Introduction
Pyrochlore is one of the principal sources of niobium and is found primarily in carbonatites and associated alkaline rocks such as pyroxenites, ijolites, nepheline syenites and phoscorites (Mariano, Reference Mariano1989; Chakhmouradian et al., Reference Chakhmouradian, Reguir, Kressall, Crozier, Pisiak, Sidhu and Yang2015; Mitchell, Reference Mitchell2015). The carbonatite-hosted Brazilian deposits of Araxá and Catalão-II and the Canadian St. Honoré deposit contribute 99% of the world Nb-production (Mitchell, Reference Mitchell2015). Pyrochlore is also found in alkaline–peralkaline granites, saturated syenites, albitites and fenites (Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002).
The general formula of the pyrochlore-group mineral is A 2B 2X6Y where: A = cubic coordination of Ca2+, Na+, K+, Sr2+, Ba2+, Th4+, U4+, Pb2+, Fe2+, Bi3+ and REE (rare earth elements) together with vacant sites; B = octahedral coordination of Nb, Ta, Ti, Zr, Sb5+, W, Fe3+, Al; X = O, OH–, F; and Y = O, OH–, F, H2O and vacancies (Hogarth, Reference Hogarth1977; Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010). Silicon is common in pyrochlore-group minerals, although its structural role remains ambiguous (see below). Three sub-groups are defined on the basis of the atomic proportions of Nb, Ta and Ti. These are: pyrochlore (Nb + Ta > 2Ti; Nb > Ta); microlite (Nb + Ta > 2Ti; Ta > Nb); and betafite (2Ti > Nb + Ta) (Hogarth, Reference Hogarth1977). In the recent Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) classification (Hatert and Burke, Reference Hatert and Burke2008; Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010), the pyrochlore group is given the status of a super group and consequently former sub-groups have been assigned to group status on the basis of the dominant-valency rule.
Regarding the crystallo-chemical role of Si in pyrochlore it has been considered by some mineralogists that Si should be considered as B-site cation (Lumpkin and Mariano, Reference Lumpkin and Mariano1995; Williams et al., Reference Williams, Wall, Woolley and Phillipo1997; Uher et al., Reference Uher, Černy, Chapman, Hatar and Miko1998; Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002). In contrast, others have suggested that the Si is not bound structurally and is present as impurities in an amorphous or dispersed state (Voloshin et al., Reference Voloshin, Pakhomovsky, Pushcharovsky, Nadezhina, Bakhchisaraitsev and Kobyashev1989; Sorokhtina et al., Reference Sorokhtina, Kogarko and Shpachenko2010). Experimental studies have shown that in some instances only a minor fraction (30–50%) of the total Si occupies the B-site, whereas a more significant portion (50–70%) occurs in metamictised areas (Bonazzi et al., Reference Bonazzi, Bindi, Zoppi, Capitani and Olmi2006). A recent structural study has shown that tetrahedrally coordinated Si is present at both of the A- and B-sites affected by metamictisation (Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014).
Si-rich pyrochlore have been reported from: the Bingo carbonatite, Congo; the Prašiviá massif, Slovakia; Mont Saint-Hilaire, Canada; the Gremiakha-Vyrmes massif, Kola Peninsula, Russia; and the Mariupol massif, Ukraine (Lumpkin and Mariano, Reference Lumpkin and Mariano1995; Williams et al., Reference Williams, Wall, Woolley and Phillipo1997; Uher et al., Reference Uher, Černy, Chapman, Hatar and Miko1998; Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002; Sorokhtina et al., Reference Sorokhtina, Kogarko and Shpachenko2010; Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014).
Pyrochlores in carbonatites are usually non-stoichiometric and never attain the ideal end-member composition of A 2B 2X 6Y. Their composition varies from [A](Ca, Na, U, Th, REE, Ba, Sr)2–x [B](Nb, Ti, Ta, Zr, Fe3+)2O6(OH,F)1–y in primary pyrochlore, enriched in Na, Ca and F to [A](Ba,Sr,REE,Pb,K,Ca,U,Th)Σ«2[B](Nb,Ti,Ta,Zr,Fe3+,Si)2(O,OH)6(OH,F)Σ«1⋅zH2O in late-stage pyrochlore (Hogarth et al., Reference Hogarth, Williams and Jones2000; Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004; Melgarejo et al., Reference Melgarejo, Costanzo, Bambi, Gonçalves and Neto2012; Chakhmouradian et al., Reference Chakhmouradian, Reguir, Kressall, Crozier, Pisiak, Sidhu and Yang2015; Mitchell, Reference Mitchell2015; Mitchell et al., Reference Mitchell, Wahl and Cohen2020). This compositional variation implies an increase in Ba, Sr, REE, Pb, Si, H2O and vacancies resulting in deviation from ideal stoichiometry associated with the progressive alteration of magmatic pyrochlore (Lumpkin and Ewing, Reference Lumpkin and Ewing1995; Lumpkin and Mariano, Reference Lumpkin and Mariano1995; Williams et al., Reference Williams, Wall, Woolley and Phillipo1997; Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002; Chakhmouradian et al., Reference Chakhmouradian, Reguir, Kressall, Crozier, Pisiak, Sidhu and Yang2015; Mitchell, Reference Mitchell2015). Pyrochlore-group minerals are excellent indicators for characterising the ortho-, late- and post-magmatic processes affecting carbonatites and the associated rocks (Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004; Mitchell, Reference Mitchell2015; Walter et al., Reference Walter, Parsapoor, Braunger, Marks, Wenzel, Martin and Markl2018; Giovannini et al., Reference Giovannini, Mitchell, Neto, Moura, Pereira and Porto2020).
