Vancomycin-resistant enterococci (VRE) infections are endemic in hospitals across the United States.Reference Cetinkaya, Falk and Mayhall 1 VRE are the second most common antimicrobial-resistant pathogens causing healthcare-associated infections in the United States.Reference Hidron, Edwards and Patel 2 , Reference Sievert, Ricks and Edward 3 According to the National Healthcare Safety Network data in 2009-2010, 38.6% of enterococci isolated from device-associated healthcare-associated infections and 23.1% of those isolated from surgical site infections were vancomycin resistant.Reference Sievert, Ricks and Edward 3
Multiple epidemiological investigations of VRE infections have been published; however, most prior studies were performed before newer antibiotics such as quinupristin-dalfopristin, linezolid, or daptomycin were used widely.Reference Salgado and Farr 4 Most studies that reported the incidence of VRE infections were completed at single centers and evaluated small patient populations. Additionally, some studies claiming to report the incidence of VRE infections did not report a denominator-based incidence rate but instead reported the proportion of enterococcal isolates from infections that were vancomycin resistant.Reference Monnet and Frimodt-Møller 5 , 6 Furthermore, only a few studies evaluated outcomes, and some of these studies either included both colonized patients and infected patients or included patients infected with vancomycin-susceptible enterococci (VSE) as the comparator and did not include an uninfected control group.Reference Salgado and Farr 4 , Reference Neidell, Cohen and Furuya 7 Studies that use patients with VSE infections as the comparator can assess only the impact of antimicrobial resistance, but not the effect of antimicrobial resistance in addition to the infection itself.Reference Kaye, Engemann, Mazaffari and Carmeli 8
To address gaps in our understanding about the current burden associated with VRE infections in the United States, we conducted a systematic literature review of studies that were conducted in the United States, were published during or after 2000, and reported the incidence of VRE infections or outcomes related to these infections. Our goals were to describe the recent incidence of VRE infections, and to evaluate the clinical and economic outcomes attributable to VRE infections.
METHODS
Search Strategy
We conducted a systematic review according to the Meta-analysis of Observational Studies in EpidemiologyReference Stroup, Berlin and Morton 9 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.Reference Moher, Liberati, Tetzlaff and Altman 10 guidelines. See supplementary document for a detailed description of the search strategy. We reviewed reference lists from each article we retrieved to identify additional studies.
Inclusion and Exclusion Criteria
Studies were included if they (1) were conducted in the United States, (2) reported data from any year from 2000 through 2015, and (3) evaluated the incidence of VRE infections or outcomes attributable to VRE infections, including mortality, length of stay (LOS), discharge to a long-term care facility (LTCF), readmission, recurrence, or costs. We included multicenter studies that had at least 8 sites when we assessed the incidence of VRE infections. For studies presenting outcome data, we included single-center studies because most multicenter studies that assessed outcomes evaluated the same patient population (Detroit Medical Center). We excluded studies that (1) used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes to define VRE infections, (2) combined patients with VRE colonization with those who had infections, (3) did not report original data, (4) did not have a denominator or an uninfected control group, or (5) were published in a language other than English. We included studies that did not have an uninfected control group if they assessed the postinfection (after the first positive culture of VRE) outcomes of LOS, costs, or recurrence. For LOS or costs, we excluded studies if they did not measure postinfection LOS or costs, or did not match cases with controls on either the time at risk (time from admission to infection for cases, time from admission to discharge for uninfected controls), or on propensity scores. The current study did not require institutional review board approval.
Data Extraction and Quality Assessment
One author (H.-Y.C.) reviewed the titles and abstracts of all articles to determine whether they met the inclusion criteria. For each included study, 2 of 4 reviewers (H.-Y.C., R.N., E.N.P., M.L.S.) independently abstracted data on study design, population, setting, location, definition of VRE infection, incidence data, and clinical and economic outcomes. Reviewers resolved disagreements by consensus. We assessed the risk of bias using the Newcastle-Ottawa toolReference Stang 11 for all studies and the Consensus on Health Economic CriteriaReference Evers, Goossens, de Vet, van Tulder and Ament 12 for studies evaluating costs.
Meta-analysis of Mortality
We performed a meta-analysis of the studies that provided mortality data. We abstracted adjusted odds ratios (adjusted ORs) from the literature or raw data when adjusted ORs were not available. We pooled data using both random-effects and fixed-effects models with inverse variance weighting, and we used the Cochran Q statistic and the I 2 statistic to assess heterogeneity. Publication bias was determined by visually evaluating the funnel plot.
