Introduction
Head and neck squamous cell carcinomas (SCCs) rank seventh in terms of cancer incidence worldwide, with an estimated 633 000 new cases diagnosed annually.Reference Rettig and D'Souza1 Over the past three decades, their incidence has regressed significantly in Western countries. For example, in the US male population, the incidence of non-oropharyngeal head and neck SCCs has decreased by 20.3 per cent in the 1990s.Reference Ernster, Sciotto, O'Brien, Finch, Robinson and Willson2 This decrease paralleled a decrease in tobacco smoking and alcohol consumption. However, during the same timeframe, the overall decrease in head and neck SCC incidence was paradoxically associated with an increase in incidence of oropharyngeal SCCs, especially in a subset of younger patients who were free of the classically established risk factors.Reference Argiris, Karamouzis, Raben and Ferris3 In fact, the incidence of oropharyngeal SCCs increased by 10.8 per cent in the US male population during the 1990s.Reference Ernster, Sciotto, O'Brien, Finch, Robinson and Willson2 This epidemiological finding led to the search for different risk factors for oropharyngeal SCC. The role of human papilloma virus (HPV) infection in the pathogenesis of oropharyngeal SCC has since been widely validated and confirmed.Reference Mehanna, Jones, Gregoire and Ang4, Reference Van Monsjou, Balm, Van Den Brekel and Wreesmann5
Approximately 5 per cent of healthy individuals have been diagnosed with oral and oropharyngeal HPV infection.Reference D'Souza, Agrawal, Halpern, Bodison and Gillison6, Reference Hansson, Rosenquist, Antonsson, Wennerberg, Schildt and Bladström7 This infection has a slightly higher prevalence among men, and among individuals aged 30–34 years and 60–64 years.Reference Gillison, Broutian, Pickard, Tong, Xiao and Kahle8 In a 2013 systematic review, Mehanna et al. showed a significant increase of HPV infection prevalence in oropharyngeal SCC patients in North America and Europe: from 40.5 per cent before 2000, to 64.3 per cent between 2000 and 2004, and 72.2 per cent between 2005 and 2009.Reference Mehanna, Beech, Nicholson, El-Hariry, McConkey and Paleri9 On the other hand, the prevalence of HPV infection has not changed in non-oropharyngeal head and neck SCC patients, with a mean value of 20 per cent.Reference Mehanna, Beech, Nicholson, El-Hariry, McConkey and Paleri9 The currently supported hypothesis to explain this difference in HPV prevalence between head and neck SCC subsites is that the Waldeyer's ring acts as a reservoir for the HPV, with HPV inhabiting the normal crypt epithelia.Reference Syrjänen10
The oropharyngeal SCC population has some distinct characteristics compared to the classical head and neck SCC population: young age, non-smoker, non-alcoholic and multiple sex partners.Reference Argiris, Karamouzis, Raben and Ferris3 Compared to a control group, the oropharyngeal SCC cancer group has a significantly higher prevalence of oral HPV infection, a higher lifetime number of oral sex partners and a higher seropositivity for HPV-16.Reference D'Souza, Kreimer, Viscidi, Pawlita, Fakhry and Koch11 In fact, certain sexual behaviours, such as a high lifetime number of vaginal sex or oral sex partners, are known to be implicated in the viral transmission.Reference D'Souza, Kreimer, Viscidi, Pawlita, Fakhry and Koch11–Reference Rosenquist, Wennerberg, Schildt, Bladström, Göran Hansson and Andersson13
Human papilloma virus is an encapsulated double-stranded DNA with two main carcinogenic types: HPV-16 and HPV-18.Reference Allen, Lewis, El-Mofty, Haughey and Nussenbaum14 Human papilloma virus induced carcinogenesis is different from the tobacco- or alcohol-induced type. Once integrated to the host genome, the viral genes are expressed. This produces two main oncoproteins, E6 and E7, which inactivate the products of tumour suppressor genes p53 and Rb respectively. This allows the infected cell to acquire a malignant phenotype.Reference Braakhuis, Snijders, Keune, Meijer, Ruijter-Schippers and Leemans15–Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17 The p16INK4A overexpression resulting from this malignant phenotype is currently considered a highly sensitive, indirect marker of HPV infection.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17, Reference Psyrri, Gouveris and Vermorken18
Human papilloma virus related oropharyngeal SCCs are associated with a better prognosis.Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tân19, Reference Weinberger, Yu, Kountourakis, Sasaki, Haffty and Kowalski20 In their systematic review, O'Rorke et al. found that HPV was an independent good prognosis factor for oropharyngeal SCC.Reference O'Rorke, Ellison, Murray, Moran, James and Anderson21 Ang et al. demonstrated better overall survival and progression-free survival in 323 HPV-related oropharyngeal SCC patients treated by concomitant radiation and chemotherapy.Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tân19 They further defined three subgroups according to prognosis implication: low risk (HPV-positive non-smokers), intermediate risk (HPV-positive smokers) and high risk (HPV-negative smokers).