In India, 26 carbonatite complexes have been reported (Krishnamurthy, Reference Krishnamurthy2019). Many of these complexes, such as Amba Dongar (Gujarat); Sevattur (Tamil Nadu); Beldih (West Bengal); and Samchampi (Assam) contain pyrochlore (Doroshkevich et al., Reference Doroshkevich, Viladkar, Ripp and Burtseva2009; Viladkar and Bismayer, Reference Viladkar and Bismayer2010, 2014). In this work, we report the composition and proposed genesis of diverse U-, Pb-, Ba- and Si-rich pyrochlores from the Sevattur carbonatite complex. Previously, U-rich pyrochlore with a maximum concentration of 18 wt.% UO2 has been described from this complex by Viladkar and Bismayer (Reference Viladkar and Bismayer2014). We also describe the processes of pyrochlore alteration involving U and Nb leaching coupled with Ba and Si enrichment, leading to the formation of the rare Nb-Ti-silicates belkovite and baotite. Belkovite has been reported previously from the Vuorijärvi and Seblyavr carbonatites of the Kola Peninsula, Russia (Voloshin et al., Reference Voloshin, Subbotin, Pakhomovsky, Bakhchisaraytsev, Yamnova and Pushcharovsky1990; Sorokhtina et al., Reference Sorokhtina, Voloshin and Pakhomovsky1998), and here we report a third occurrence from the Sevattur carbonatite complex. Additionally, we provide charge-balanced end-members for all the compositionally diverse pyrochlore-group minerals, belkovite and baotite using the ‘Site Total Charge’ method (Bosi et al., Reference Bosi, Biagioni and Oberti2019a,Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Millsb). This approach makes it possible to simplify the composition of pyrochlore-group minerals and is helpful in understanding the reactions producing the pyrochlore alteration assemblages observed at Sevattur.
Regional geology and petrography
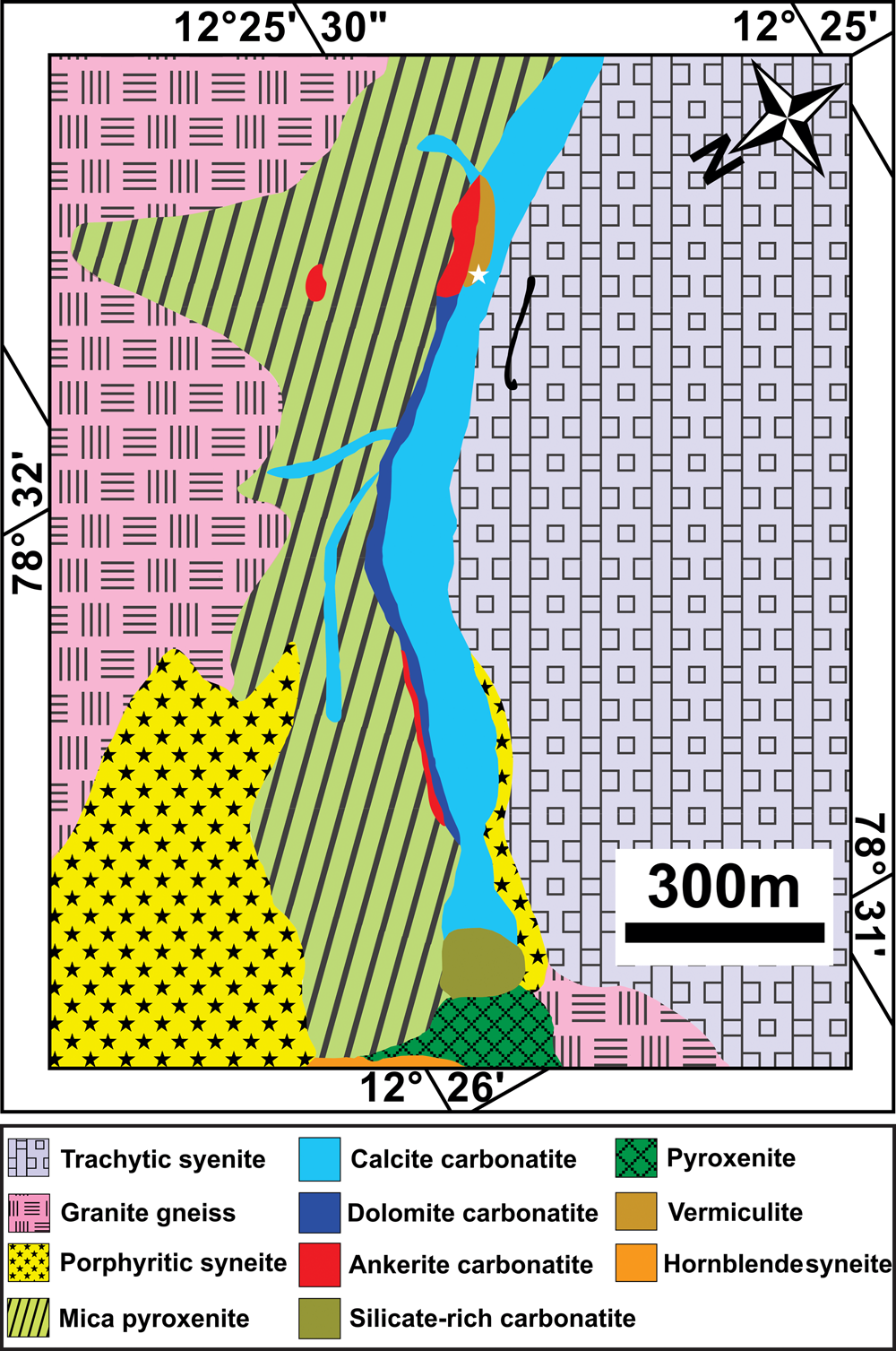
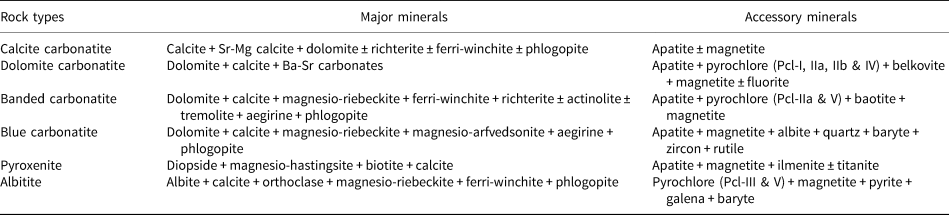