RESULTS
We screened 7,324 unique studies for eligibility (Figure 1). Eighteen studies were eligible for inclusion, including 5 multicenter studies reporting the incidence of VRE infectionsReference Hidron, Edwards and Patel 2 , Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13 – Reference Wang, Eldridge and Metersky 16 and 13 studies (4 multicenter and 9 single-center) evaluating relevant outcomes.Reference Hayakawa, Marchaim and Martin 17 – Reference Ford, Lopansri and Haydoura 29
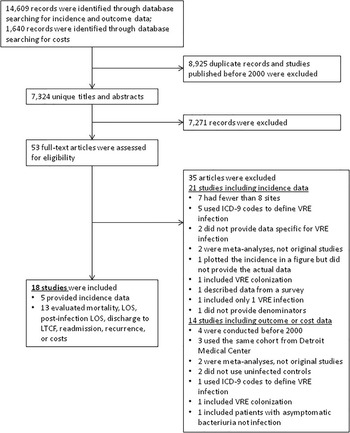
FIGURE 1 Flow diagram of search strategy. ICD-9, International Classification of Diseases, Ninth Revision; LOS, length of stay; LTCF, long-term care facility; VRE, vancomycin-resistant enterococci.
Five studies used the National Healthcare Safety Network definition of hospital-acquired VRE infections,Reference Hidron, Edwards and Patel 2 , Reference Jain, Kralovic and Evans 15 , Reference Song, Srinivasan, Plaut and Perl 21 – Reference DiazGranados and Jernigan 23 8 studies included patients with VRE recovered from sterile sites,Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13 , Reference Wang, Eldridge and Metersky 16 , Reference Hayakawa, Marchaim and Martin 17 , Reference Britt, Potter, Patel and Steed 20 , Reference Butler, Olsen and Merz 25 , Reference Santayana, Grim, Janda, Layden, Lee and Clark 27 – Reference Ford, Lopansri and Haydoura 29 4 studies included patients with VRE recovered from sterile sites or urine,Reference Hayakawa, Marchaim and Vidaillac 14 , Reference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 , Reference Scheetz, Knechtel, Postelnick, Malczynski and Qi 26 and 1 study did not define VRE infection.Reference Gearhart, Martin and Rudich 24 Overall, the risk of bias among all studies evaluated was low (Table 1).
TABLE 1 Risk of Bias Assessment Using Newcastle-Ottawa ToolReference Stang 11

NOTE. A star (*) indicates the study had a low risk of bias and high quality in that category. A maximum of 2 stars can be given for comparability category. NA=Not applicable because the study did not assess outcome.
a The 5 studies reporting incidence each had a low risk of bias in the selection of the study populations.
b The outcome studies had some risk of bias because 6 studies did not provide information about patients who were lost to follow up.Reference Hayakawa, Marchaim and Martin 17 - Reference Omotola, Li and Martin 19 , Reference Raad, Hachem and Hanna 22 , Reference Gearhart, Martin and Rudich 24 , Reference Butler, Olsen and Merz 25
c The 2 studies reporting costs had low risk of bias because 1 studyReference Butler, Olsen and Merz 25 met 14 of the 19 Consensus Health Economic CriteriaReference Evers, Goossens, de Vet, van Tulder and Ament 12 and another study met 17 criteria.Reference Song, Srinivasan, Plaut and Perl 21
Incidence of VRE Infections
The incidence varied by study location, population, and the denominator used (ie, person-years, patient-days, device-days, or number of hospitalizations) (Table 2). Thus, we could not calculate a summary incidence estimate. The incidence of VRE infections in Atlanta increased from 0.77 per 100,000 person-years in 1997 to 1.60 per 100,000 person-years in 2000 (P=.001). The increasing trend was significant in the African Americans but not in the white residents, and the overall incidence was significantly higher in the African Americans than in the white populations (2.59 vs 0.70 per 100,000 person-years).Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13 Among patients admitted to the 8-hospital Detroit Medical Center system, the incidence of VR Enterococcus faecalis infections increased from 0.72 per 1,000 patient-days in 2003 to 1.68 per 1,000 patient-days in 2009 (P<.001), and the incidence of VR Enterococcus faecium increased from 1.97 to 2.67 per 1,000 patient-days (not statistically significant).Reference Hayakawa, Marchaim and Vidaillac 14 Consistent with previous literature, VR E. faecium caused a higher proportion of the infections than did VR E. faecalis in Atlanta (83% vs 6%)Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13 and in the southeast Michigan area (71% vs 29%).Reference Hayakawa, Marchaim and Vidaillac 14
TABLE 2 Multicenter Studies That Evaluated Incidence Data on VRE Infections

NOTE. BSI, bloodstream infection; CAUTI, catheter-associated urinary tract infection; CDC, Centers for Disease Control and Prevention; CHF, congestive heart failure; CLABSI, central line–associated bloodstream infection; CSF, cerebrospinal fluid; ICU, intensive care unit; MI, myocardial infarction; NHSN, National Healthcare Safety Network; VR, vancomycin-resistant; VRE, vancomycin-resistant enterococci.