Human papilloma virus related oropharyngeal SCC is a distinctive subgroup with different carcinogenesis, risk factors, clinical features and prognosis profile. Furthermore, the association between HPV and oropharyngeal SCC has important implications for prevention, treatment and prognosis.
It is thus crucial to detect HPV infection in oropharyngeal SCC patients. In the absence of a standardised detection technique, a key element in choosing the optimal technique to detect HPV involvement in oropharyngeal SCC is to differentiate between certain highly sensitive tests able to detect the mere presence of the virus without any carcinogenetic involvement and other more specific tests providing proof of viral transcriptional activity and thus evidence of clinically relevant infections.Reference Braakhuis, Brakenhoff, Meijer, Snijders and Leemans22–Reference Melkane, Mirghani, Aupérin, Saulnier, Lacroix and Vielh24 The association of viral DNA detection by polymerase chain reaction and p16 detection by immunohistochemistry is a validated diagnostic algorithm, which aims to reliably determine biologically relevant HPV infections in formalin-fixed paraffin-embedded biopsy material taken from oropharyngeal SCC patients.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17, Reference Melkane, Mirghani, Aupérin, Saulnier, Lacroix and Vielh24
To date, epidemiological data concerning the HPV prevalence in oropharyngeal SCC are mainly limited to North America and Europe. And while the Middle-Eastern population is characterised by different social and sexual behaviours, a study regarding the HPV prevalence in this population has yet to be published. With the present study, and for the first time in a Middle-Eastern population, we aimed to evaluate the prevalence of the p16 overexpression and oncogenic HPV DNA viral load in a homogeneous single-institution oropharyngeal SCC cohort, in Lebanon, and compare it to the available data from a Western population.
Materials and methods
Patient selection
A retrospective chart review was conducted on all patients diagnosed or treated for oropharyngeal SCC between January 2010 and 2016 at the Hotel Dieu de France Hospital, Beirut, Lebanon. Only patients with a single and previously untreated primary lesion of the oropharynx were considered eligible for the study. Formalin-fixed paraffin-embedded tumour samples were collected from the pathology archives and available amounts of tissue were assessed. This work had been reviewed and approved by the institutional ethical committee at the Hotel Dieu de France Hospital.
Tissue sample preparation and analysis
From each patient's tissue sample, a section was first obtained and reviewed by the pathologist to ensure that the tumour core was reached. The section was used for formalin-fixed paraffin-embedded tissue sample preparation for immunohistochemistry. The rest of the paraffin block was macrodissected with trimming of excessive wax. Serial sections were then collected and directly processed for DNA extractions. Disposable, sterile microtome blades and scalpels were changed before every block to avoid contamination.
The p16 expression determined by immunohistochemistry was evaluated for all samples by two different pathologists blinded to each other's results. There was no inter-rater variability and the results were concordant for all patients. The HPV DNA viral load data obtained by polymerase chain reaction were analysed by a single bioengineer. Both procedures were conducted simultaneously in separate laboratories in a blind fashion, to avoid any bias. For p16INK4A expression evaluation, tumours were classified into two groups: p16 INK4A-positive (strong, diffuse, nuclear and cytoplasmic staining in more than 10 per cent of carcinoma cells) or p16 INK4A-negative.Reference Lassen, Eriksen, Hamilton-Dutoit, Tramm, Alsner and Overgaard25
The E6 and E7 regions of the oncogenic HPV strains were targeted for the polymerase chain reaction analysis, and tumours were then classified dichotomously as HPV DNA positive or HPV DNA negative. In the positive group, the HPV strain was also determined.
Statistical analysis
Associations between the clinicopathological features of patients and both p16 expression and HPV DNA viral load status (positive or negative) were evaluated using the Fisher exact test and the two-sample t-test. A p-value of less than 0.005 was considered statistically significant. All statistical analyses were performed with SPSS® Statistics Desktop software, version 22.0.