The Neoproterozoic Sevattur carbonatite complex (12.42°N, 78.52°E) occurs as an arcuate-shaped body (~1600 × 300) m2 within the NE–SW trending discontinuous Dharmapuri Shear Zone. The complex is composed of different generations of carbonatites together with pyroxenites, diverse syenites, fenites and vermiculite-bearing mica pyroxenite (Fig. 1) (Udas and Krishnamurthy, Reference Udas and Krishnamurthy1970; Krishnamurthy, Reference Krishnamurthy1977; Subramanium et al., Reference Subramaniam, Viladkar and Upendran1978; Viladkar and Subramanium, Reference Viladkar and Subramanian1995; Schleicher et al., Reference Schleicher, Kramm, Pernicka, Schidlowski, Schmidt, Subramanian, Todt and Viladkar1998; Ackerman et al., Reference Ackerman, Magna, Rapprich, Upadhyay, Krátký, Čejková, Erban, Kochergina and Hrstka2017; Schleicher, Reference Schleicher2019). The carbonatites are represented predominantly by calcite carbonatite, followed by dolomitic carbonatite and silica-rich carbonatite with minor occurrences of ankerite carbonatite (Fig. 1) (Viladkar and Subramanian, Reference Viladkar and Subramanian1995; Viladkar and Bismayer, Reference Viladkar and Bismayer2014; Ackerman et al., Reference Ackerman, Magna, Rapprich, Upadhyay, Krátký, Čejková, Erban, Kochergina and Hrstka2017). The carbonatites were emplaced during multiple intrusions as evidenced by the presence of different apatite and calcite generations hosted in the calcite and dolomite carbonatites (Schleicher, Reference Schleicher2019). On the basis of texture, two varieties of calcite carbonatite are identified: a fine-grained variety with an average grain size between 50–500 μm; and a relatively coarse-grained variety consisting dominantly of large (>0.1–1 cm) euhedral calcite crystals. In both varieties, minor dolomite is present together with accessory apatite and magnetite. Dolomite carbonatite is medium-to-coarse grained (>0.1–10 mm) and composed of, in order of decreasing modal abundance: dolomite; calcite; apatite; minor Ba-Sr carbonates; and accessory pyrochlore (Fig. 2a,b) (Table 1).