Among patients admitted to all Veterans Affairs (VA) hospitals, the incidence of VRE decreased between 2007 and 2010 from 1.51 to 0 per 1,000 patient-days for patients admitted to intensive care units (ICUs) (P<.001) and decreased from 0.33 to 0.09 per 1,000 patient-days for patients admitted to non-ICU units (P<.001).Reference Jain, Kralovic and Evans 15 Between 2005 and 2011, the incidence of VRE infections did not change significantly among Medicare patients who had 1 of the 4 conditions (ie, acute myocardial infarction, congestive heart failure, pneumonia, or conditions requiring surgery).Reference Wang, Eldridge and Metersky 16
The National Healthcare Safety Network reported that the pooled incidence of VR E. faecium central line–associated bloodstream infections (BSIs) during 2006 and 2007 was 0.18 (range, 0.06 to 0.37) per 1,000 device-days in ICUs and 0.14 (range, 0.13 to 0.15) per 1,000 device-days in non-ICUs. The pooled incidence of VR E. faecium catheter-associated urinary tract infections was 0.14 (range, 0.05 to 0.18) per 1,000 device-days in ICUs and 0.25 (range, 0.12 to 0.45) per 1,000 device-days in non-ICUs.Reference Hidron, Edwards and Patel 2
Outcomes Attributable to VRE Infections
Table 3 summarizes the results of 13 studies that reported outcomes or costs attributable to VRE infections. The 3 multicenter studies from Detroit Medical Center used different subgroups of patients: BSIs caused by VR E. faecalis or VR E. faecium in 2008–2010,Reference Hayakawa, Marchaim and Martin 17 all VR E faecalis infections in 2008–2009,Reference Hayakawa, Marchaim and Palla 18 and all community-onset VR E. faecalis in 2008–2009.Reference Omotola, Li and Martin 19 The study populations of the single-center studies included all hospitalized patients,Reference Song, Srinivasan, Plaut and Perl 21 patients with liver transplants or stem cell transplants,Reference Gearhart, Martin and Rudich 24 , Reference Vydra, Shanley and George 28 nonsurgical patients,Reference Butler, Olsen and Merz 25 or patients with leukemia.Reference Ford, Lopansri and Haydoura 29 Five studies evaluated only VRE BSIs,Reference Hayakawa, Marchaim and Martin 17 , Reference Song, Srinivasan, Plaut and Perl 21 , Reference Butler, Olsen and Merz 25 , Reference Vydra, Shanley and George 28 , Reference Ford, Lopansri and Haydoura 29 1 study evaluated community-onset VRE infections,Reference Omotola, Li and Martin 19 and 2 studies evaluated all VRE infections.Reference Hayakawa, Marchaim and Palla 18 , Reference Gearhart, Martin and Rudich 24
TABLE 3 Studies That Evaluated Outcomes Attributable to VRE Infections

NOTE. APR-DRG, All Patient Refined-Diagnosis Related Group; BSI, bloodstream infection; DMC, Detroit Medical Center; HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; LTCF, long-term care facility; OR, odds ratio; VRE, vancomycin-resistant enterococci.
a Readmissions within 6 months following VRE isolation for infected patients or within 6 months after admission for uninfected patients.
b VRE-infected patients and uninfected patients were matched by hospital or outpatient facility, unit or clinic, calendar year, and time at risk (ie, time from admission to culture for infected patients, time from admission to discharge for uninfected patients).
c Deaths within 90 days after VRE isolation for infected patients or within 90 days after admission for uninfected patients.
d Patients with VRE BSI and uninfected patients were matched on time at risk and at least 3 of the following criteria: age (±10 years), calendar year (±2 years), principal International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code at admission, primary ICD-9 procedure code during hospitalization, or APR-DRGs.
e Adjusted for severe illness (APR-DRG complexity level 4), being transferred from another healthcare facility, and staying in an ICU.