Ethical considerations
The institutional review board gave its approval on the study protocol and patient confidentiality was protected.
Results
Patients’ characteristics
Thirty patients met the criteria for eligibility and were included in the study: 21 were men and 9 were women. Mean age at diagnosis was 60 years (range, 36–89 years), and no significant difference was found between the sexes. Seventeen patients (56.67 per cent) were smokers. Thirteen patients (43.33 per cent) consumed alcohol; all of them were concomitant smokers. Thirteen patients (43.33 per cent) did not use either carcinogen.
Tumour characteristics
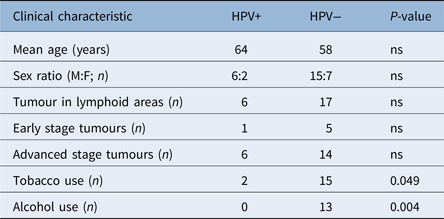
Twenty-three tumours (76.67 per cent) were found in lymphoid areas: 12 in the tonsillar fossa and 11 in the base of the tongue. The remaining seven tumours were found in the soft palate. All of the tumours were SCCs. On histopathology, 14.81 per cent of the lesions were well differentiated, 55.56 per cent were moderately differentiated and 29.63 per cent were poorly differentiated. Twenty-three per cent of patients were diagnosed with early stage disease: tumour–node–metastasis (TNM) stages I (11.54 per cent) and II (11.54 per cent). Seventy-seven per cent of patients were diagnosed with advanced stage disease: TNM stages III (19.23 per cent) and IV (57.69 per cent). These findings are summarised in Table 1.
Table 1. Summary of patient and tumour characteristics

*All alcohol drinkers also smoked. †Staging information was missing for four patients. ‡Tumour differentiation details were missing for three patients. IHC = immunohistochemistry; HPV = human papilloma virus; PCR = polymerase chain reaction
Immunohistochemistry and polymerase chain reaction findings
Based on the predefined criteria, 14 patients (46.67 per cent) tested positive for the p16INK4A protein on immunohistochemistry. Eight patients (26.67 per cent) were found to have HPV DNA on polymerase chain reaction. Human papilloma virus 16 was responsible for the majority of infections (75 per cent); other HPV subtypes were less frequently involved.
As discussed earlier, an oncologically relevant HPV infection in an oropharyngeal SCC patient is defined by the presence of HPV DNA in the tumour as well as overexpression of p16INK4A. Hence, the HPV-positive group comprised patients with a p16-positive/HPV DNA positive profile. The prevalence of this profile was 26.67 per cent, while 53.33 per cent and 20 per cent of patients were p16-negative/HPV DNA negative and p16-positive/HPV DNA negative, respectively.
Gender, age, tumour location and tumour TNM stage at diagnosis were not significantly associated with HPV status. Tobacco exposure was significantly lower in the HPV-positive group than the HPV-negative group (25 per cent vs 68 per cent; p = 0.049). None of the HPV-positive patients consumed alcohol, whereas 59 per cent of HPV-negative patients did (p = 0.004; Table 2).
Table 2. Clinical associations with HPV-positive oropharyngeal SCC
HPV = human papilloma virus; SCC = squamous cell carcinoma; ns = non-significant; M = males; F = females
Discussion
Human papilloma virus prevalence
In this study sample, the prevalence of HPV-related oropharyngeal SCCs was only 26.67 per cent. The majority of oropharyngeal SCCs in the Lebanese population were not HPV-related tumours, but rather secondary to tobacco or alcohol consumption.
In a recent study from the Gustave Roussy Institute in France, the prevalence of HPV-related oropharyngeal SCC was as high as 48 per cent.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17 In another multi-institutional French study, published in 2011, St Guily et al. reported a prevalence of 46.5 per cent for HPV-related oropharyngeal SCCs.Reference St Guily, Jacquard, Prétet, Haesebaert, Beby-Defaux and Clavel26 Moreover, in the 2005 meta-analysis by Kreimer et al., the overall reported prevalence of HPV infection in oropharyngeal SCCs was 35.6 per cent.Reference Kreimer27 This difference in prevalence values could in part be due to the variability of detection techniques between studies or a result of geographic differences in the distribution of risk factors.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17
While some inter-study variability in prevalence rates is found in Western publications, these rates remain in a somewhat narrow range.Reference St Guily, Jacquard, Prétet, Haesebaert, Beby-Defaux and Clavel26, Reference Kreimer27 In contrast, the results of the current study show that the prevalence of HPV-related oropharyngeal SCCs in the Lebanese population is considerably lower and accounts approximately for half of that found in Western countries.