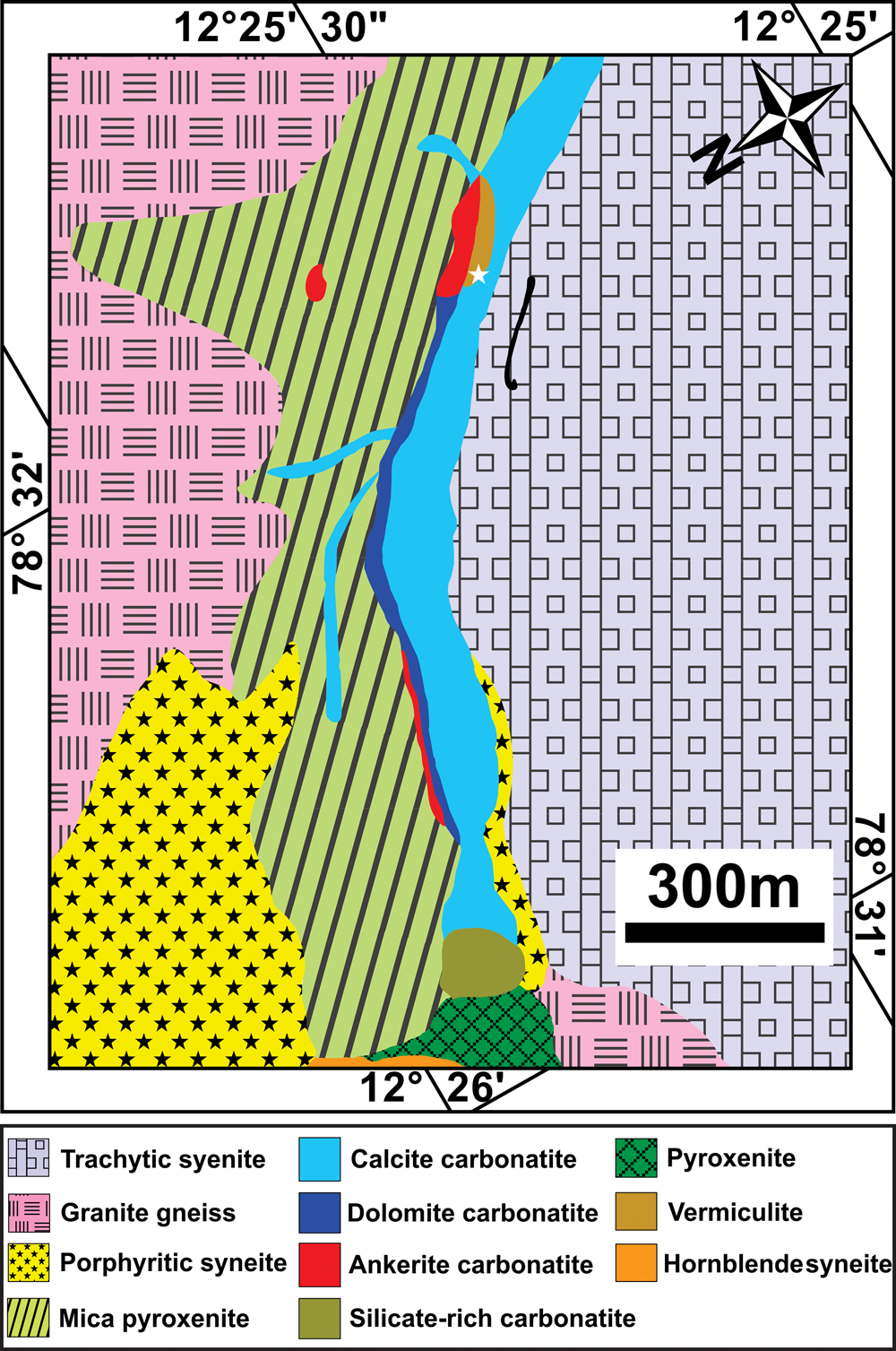
Fig. 1. (a) General geological map of a part of the Sevattur carbonatite complex illustrating the relations among the lithological units. The vermiculite mine is marked with a white star (modified after Ramasamy et al., Reference Ramasamy, Gwalani and Subramanian2001).
