INTRODUCTION
Polychaetes from the Mariana Islands of Guam and Saipan have been characterized in previous studies by Kohn & White (Reference Kohn and White1977), Emery (Reference Emery1962), Bailey-Brock (Reference Bailey-Brock1999, Reference Bailey-Brock2003), Sardá et al. (Reference Sardá, Avila and Paul2002), Magalhães & Bailey-Brock (Reference Magalhães and Bailey-Brock2013), Bailey-Brock & Magalhães (Reference Bailey-Brock and Magalhães2013) and Magalhães & Rizzo (Reference Magalhães and Rizzo2012). The latest compilation reports a total of 104 species from Guam and 51 from Saipan (Bailey-Brock, Reference Bailey-Brock2003). Although Kohn & White (Reference Kohn and White1977) have reported high densities of polychaetes in reef rocks (43,500 m−2) and algal mat habitats (21,400 m−2), a greater sampling effort is still necessary in order to investigate the hidden biodiversity of this region.
The cirratulids of the Mariana Islands have been poorly characterized and a thorough taxonomic study has only been possible recently (Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013). The most commonly reported cirratulid in the literature of the western Pacific is Dodecaceria laddi and it seems to be a common boring species of shallow water coral rubbles of the Mariana Islands (Bailey-Brock, Reference Bailey-Brock1999, Reference Bailey-Brock2003). Timarete punctata, Timarete caribous (most likely Timarete hawaiensis), Chaetozone sp. and Aphelochaeta saipanensis are also reported to occur in the islands (Bailey-Brock, Reference Bailey-Brock1999, Reference Bailey-Brock2003; Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2013). A recent study in Apra Harbor, Guam has reported the occurrence of at least 10 unidentified cirratulid species (Bailey-Brock et al., Reference Bailey-Brock, Cooke, Eberhardt, Lin and Magalhães2012) and some of these are described herein as a new species and two new records.
MATERIALS AND METHODS
Specimens were collected by scuba divers in December 2010 at several stations within Apra Harbor, Guam. The sediment was preserved in 15% buffered formalin with Rose Bengal and later stored in ethanol 70%. Preserved specimens were examined with light microscopy and some specimens were prepared for scanning electron microscopy (SEM). Specimens were dehydrated in a graded ethanol series, dried in a SAMDRI-795 critical point dryer using liquid CO2 and gold-coated for about 2 min. SEM observations were carried out using the Hitachi S-4800 at the Biological Electron Microscopy Facility (BEMF), University of Hawaii at Manoa.
Specimens were bathed in a saturated solution of methyl green and 70% ethanol for a minimum of 60 s and the MGSP (methyl green staining pattern) was determined after the specimens were seated for a few seconds into clean 70% ethanol. Type and voucher material are deposited at the United States National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM) and the Bernice Pauahi Bishop Museum, Honolulu, Hawaii, USA (BPBM).
RESULTS
SYSTEMATICS
Family CIRRATULIDAE Ryckholt, 1851
Genus Caulleriella Chamberlin, 1919
Caulleriella pacifica E. Berkeley, Reference Berkeley1929
(Figure 1)
Caulleriella viridis var. pacifica E. Berkeley, Reference Berkeley1929: 307.
Caulleriella pacifica: Blake, Reference Blake, Blake, Hilbig and Scott1996: 310–312, fig. 8.18 (and references therein).