f Adjusted for being transferred from another healthcare facility.
g Adjusted for severe illness (APR-DRG complexity level 4).
h Adjusted for severe illness (APR-DRG complexity level 4) and staying in an ICU.
i VRE-infected patients and uninfected patients were matched (1:2) by age, gender, underlying disease, United Network for Organ Sharing status, primary or re-transplant, transplant date.
j Patients with VRE BSI and uninfected patients were matched on the basis of their propensity to develop VRE BSI (propensity scores matching).
k Non-relapse mortality is defined as deaths that could not be attributed to disease relapse or progression.
l Adjusted for acute graft-vs-host disease (GVHD), chronic GVHD, engrafted by day 42, age, sex, diagnosis, cytomegalovirus, donor type, and Karnofsky performance score.
m VRE-infected patients and uninfected patients were matched (1:3) by leukemia type, age, admitting Karnofsky performance status, and initial treatment regimen.
Mortality
Figure 2 summarizes the ORs from 6 studies that reported mortality data. These studies included a total of 1,182 VRE-infected patients and 1,840 uninfected controls. Compared with uninfected controls, patients who had VRE infections had a 2.5-fold higher risk of death (random-effects model; pooled OR, 2.55 [95% CI, 1.91–3.39]). The heterogeneity among studies was negligible (P=.54 for Q statistic test and I 2 =0%). The funnel plot (Figure 3) was not consistent with publication bias. The pooled mortality estimate from the 4 single-center studiesReference Song, Srinivasan, Plaut and Perl 21 , Reference Gearhart, Martin and Rudich 24 , Reference Vydra, Shanley and George 28 , Reference Ford, Lopansri and Haydoura 29 was higher (pooled OR, 3.15 [95% CI, 2.15–4.60]) than the estimates from the 2 multicenter studies (OR, 1.81 [95% CI, 1.06–3.08] and OR, 2.20 [95% CI, 1.04–4.65]),Reference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 which was not surprising because small single-center studies often overestimate true effects.

FIGURE 2 Forest plot of 6 studies providing mortality data.Reference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 , Reference Song, Srinivasan, Plaut and Perl 21 , Reference Gearhart, Martin and Rudich 24 , Reference Vydra, Shanley and George 28 , Reference Ford, Lopansri and Haydoura 29 IV, inverse variance; VRE, vancomycin-resistant enterococci.

FIGURE 3 Funnel plot of 6 studies providing mortality data.Reference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 , Reference Song, Srinivasan, Plaut and Perl 21 , Reference Gearhart, Martin and Rudich 24 , Reference Vydra, Shanley and George 28 , Reference Ford, Lopansri and Haydoura 29 OR, odds ratio.
Postinfection LOS and LOS Attributable to VRE
Five studies that did not include uninfected control patients found postinfection LOS ranging from 9 to 22 days. The median postinfection LOS for patients with VRE BSI ranged from 9.1 to 13 days in 2 multicenter studiesReference Hayakawa, Marchaim and Martin 17 , Reference Britt, Potter, Patel and Steed 20 and was 17 days in a single-center study.Reference DiazGranados and Jernigan 23 In 2 other single-center studies, the postinfection LOS for patients infected with linezolid-resistant or linezolid-intermediate VRE was 3 to 4 days longer than that for patients infected with linezolid-susceptible VRE (median, 13 vs 9 daysReference Scheetz, Knechtel, Postelnick, Malczynski and Qi 26 ; mean, 22 vs 19 daysReference Santayana, Grim, Janda, Layden, Lee and Clark 27 ).
Four studies assessed LOS attributable to VRE infections by either matching infected patients and uninfected patients on time at risk or by matching on propensity scores. These studies found that LOS for patients with VRE infections was 3 to 4.6 days (median difference) longerReference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 , Reference Butler, Olsen and Merz 25 or 1.4 times (multiplicative increase) longerReference Song, Srinivasan, Plaut and Perl 21 than for uninfected patients.