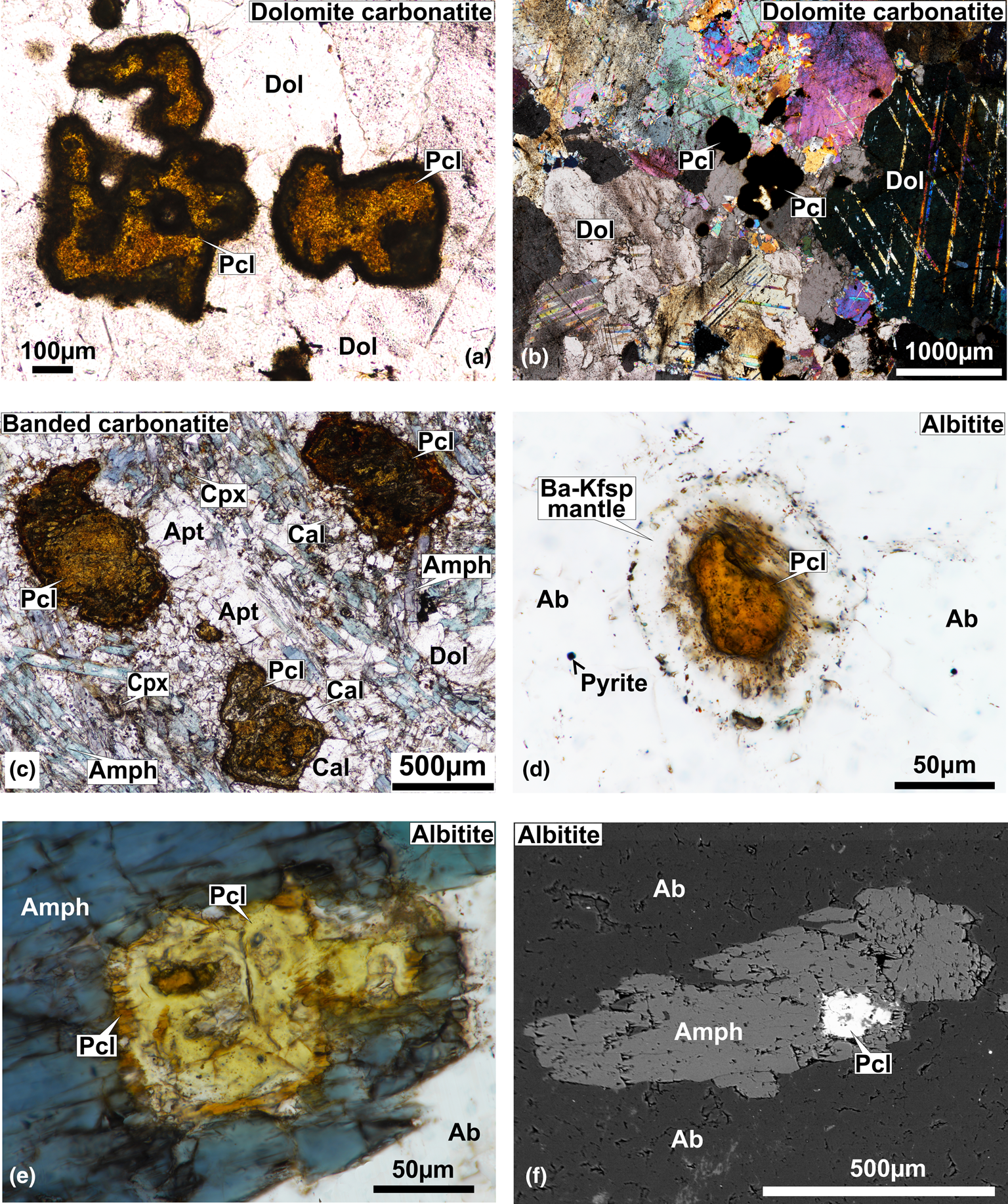
Fig. 2. Transmitted light (a–e) and back-scattered electron (BSE) images (f) of pyrochlore and associated minerals from Sevattur. Photomicrographs of anhedral–subhedral pyrochlore (Pcl) grains within the dolomite (Dol) matrix in dolomite carbonatite in plane polarised light (PPL) (a) and cross polarised light (b). (c) PPL photomicrograph illustrating clusters of pyrochlore grains associated with calcite (Cal), clinopyroxene (Cpx), amphibole (Amph) and apatite (Apt) in a dolomite (Dol) matrix in banded carbonatite. Note the late-formed, elongated and acicular blue–green amphiboles (Amph). (d) Albitite-hosted pyrochlore crystal with a thick orbicular mantle of Ba-rich potassium feldspar (Ba-Kfsp) (PPL image). (e) An amphibole surrounding an inclusion of pyrochlore with a lemon-yellow core and orange coloured rim, in albitite (PPL image). (f) Back-scattered electron (BSE) image of the same amphibole and pyrochlore crystals as in (e).
Table 1. Mineral assemblages in different lithological units.
Field observations indicate the presence of two varieties of silicate-rich carbonatite: a banded variety with alternate bands of carbonates and silicates (aegirine, magnesio-riebeckite, ferri-winchite, richterite and phlogopite) (banded carbonatite); and a blue-coloured variety (blue carbonatite) characterised by the abundant blue sodic-amphiboles and aegirine (Fig. 2c) (Table 1). The banded carbonatite is devoid of metamorphic features such as gneissosity, recrystallisation bands, and strained grains. Therefore, the banding observed is either a magmatic or a rheomorphic feature but certainly not related to post-formational metamorphic events. In both the varieties of silicate-rich carbonatite, apatite and magnetite are the typical accessory phases, whereas pyrochlore is restricted to the banded carbonatite.