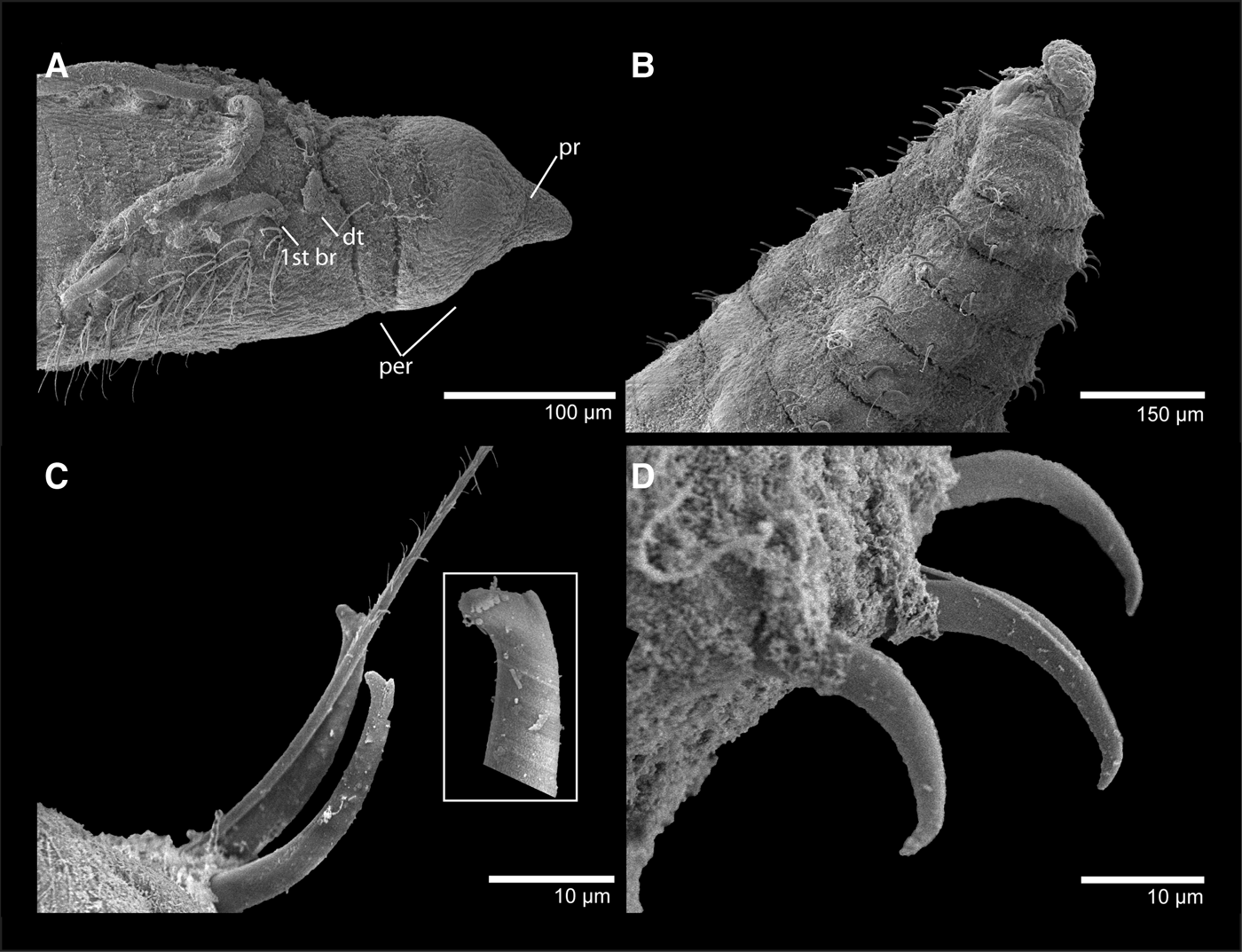
Fig. 1. Caulleriella pacifica. (A) anterior end, dorso-lateral view; (B) posterior end showing pygidium; (C) bidentate hooks and companion capillary; (D) detail of curved hooks from posterior end.
MATERIAL EXAMINED
Pacific Ocean – Apra Harbor, Guam, Mariana Islands, Dec/2010: station B20, 13°26′57.08″N 144°39′35.43″E, 15.5 m (2 complete, BPBM-R3652); station B30, 13°26′52.1″N 144°39′35.43″E, 15.8 m (1 complete, BPBM-R3653); station B16, 13°26′50″N 144°39′35.43″E, 17.4 m (2 complete on SEM stub).