The primary risk factor for developing HPV-related oropharyngeal SCC is oral HPV infection. Oral sex and multiple sexual partners are the major causes of oral HPV infections.Reference Taberna, Mena, Pavón, Alemany, Gillison and Mesía28 A study conducted among Lebanese university students showed that only 20 per cent of them were engaged in regular sexual activity, while an additional 15 per cent had had only one sexual encounter.Reference Salameh, Zeenny, Salamé, Waked, Barbour and Zeidan29 These numbers, while similar to those seen in other countries witnessing a health transition, such as China,Reference Zhang, Pan, Cui, Law, Farrar and Ba-Thein30 are lower than those reported in Western countries.Reference Salameh, Zeenny, Salamé, Waked, Barbour and Zeidan29, Reference Lally, Nathan-V, Dunne, McGrath, Cullen and Meagher31 These differences in sociocultural and sexual behaviours between the Middle-Eastern Lebanese population and the Western population may constitute a potential explanation for the lower rates of HPV-related oropharyngeal SCCs seen in the Lebanese population when compared to Western countries.
Associations with clinical features
Some previously published studies found that oropharyngeal SCC patients who are HPV-positive tend to be predominantly male and younger than HPV-negative patients.Reference Mellin, Friesland, Lewensohn, Dalianis and Munck-Wikland32–Reference Smith, Ritchie, Summersgill, Klussmann, Lee and Wang34 In the current study, however, age and sex showed no significant associations with HPV positivity. In line with previously published reports,Reference Gillison, D'Souza, Westra, Sugar, Xiao and Begum33 HPV positivity was associated with the absence of both tobacco and alcohol consumption. In fact, it is now well established that while tobacco and alcohol consumption are the primary risk factors for HPV-negative head and neck SCC, sexual habits have become the primary risk factors for HPV-positive oropharyngeal SCCs.Reference Smith, Ritchie, Summersgill, Klussmann, Lee and Wang34
Human papilloma virus positive oropharyngeal SCCs are preferentially located to the lymphoid areas of the oropharynx. Although tumour location and HPV status were not associated in our results, the widely accepted theory is that Waldeyer's ring acts as a natural habitat and reservoir for the virus.Reference Hobbs, Sterne, Bailey, Heyderman, Birchall and Thomas35, Reference Paz, Cook, Odom-Maryon, Xie and Wilczynski36 In several published reports, there was a significant association between HPV positivity and a more advanced TNM stage at diagnosis.Reference Paz, Cook, Odom-Maryon, Xie and Wilczynski36, Reference Hafkamp, Manni, Haesevoets, Voogd, Schepers and Bot37 In the vast majority of these reports, the advanced stage at diagnosis is attributed to the positive nodal status, rather than a large tumour size or local aggressiveness.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17 However, this finding was not reproduced in our results, probably because of the smaller sample size of the study.
Prognosis, survival and therapeutic repercussions
Human papilloma virus positive status has served as a significant prognostic factor for survival in oropharyngeal SCCs.Reference Fakhry, Westra, Li, Cmelak, Ridge and Pinto38, Reference Fundakowski and Lango39 In fact, HPV-related oropharyngeal SCCs have better treatment response rates, which translate into improved survival rates that are persistent regardless of the treatment modality.Reference Fundakowski and Lango39, Reference Dayyani, Etzel, Liu, Ho, Lippman and Tsao40 This is mainly explained by the inherent immunogenicity, increased radiosensitivity and absence of field cancerisation in the HPV-related oropharyngeal SCCs.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17, Reference Agrawal, Koch, Xiao, Westra, Trivett and Symer41, Reference Lassen, Eriksen, Krogdahl, Therkildsen, Ulhøi and Overgaard42
In a report published in 2008, Fakhry et al. showed that locoregional failure rates at three years were significantly lower in HPV-related oropharyngeal SCC patients compared to HPV-negative patients (13.6 per cent vs 35 per cent, respectively).Reference Fakhry, Westra, Li, Cmelak, Ridge and Pinto38 In addition, the three-year progression-free survival rates have shown important differences between HPV-positive and HPV-negative oropharyngeal SCCs, with rates ranging from 75 to 82 per cent and from 45 to 57 per cent, respectively.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17, Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tân19, Reference Fundakowski and Lango39, Reference Licitra, Perrone, Bossi, Suardi, Mariani and Artusi43–Reference Heiduschka, Grah, Oberndorfer, Kadletz, Altorjai and Kornek45 Conversely, the rates of distant metastasis in HPV-positive oropharyngeal SCCs are similar to those in the HPV-negative oropharyngeal SCCs, with lung metastasis being the most common.Reference Fakhry, Zhang, Nguyen-Tan, Rosenthal, El-Naggar and Garden46 However, even in a scenario of recurrent or metastatic disease, an HPV-positive status is still a favourable prognostic factor.Reference Fundakowski and Lango39