The origins of these silicate-rich carbonatites remain undetermined. Similar assemblages can be found in many carbonatites which have not been metamorphosed and which are a direct consequence of magmatic differentiation from a parent carbonated silicate melt, e.g. Cargill, Canada (Pressacco, Reference Pressacco2001; Rukhlov and Bell, Reference Rukhlov and Bell2010); Belaya Zima, Russia (Doroshkevich et al, Reference Doroshkevich, Veksler, Klemd, Khromova and Izbrodin2017); and Fen, Norway (Andersen, Reference Andersen1986). However, a similar assemblage could also form during multiple stages of carbonatitic magmatism as a consequence of extensive fenitisation (Elliot et al., Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018). To avoid any genetic inferences and hypothetical relationships with the calcite and dolomite carbonatites we use the terminology ‘banded and blue carbonatite’ for these silicate-rich carbonatites.
During field investigations, we found freshly exposed albitite veins cross-cutting the blue carbonatite in the western and south-eastern walls of the vermiculite mine (12.422°N, 78.528°E) (Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021). These veins are of variable thickness (2–12 cm) and do not have surficial exposures. The veins are medium or coarse grained and composed dominantly of albite (~95 vol.%). The albite exhibits a bimodal distribution in grain size. Smaller euhedral-to-subhedral grains (~300 μm) form the majority of the matrix together with large, elongated laths (>0.5–1 cm) of macrocrysts. Other minerals present are: calcite; magnesio-riebeckite; ferri-winchite; pyrochlore; baryte; phlogopite; magnetite; and pyrite (Fig. 2e,f). Calcite and biotite occupy the interstices among the smaller albite grains and also occur as fracture fillings in the macrocrysts. Euhedral-to-subhedral blue amphiboles (100–300 μm) occur as discrete grains or in clusters within the albite matrix.
Experimental methods
The compositions of pyrochlore, belkovite and baotite were determined using a CAMECA SX100 electron microprobe (EMP) at the Institute Instrumentation Centre (IIC), Indian Institute of Technology Roorkee. This instrument is equipped with four wavelength dispersive (WD) spectrometers and one energy dispersive (ED) spectrometer. Analyses were undertaken with a 20 kV accelerating voltage, 10 nA beam current and a focussed 1–10 μm beam diameter depending on the size of the mineral analysed. Prior to EMP data collection, the polished sections were examined in transmitted light microscope. Selection of analytical points was guided by back-scattered electron (BSE) images. The following X-ray lines and standards were used for quantification of the major elements: Nb-metal (NbLα), Ta metal (TaMα), UO2 (UMα), ThO2 (ThMα), zircon (ZrLα), jadeite (Si-Kα), rutile (TiKα), jadeite (AlKα), baryte (BaLα), celestine (SrLα), galena (PbKα), hematite (FeKα), rhodonite (MnKα), periclase (MgKα), diopside (CaKα), jadeite (NaKα), orthoclase (KKα), fluorite (FKα) and synthetic REE glass (LaLα, CeLα, PrLα, NdLα, SmLα, EuLα, GdLα, TbLβ, DyLα, HoLα, ErLα, TmLα, YbLα and LuLα). The precision of the analyses from the repeated analysis of standards suggests that standard deviation is less than ± 0.5% for major, and less than 0.1% for minor elements. Peak counting times for major elements were 10–20 s and 30–60 s for minor elements (half of the values for the background), with shorter times for volatile elements.
Additional analyses of pyrochlore, baotite and belkovite were carried out at Lakehead University, Canada by quantitative X-ray energy dispersive spectrometry (EDS) using a Hitachi FE-SU70 scanning electron microscope equipped with AZtec software (Oxford Instruments). An accelerating voltage of 20 kV and beam current of 0.3 nA were used to collect X-ray spectra for 120 s for all the major elements. For F, a CaF2 standard was used with a collection time of 250 s. Analytical standards used for calibration were: apatite (P, Ca); SrTiO3 (Sr, Ti); ThNb4O12 (Th, Nb); Mn-rich fayalite (Mn, Fe, Mg and Si); wollastonite (Si, to ratify the excess silica in belkovite); jadeite (Na, Al); benitoite (Ba, Ti); REE phosphate glasses; Ta and U metals (Mitchell and Smith, Reference Mitchell and Smith2017). Any Na-bearing minerals were analysed with a beam rastered over areas ranging from 100 to 1000 μm2 to minimise Na volatilisation.