DESCRIPTION
Complete specimens 1.8–8 mm long, 0.1–0.25 mm wide for 48–89 chaetigers. Body slightly rounded dorsally and ventrally flattened; posterior 10–20 segments with shallow ventral groove. Thorax indistinct from abdomen. Posterior end tapering before pygidium; pygidium broad ventral disk with dorsal anal aperture. Colour in alcohol white to pale yellow.
Prostomium short, conical, as long as two anterior chaetigers; a pair of black, small, rounded eyespots present, nuchal organs elongated, latero-dorsal. Peristomium with two annuli, first annulus two times longer than second, dorsally inflated. Chaetiger 1 elongate and bearing dorsal tentacles and first pair of branchiae, postero-lateral to dorsal tentacles.
Notopodia and neuropodia widely separated. Chaetae simple capillaries and bidentate hooks. Notopodial bidentate hooks from chaetiger 14–23, 2–3 bidentate hooks with two companion capillaries; hooks decreasing in number to 1–2 hooks and companion capillaries present on very posterior end chaetigers. Neuropodial bidentate hooks from chaetiger 1, numbering four (rarely five) bidentate hooks and 3–4 companion capillaries; bidentate hooks not decreasing in number posteriorly but accompanied by single companion capillary.
MGSP
No distinct staining reaction observed; all specimens staining uniformly with a light green.
DISTRIBUTION
This species has been previously recorded from Puget Sound and Western Canada (Berkeley & Berkeley, Reference Berkeley and Berkeley1950; Banse & Hobson, Reference Banse and Hobson1968) and newly recorded from Guam in the western Pacific.
REMARKS
The specimens from Apra Harbor, Guam are very similar to those described from Puget Sound by Blake (Reference Blake, Blake, Hilbig and Scott1996) in regards to shape of the anterior end, segmental origin of neuropodial hooks and shape of the pygidium. Specimens from Guam presented segmental origin of notopodial hooks from chaetigers 14–23 while specimens described in Blake (Reference Blake, Blake, Hilbig and Scott1996) had notopodial hooks from chaetiger 50. Also, the specimens from Guam had companion capillaries in posterior neuropodia while Blake (Reference Blake, Blake, Hilbig and Scott1996) reports these to be absent. These morphological differences are herein regarded as ontogenetic because the specimen described in Blake (Reference Blake, Blake, Hilbig and Scott1996) had 240 chaetigers while the material from Guam had up to 89 chaetigers. Several studies have shown that the chaetal distribution in cirratulids varies with development (e.g. Blake, Reference Blake, Blake, Hilbig and Scott1996; Magalhães & Bailey-Brock, Reference Magalhães and Bailey-Brock2010).
Although the segmental origin of notopodial hooks in larger specimens of C. pacifica seems to occur more posteriorly, a juvenile specimen with 48 chaetigers also presented the segmental origin of neuropodial hooks on chaetiger 1.
Genus Chaetozone Malmgren, 1867
Chaetozone flagellifera Gallardo, Reference Gallardo1968
(Figure 2)
Chaetozone flagellifera Gallardo, Reference Gallardo1968: 105, Pl. XLVII, figs. 7–8, Pl. XLVII, ×figs. 1–4.

Fig. 2. Chaetozone flagellifera. (A) anterior end, dorso-lateral view; (B) posterior end with pygidium; (C–D), Neuropodial aristate chaetae.
MATERIAL EXAMINED
Pacific Ocean − Apra Harbor, Guam, Mariana Islands, Dec/2010: station B3, 13°26′46.08″N 144°39′44.34″E, 14.6 m (3 on stub, USNM 1251835; 1 in ethanol, USNM 1251834).
DESCRIPTION
Complete specimen 12 mm long, 0.4 mm wide for 108 chaetigers and an anterior fragment 5.5 mm long, 0.6 mm wide for 34 chaetigers. Body elongate divided in two regions; thoracic region of about 15 chaetigers, flattened dorsal-ventrally, widest at chaetiger 15; abdominal region rounded dorsally and flattened ventrally. Body white to pale yellow in alcohol. Posterior end segments slightly inflated with a shallow ventral groove; pygidium with small ventral lobe and anal aperture placed dorso-terminally.