The striking difference in clinical presentation and treatment response between HPV-negative and HPV-positive oropharyngeal SCCs has led authors to consider the latter as a distinct biological and clinical entity.Reference Fundakowski and Lango39 Therefore, new staging systems have been validated to better take into account the disease severity and prognostic differences between HPV-positive and HPV-negative oropharyngeal SCCs, although none of these staging systems are universally adopted at this time.Reference O'Sullivan, Huang, Su, Garden, Sturgis and Dahlstrom47
Different therapeutic and preventive approaches are currently being developed for the HPV-related oropharyngeal SCCs. While the present treatment guidelines do not recommend or take into consideration treatment modifications based on HPV status, this will probably change in the future.Reference Fundakowski and Lango39 In fact, several clinical trials targeting HPV-positive oropharyngeal SCC patients are currently underway to evaluate radiation de-escalation, modified surgical interventions and alterations in systemic therapy for this particular patient group. Decreasing radiation dose, replacing cisplatin with rituximab, revisiting the need for chemotherapy added to radiotherapy for adjuvant therapy, employing less invasive surgery using transoral robotic surgery, and introducing immunotherapy to the treatment arsenal are among the proposed de-intensification strategies presently being assessed.Reference Kofler, Laban, Busch, Lörincz and Knecht48 Based on the inherent better prognosis of the HPV-related oropharyngeal SCCs, these carefully planned strategies aim to maximise disease control, while minimising both long- and short-term toxicity.Reference Brockstein and Vokes49, Reference Givens, Karnell, Gupta, Clamon, Pagedar and Chang50 Unfortunately, there is currently not sufficient evidence to validate the introduction of de-intensification protocols according to HPV status in treatment guidelines.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17
• Human papilloma virus (HPV) is an established risk factor for oropharyngeal squamous cell carcinoma (SCC)
• It accounts for more than 50 per cent of newly diagnosed oropharyngeal SCCs in Western populations
• To date, no data have been published on the prevalence of HPV involvement in oropharyngeal SCC in a Middle-Eastern population
• In this first Middle-Eastern study, HPV-positive oropharyngeal SCC prevalence was 27 per cent
• This rate is much lower compared to that in Western populations
In the face of the growing interest in preventive medicine, more attention has been given to the potential of HPV vaccines for preventing oropharyngeal SCC. To our knowledge, the three available prophylactic HPV vaccines are only approved by the US Food and Drug Administration for the prevention of anogenital cancer, pre-cancer and warts, but not for oropharyngeal SCC.Reference Guo, Eisele and Fakhry51 To the best of our knowledge, a limited number of studies have been conducted to date to evaluate the impact of the HPV vaccine on oral HPV infection or oropharyngeal SCC.Reference Guo, Eisele and Fakhry51 There is, however, some promising biological evidence regarding vaccine efficacy for oral HPV infection. In animal studies, passive antibody transfers and vaccination for canine oral papillomavirus were shown to provide protection against oral infection and the development of oral pre-cancerous lesions.Reference Suzich, Ghim, Palmer-Hill, White, Tamura and Bell52 While the epidemiology of oropharyngeal SCCs may be eventually altered by the long-term effects of the female anti-HPV vaccination, a similar systematic male vaccination protocol should be implemented to efficiently prevent transmission of oncogenic HPV subtypes.Reference Melkane, Auperin, Saulnier, Lacroix, Vielh and Casiraghi17 The current body of evidence is, however, insufficient to determine the efficacy of these vaccines exclusively within the context of oral HPV infection or oropharyngeal SCC.Reference Guo, Eisele and Fakhry51
Acknowledgements
The authors would like to thank Dr Amine Haddad and Dr Bassam Tabchy for granting access to their patient databases.
Competing interests
None declared




