Porsolt test.
The inability to replicate the effects of acute treatment with antidepressants in the Porsolt test seems unrelated with experimental conditions because Swiss control mice behaved according to our own previous observations and those from other research groups (Reference Porsolt, Bertin and Jalfre5,Reference Costa, Vieira and Bohner9,Reference Castagne, Moser and Porsolt10). As observed by Costa et al. (Reference Costa, Vieira and Bohner9), Swiss control mice had progressively lower scores of climbing and swimming from the beginning to the end of the test. In addition, activity abundant during the first 2 min of the test disappeared until the end of the session (Reference Costa, Vieira and Bohner9). In the present study, the behavioural patterns of untreated mice in Porsolt test remained present in Swiss mice treated with sucrose 10% or saline 0.9% indicating that the VEH had no effect per se. Scores of climbing remained low over repetition of the test, on the seventh and fourteenth days later, while scores of swimming and immobility changed. In agreement with the initial hypothesis, the immobility time of Swiss mice increased over retesting, as observed in male Wistar rats (Reference Mezadri, Batista, Portes, Marino-Neto and Lino-de-Oliveira11,Reference Possamai, dos Santos, Walber, Marcon, dos Santos and Lino de Oliveira12). Data from the control groups were quite consistent, across the experiments in mice housed in the laboratory conditions for 70 days, indicating that fluctuations in the baseline may not explain the failure to detect any effects of the pharmacological treatments.
Unexpectedly, the present samples contained subgroups of mice with high and low levels of immobility, classified according previous reports in rats and mice (Reference Enriquez-Castillo, Alamilla and Barral25,Reference Flores-Serrano, Vila-Luna, Alvarez-Cervera, Heredia-Lopez, Gongora-Alfaro and Pineda26,Reference El Yacoubi, Bouali and Popa32,Reference Jayatissa, Bisgaard, Tingstrom, Papp and Wiborg33). In the control groups, close to 50% of mice were classified in either the HI or LI categories. Curiously, a larger number of HI mice were found in those groups treated with sub-effective doses than with effective doses of antidepressants. Conversely, LI mice were more frequent in the groups treated with high doses of antidepressants. The mixture of phenotypes may increase the standard error and reduce the accuracy of the measurement of immobility time in the test and retests. Inaccuracy may account for data variability and contribute to the lack of significant results. In addition, the effective doses of BUP and DMI reduced the immobility time in LI mice but failed to affect immobility time in HI mice. The absence of the effects of BUP and DMI in the HI subgroup may help to explain the unexpected results in the group of Swiss mice. In addition, in opposition to the initial expectation and independent of the dose, chronic treatment with BUP and DMI failed to reduce the immobility time in the retests. Ineffective or effective doses of FLX were unable to reduce the immobility time in LI or HI mice after acute or chronic treatment. These data are in contrast with data obtained previously by our research group (Reference Costa, Vieira and Bohner9), indicating that the colony of Swiss mice may have changed over time. Indeed, phenotypic characteristics of ‘helpless’ and ‘non-helpless’ mice, derived from a stock of Swiss albino CD-1 mice, changed across generations (Reference El Yacoubi, Bouali and Popa32). In addition, Lucky et al. (Reference Lucki, Dalvi and Mayorga7), comparing strains of mice from different suppliers in the Porsolt test, observed that Swiss-derived stocks of mice (CD-1, CF-1, Swiss-Webster) were unresponsive to a range of dosed of FLX while some of them responded to DMI (CD-1, CF-1) or to both (Swiss-NIH). The present study seems to be the first reporting a stock of Swiss resistant to the influence of monoaminergic antidepressants due the presence of subpopulations with low or high susceptibility to the treatments.
Laboratory animals resistant to the effects of monoaminergic antidepressants might be an opportunity for the discovery of new targets for these drugs (Reference Caldarone, Zachariou and King34,Reference Willner and Belzung35). Although considered a promising candidate mechanism (Reference Covington, Maze and LaPlant36), the inhibition of histone deacetylases was ineffective in traditional animal models for antidepressant detection (Reference Schroeder, Lin, Crusio and Akbarian37–Reference Qiu, Xiao, Li and Li41). For example, high doses of SB (>100 mg/kg) failed to reduce the immobility time in the Porsolt test in C57BL/6J mice (Reference Schroeder, Lin, Crusio and Akbarian37–Reference Covington, Vialou, LaPlant, Ohnishi and Nestler39) and ICR mice (Reference Han, Sung, Chung and Kwon40). Here, the immobility time of Swiss mice treated acutely or chronically with SB at doses of 1 or 3 mg/kg was similar to that of the VEH group in the repeated FST. Although the doses of 10 or 30 mg/kg of the SB reduced the immobility time of Swiss mice by more than 20% in the retest 1 in experiments 4 and 5, the confirmatory study failed to find such an effect. However, the SB at doses of 10 or 30 mg/kg increased the number of LI mice in the test, retest 1 and retest 2 compared with lower doses of SB. Together, these data indicate that a subgroup of Swiss mice that are resistant to monoaminergic antidepressants in the Porsolt test may be susceptible to histone deacetylases inhibitor. Studies that are more appropriate should be performed to verify the last hypothesis. Independent of the reason for the failed treatments, this is the first study showing the resistance of Swiss mice to a range of monoaminergic antidepressants.
Acknowledgements
Authors thank Prof. Dr. José Marino Neto and Prof. Dr. Fernando F. Melleu for the discussions on the data.
Authors’ Contribution: P.R.S. performed behavioural experiments, analysed data, discussed the data, wrote the manuscript; N.Z. performed experiment 7 and confirmatory experiments, scored behaviours from the videos from experiment 7 and confirmatory experiments, analysed data; discussed data; L.C.T. scored behaviours from the videos; K.D. prepared the solutions, analysed data, discussed data: C.L.O selected research theme, delineate experimental design, obtained grants, performed the statistical analysis, discussed data, wrote the manuscript, approved final version of the manuscript.
Financial Support
P.R.S., N.Z. and K.D received Masters’ Fellowships, and L.C.T. received PIBIC fellowship from CNPq. This work received financial support from Alexander von Humboldt Foundation (Equipment Grants) and CNPq (Proc. 472446/2012-6) to C.L.O. All procedures carried out in the present study complied with the current laws in Brazil.
Conflicts of Interest
The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Although P.R.S., N.Z., K.D., L.C.T. received fellowships from Brazilian public agencies during the work, the financial agencies did not interfere with the contents of the project or the publication.
Ethical Standards
The authors prepared the manuscript using ARRIVE guidelines to keep the report as transparent as possible. Protocols were according to the Brazilian guide for the care and use of laboratory animals, Brazilian laws on animal research and approved by the Ethical Committee of our University (CEUA/UFSC-PP00764). The authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. Although P.R.S., N.Z, K.D., L.C.T. received fellowships and C.L.O. received grants from public agencies during the work, the financial agencies did not interfere with the contents of the project or the publication.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2017.33