Pyrochlore – composition and parageneses
On the basis of their composition, two main types of pyrochlore are present at Sevattur. Pyrochlore hosted in dolomite and banded carbonatites are characterised by elevated Ta contents (up to 13.4 wt.% Ta2O5), whereas those present in albitite are essentially Ta poor (<1 wt.% Ta2O5). The silicate-rich blue carbonatite is devoid of pyrochlore. Carbonatite-hosted pyrochlores are relatively large (0.1–1 mm) (Fig. 2a–c), whereas the majority of the albitite-hosted pyrochlores (Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021) are small (<50 μm), and large grains (>100 μm) are seldom present (Fig. 2d–f). The habit of these pyrochlores are variable, ranging from anhedral (Fig. 2a) to subhedral (Fig. 2c). They appear lemon-yellow to orange/brown in plane polarised light (Fig. 2a–f). These pyrochlores are also characterised by a mantle of Ba-bearing potassium feldspar (Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021) (Fig. 2d). However, there are few examples in which this mantle is partly or entirely disrupted. In albitite, pyrochlores are also present as inclusions in amphibole (Fig. 2e,f), implying that these amphiboles postdate pyrochlore formation.
On the basis of texture and compositional variation, we have identified five types of pyrochlore in the carbonatites and albitite: Pcl-I to Pcl-V (Fig. 3a–h).
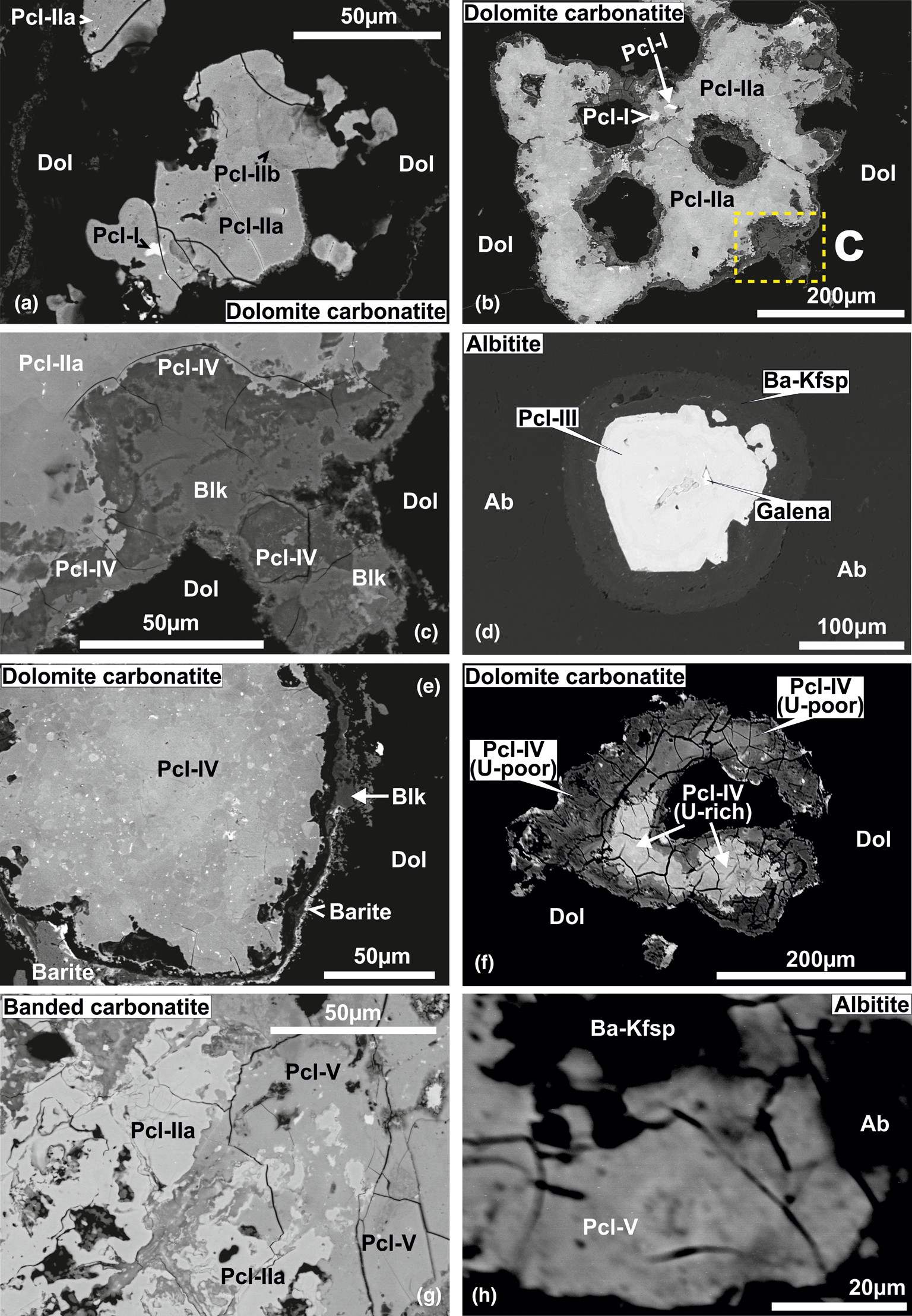