Prostomium conical, as long as 3–4 anterior segments, with a pair of small postero-lateral nuchal organs, eyespots lacking. Peristomium with three sub-equal annuli, as long as 5–6 anterior chaetigers; third peristomial annulus slightly inflated and projecting towards chaetiger 1. Branchial filaments arising on posterior end of peristomium, a single pair per segment and only observed on anterior third. A pair of feeding tentacles present on posterior end of chaetiger 1, behind peristomial elevation.
Notopodium and neuropodium clearly separated anteriorly but with smaller gap posteriorly. Thoracic region with fimbriated capillaries only; capillaries of similar length. Neuropodial aristate hooks begin from chaetiger 15 or 17, usually 4–6, decreasing in number to three in posterior chaetigers; companion capillaries few and shorter than respective capillaries on notopodia. Modified chaetae absent on notopodia; capillaries on posterior end less numerous but of similar length to those in anterior chaetigers.
MGSP
Distinctive staining reaction leaving posterior end of peristomium stained, segmental region of thorax stained laterally and ventrally; parapodial tori not stained. Notopodial region around insertion point of branchial filaments stained with dark green speckles. Mid- and posterior segments with green lateral green speckles on parapodial region.
DISTRIBUTION
This species was originally described from Nha Trang, south Vietnam and newly recorded to Apra Harbor, Guam.
REMARKS
The species Chaetozone flagellifera is unique in the genus by lacking modified chaetae in notopodia and by having aristate chaetae in mid- and posterior neuropodial segments. The taxonomic placement of this species is questionable since Chaetozone is currently defined as having unidentate spines arranged in cinctures (Blake, Reference Blake, Blake, Hilbig and Scott1996). The modified chaetae in C. flagellifera are unidentate sigmoid hooks with a distally attached arista and are never arranged in cinctures. Tharyx retieri Lechapt, 1994, described from off Morocco, also has aristate chaetae but these are restricted to notopodia while pseudocompound chaetae are present in neuropodia. Blake (Reference Blake, Blake, Hilbig and Scott1996) felt that T. retieri should be included in Chaetozone due to the nature of the spines and origin of the branchiae in relation to feeding tentacles. Uebelacker & Johnson (Reference Uebelacker and Johnson1984) also report on an undescribed cirratulid species (as Genus A) as having aristate chaetae from chaetiger 6–8 in neuropodia and from chaetiger 12 in notopodia. Phylogenetic analyses are necessary in order to understand the placement of species with aristate chaetae among the bitentaculate genera.
Genus Monticellina Malmgren, 1867
Monticellina lueldredgei, sp. nov.
(Figures 3 and 4)

Fig. 3. Monticellina lueldredgei, sp. nov. (A) anterior end, dorso-lateral view; (B) anterior end, lateral view; (C) posterior end with pygidium; (D), modified capillaries from posterior neuropodia.

Fig. 4. Monticellina lueldredgei, sp. nov. (A) anterior end, dorso-lateral view; (B) posterior end with pygidium; (C) detail thoracic serrated capillaries; (D) short serrated capillaries from posterior end.
MATERIAL EXAMINED
TYPE MATERIAL
Holotype: Pacific Ocean – Apra Harbor, Guam, Mariana Islands, Dec/2010, station B11, 13°26′46.08″N 144°39′35.22″E, 17.1 m (USNM 1251836).
Paratypes: same locality and date as holotype (4 complete, USNM 1251837; 3 incomplete, BPBM-R3654; 2 complete on SEM stub, USNM 1251838).
NON-TYPE MATERIAL
Pacific Ocean − Apra Harbor, Guam, Mariana Islands, Dec/2010: station B3, 13°26′46″ N 144°39′44.34″ E, 14.6 m (5 incomplete, BPBM-R3655).