Discharge to an LTCF
Two multicenter studies evaluated the likelihood that patients admitted to Detroit Medical Center from home would be discharged to LTCFs. Compared with uninfected patients, patients with VR E. faecalis infections had a 2.8-fold increased risk (11.4% vs 33.9%)Reference Hayakawa, Marchaim and Palla 18 of being discharged to a LTCF and patients with community-onset VR E. faecalis infections had a 6.5-fold increased risk (4.7% vs 26.3%).Reference Omotola, Li and Martin 19
Readmission
Only 1 multicenter study evaluated readmissions associated with VRE infections. The authors found that patients with VR E. faecalis infections were 2.9-fold more likely to be readmitted within 6 months (after the first culture positive for VRE for infected cases and after admission for uninfected controls), compared with matched controls (74.5% vs 50.8%).Reference Hayakawa, Marchaim and Palla 18
Recurrence
Two studies evaluated recurrence rates. Of patients treated for VRE BSI in VA centers, 23.6% had recurrences within 60 days after completing treatment.Reference Britt, Potter, Patel and Steed 20 Fifteen percent of patients treated for VR E. faecium at a cancer center had recurrences within 30 days.Reference Raad, Hachem and Hanna 22
Costs
Two single-center studies evaluated costs associated with VRE infections and matched on either time at riskReference Song, Srinivasan, Plaut and Perl 21 or propensity score.Reference Butler, Olsen and Merz 25 Song et alReference Song, Srinivasan, Plaut and Perl 21 found that the costs of a hospital admission were $124,257 for patients with VRE BSIs and $46,699 for uninfected controls. The adjusted analysis showed that the costs for patients with VRE BSIs were 1.6-fold higher than the costs for uninfected controls. Butler et alReference Butler, Olsen and Merz 25 found that the costs for nonsurgical patients with VRE BSIs were $9,949 USD more than the costs for uninfected patients.
DISCUSSION
Our systematic literature review found that the incidence of VRE infections varied by study. Patients with VRE infections were more likely to die in the hospital, to have longer hospital stays, to be discharged to LTCFs after being admitted from home, to be readmitted within 6 months, and to have higher hospital costs compared with uninfected patients.
Incidence
Two studies assessing the incidence of VRE infections in individual metropolitan areas found that the incidence increased during their study periods.Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13 , Reference Hayakawa, Marchaim and Vidaillac 14 In addition, the VRE infection incidence was significantly higher among African Americans than among white residents in Atlanta. The investigators postulated that African Americans had a higher rate of chronic conditions, which increased their need for healthcare and, thereby, increased their risk for staphylococcal infections and vancomycin exposure.Reference Camins, Farley, Jernigan, Ray, Steinberg and Blumberg 13
A study among a subset of Medicare patients who had few VRE infections found stable VRE infection rates during 2005–2011.Reference Wang, Eldridge and Metersky 16 The findings of this study may indicate that the incidence of VRE infections among low-risk populations has not changed significantly since 2000. In contrast, a study of all VA patients found that the incidence of VRE infections and methicillin-resistant Staphylococcus aureus (MRSA) infections decreased during 2007–2010, after VA hospitals implemented a bundle to decrease MRSA healthcare-associated infections.Reference Jain, Kralovic and Evans 15 The decline in VRE infections may have been related to the decline in MRSA infections and less frequent use of vancomycin or to improved overall infection prevention practices associated with the MRSA intervention.
To avoid misclassification bias, we did not include studies that used ICD-9-CM diagnosis codes (V09.80, V09.81, 041.04) to define VRE infection.Reference Ramsey and Zilberberg 30 – Reference Zilberberg, Shorr and Kollef 34 Administrative coding was designed for billing, not research. Prior studies have shown that codes for acute conditions, such as infections, often overestimate the incidence of these conditions.Reference Dubberke, Butler and Nyazee 35 , Reference Schweizer, Eber and Laxminarayan 36 To our knowledge, no published study has validated the ICD-9-CM codes for either VRE or enterococcal infection with lab-confirmed VRE infection. Until they have been validated, these codes should not be used to estimate the burden of VRE infections.
Most VRE infections in the United States are caused by enterococcal isolates that have the VanA plasmid, which carries the vancomycin-resistant gene. This plasmid occurs more commonly in VR E. faecalis than in other species of Enterococcus and may be transferred to S. aureus, causing the isolates to become vancomycin resistant. 37 , Reference DiazGranados, Zimmer, Klein and Jernigan 38 As of May 2015, 8 of 14 vancomycin-resistant S. aureus infections in the United States occurred in southeastern Michigan, where the incidence of VR E. faecalis is higher than in other regions.Reference Hayakawa, Marchaim and Palla 18 , 37 Thus, monitoring the regional incidence of VRE could help public health officials assess the potential for emergence and spread of vancomycin-resistant S. aureus.
Mortality
Our study, which compared the risk of mortality among VRE-infected patients with uninfected patients, found that VRE infection was significantly associated with mortality (pooled OR, 2.55). Three prior meta-analyses also evaluated mortality among VRE-infected patients but used patients with VSE infections as their comparison groups. Two of these meta-analyses only included studies that were conducted before 2003, when newer antimicrobial agents such as daptomycin, linezolid, and quinupristin-dalfopristin were not widely available. The first meta-analysis of 13 studies found that patients with VRE BSI had a 2-fold higher risk of mortality compared with patients who had VSE BSI.Reference Salgado and Farr 4 The second meta-analysis, which assessed 9 studies and adjusted for severity of illness, found that patients with VRE BSI were 2.5 times more likely to die than patients with VSE BSI.Reference DiazGranados, Zimmer, Klein and Jernigan 38 The third meta-analysis only included studies that were published after the approval of new antimicrobial agents effective against VRE.Reference Prematunge, MacDougall and Johnstone 39 That meta-analysis compared patients with VRE infections with those who had VSE infections and found a smaller unadjusted association between VRE infection and mortality (pooled OR, 1.80 [95% CI, 1.38–2.35]). Our meta-analysis evaluated studies published during the same period as the third meta-analysis. However, we assessed studies that used uninfected controls, which likely explains the stronger association we found between mortality and VRE infection. In addition, VSE and VRE have relatively low virulence. Kaye et alReference Kaye, Engemann, Mazaffari and Carmeli 8 previously found that the effect of clinical outcomes associated with MRSA surgical site infections was 2- to 3-fold greater when uninfected patients were used as controls than when patients with methicillin-susceptible S. aureus surgical site infections were used, whereas clinical outcomes of VRE wound infections were similar when controls were uninfected or when they were infected with VSE. They postulated that the magnitude of the effect was related to the virulence of the pathogen being studied.
Other Outcomes
We found that the attributable hospital LOS was 3-4.6 days or 1.4 times longer and the attributable cost was $10,000 USD or 1.6-fold more for patients with VRE infections than those for uninfected controls. Our estimates are likely to be less biased than those of prior studies because we included studies that used uninfected controls that matched on the time at riskReference Hayakawa, Marchaim and Palla 18 , Reference Omotola, Li and Martin 19 , Reference Song, Srinivasan, Plaut and Perl 21 or on a propensity score.Reference Butler, Olsen and Merz 25 Studies that do not account for the time from admission to infection overestimate the LOS attributable to the infection because of time-dependent bias. Nelson et alReference Nelson, Nelson and Khader 40 performed a systematic review to estimate the magnitude of time-dependent bias. They compared the conventional method of calculating excess LOS attributable to healthcare-associated infections with that calculated after matching patients on time at risk. They found that estimates of the LOS calculated by conventional methods were on average 12.6 days longer or 139% greater than those generated when controls were matched on time to infection. Similarly, studies that do not account for patient characteristics in the analyses or do not match on propensity scores may overestimate the LOS or cost attributable to VRE because patients infected with resistant organisms often have severe underlying diseases, which are independently predictive of adverse outcomes and increased costs.
Limitations
Our study has several potential limitations. First, the definition of VRE was not consistent across studies. Second, we could not pool incidence data because denominators and study populations varied by study. Third, the Newcastle-Ottawa risk of bias tool was not useful because the questions about comparability and outcome assessment were not applicable to the incidence studies and the questions about selection of non-infected controls and comparability were not applicable to studies including only VRE infected patients. However, we do not think these limitations would cause us to underestimate or overestimate the burden of VRE infections.
In conclusion, VRE infections still increase mortality, hospital LOS, and costs in the United States despite the current treatment options and infection prevention measures. Most published studies evaluating outcomes attributable to VRE infections had small sample sizes or did not consider the time at risk or confounders. In addition, many studies assessed outcomes attributable to vancomycin resistance instead of those attributable to VRE infections. However, our study, which evaluated studies that used uninfected patients as controls, found that VRE infection was associated with poor outcomes. Our study provides valuable information about the current burden of VRE infections in the United States and identifies gaps that should be addressed by future studies, so that we can estimate accurately the incidence and outcomes attributable to VRE infections.
ACKNOWLEDGMENTS
Financial support. Centers for Disease Control and Prevention, Safe Healthcare, Epidemiology, and Prevention Research Development (SHEPheRD) Program (contract 200-2011-42039 to M.S. and E.N.P.); and VA Health Services Research and Development Career Development Award (award 11-215 to M.L.S.).
Potential conflicts of interest. All authors report no conflicts relevant to this article.
Disclaimer: The opinions expressed are those of the authors and not necessarily those of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the US government.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/ice.2016.254