DESCRIPTION
Holotype 12 mm long, 0.2 mm wide for 81 chaetigers. Complete paratypes 2.2–6.0 mm long, 0.1–0.15 wide for 45–53 chaetigers. Thoracic chaetigers distinct from abdominals; thorax 8–14 crowded anterior segments, dorsally and ventrally expanded with shallow dorsal and ventral grooves; abdominal segments slightly longer than wide, sometimes beadlike, and not tapering before pygidium. Posterior end weakly expanded and ending abruptly into pointed pygidium with terminal anus. Colour in alcohol white to pale yellow.
Prostomium short, conical, as long as 3–4 anterior chaetigers; eyes absent, nuchal organs not observed; peristomium long with two annuli, second annulus twice longer than first one. First pair of branchiae postero-lateral to dorsal tentacles on anterior border of chaetiger 1. Dorsal tentacles arising on posterior end of peristomium, second pair of branchiae on posterior margin of chaetiger 1.
Notopodia and neuropodia with small gap. Chaetae all capillaries of two types; thoracic chaetigers with 4–6 simple capillaries per fascicle. Short capillaries with basally expanded blades and sawtooth edge from anterior abdominal notopodia and neuropodia (chaetigers 10–12; Figure 4C). Anterior abdominal notopodia with 4–5 modified capillaries reducing to 2–3 posteriorly; anterior abdominal neuropodia with six modified capillaries reducing to three posteriorly. Notopodial and neuropodial modified capillaries similar in shape but neuropodial ones slightly shorter (Figure 4C). Companion capillaries absent.
MGSP
Specimens easily recognized by the distinct staining reaction on thoracic region, taking up from chaetigers 4–10 and leaving dorsal region unstained. The rest of the body was unstained except for the light staining of the parapodial tori.
ETYMOLOGY
This species is named after the late Dr Lucius G. Eldredge, an invertebrate zoologist of the Bishop Museum and former faculty member at the University of Guam from 1965 to 1987. Dr Eldredge was always enthusiastic and supportive of our polychaete research in the western Pacific region since 1981.
DISTRIBUTION
This species is only known from Apra Harbor, Guam.
REMARKS
Monticellina lueldredgei, sp. nov. resembles M. cryptica Blake, Reference Blake, Blake, Hilbig and Scott1996 and M. acunai Dean & Blake, Reference Dean and Blake2009 by the shape of the modified capillaries with widely spaced barbs. Modified capillaries in M. lueldredgei, sp nov. appear on anterior abdominal chaetigers, 10–12 in both notopodia and neuropodia while in M. acunai in notopodia 22–34 and neuropodia 21–33. The methyl green staining patterns are very similar in all three species, staining a glandular area on the ventral part of thoracic segments. In M. lueldredgei, the distinct bands are darker from chaetigers 4–10 while in M. acunai the bands are darker in chaetigers 10–16 (Dean & Blake, Reference Dean and Blake2009) and in M. cryptica it is reported to be darker on posterior one-third of thorax (Blake, Reference Blake, Blake, Hilbig and Scott1996). Magalhães & Bailey-Brock (Reference Magalhães and Bailey-Brock2013) report M. nr. cryptica for Hawaii but point out some slight morphological differences from the type specimens described in Blake (Reference Blake, Blake, Hilbig and Scott1996). The material from Guam differs most noticeably from the Hawaiian material by the segmental origin of the modified chaetae (chaetigers 18–20 in M. nr. cryptica) and by the absence of a distinctive methyl green staining pattern in the Hawaiian specimens.
ACKNOWLEDGEMENTS
This is contributed paper WRRC-CP-2015-07 of the Water Resources Research Center and contributed paper 2014-18 of the Department of Biology, University of Hawaii at Manoa, Honolulu. Collections within Apra Harbor were made by CSA International. The staff at the Wormlab in the Department of Zoology at the University of Hawaii at Manoa processed the samples collected from Apra Harbor. Tina M. Carvalho (BEMF, University of Hawaii) provided guidance with SEM procedures. This research was funded by a grant from CSA International administered through the Water Resource Research Center (WRRC). Jonathon Roberts, UH Manoa Office of Technology Transfer & Economic Development (OTTED), prepared the contract with CSA International. We thank two anonymous reviewers for the suggestions that improved a previous version of this manuscript.