Fig. 3. BSE images illustrating the different textural modes of occurrences of pyrochlore-group minerals at Sevattur. (a) Pb-rich Pcl-I inclusions (high-AZ; AZ: average atomic weight) within U-rich Pcl-IIa (intermediate-AZ). Note that Pcl-IIb is formed by alteration of Pcl-I and Pcl-IIa along the grain margin (low-AZ areas). (b) and (c) Anhedral Pcl-IIa with variable AZ areas illustrating patchy zoning. Note the extensive alteration along the grain margin. (c) Enlarged view of a part of the altered grain margin (dashed lines in (b)); the alteration assemblage of Ba-rich Pcl-IV and belkovite (Blk) formed after Pcl-IIa. (d) A high-AZ U-Ti-rich euhedral Pcl-III crystal with an inclusion of galena, mantled by low-AZ Ba-rich potassium feldspar (Ba-Kfsp). (e) Pcl-IV in association with belkovite (low-AZ) and baryte (high-AZ) along the grain margin in dolomite carbonatite. Note the variable AZ areas in Pcl-IV illustrating patchy zoning. (f) A highly metamictised and fractured Pcl-IV with a relict U-rich core (high-AZ) and U-poor rim (low-AZ) in dolomite carbonatite. (g) An extensively metamictised grain of Pcl-IIa replaced by late-stage Si-rich Pcl-V in banded carbonatite. Note the relict patches of relatively unaltered Pcl-IIa. (h) Partially-disrupted Ba-rich potassium feldspar (Ba-Kfsp) mantle facilitating the formation of Pcl-V in albitite.
Pyrochlore-I
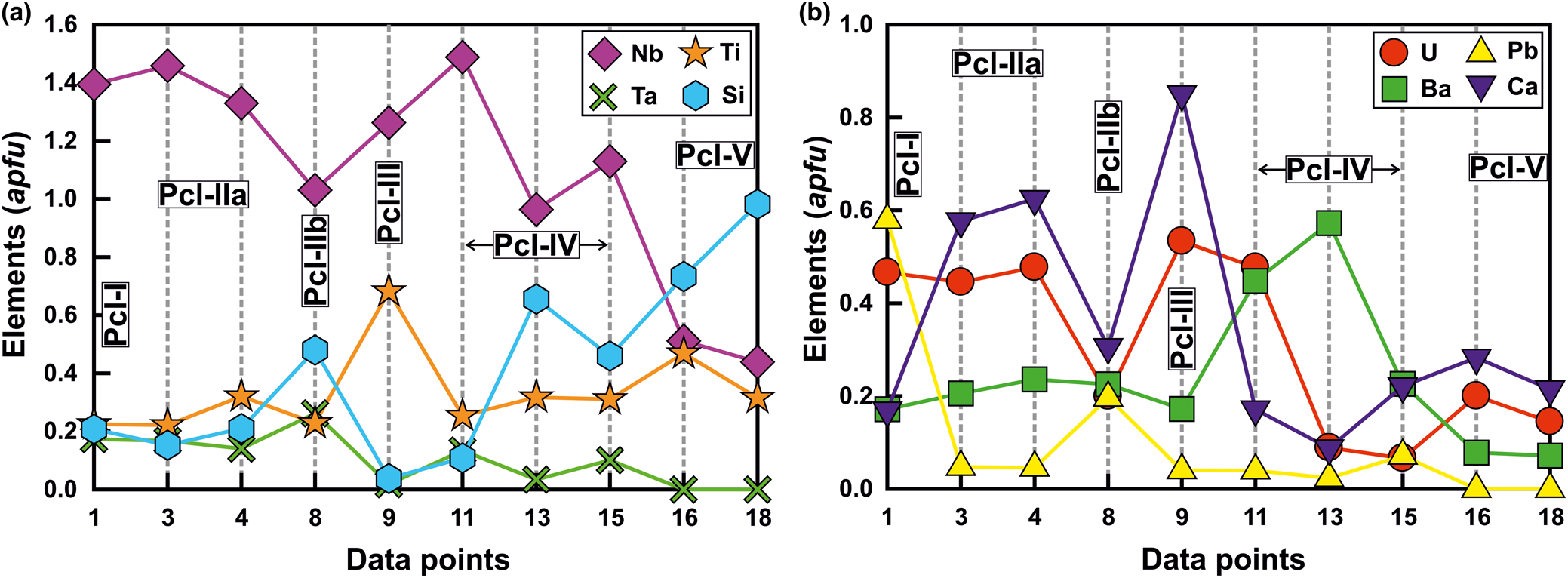
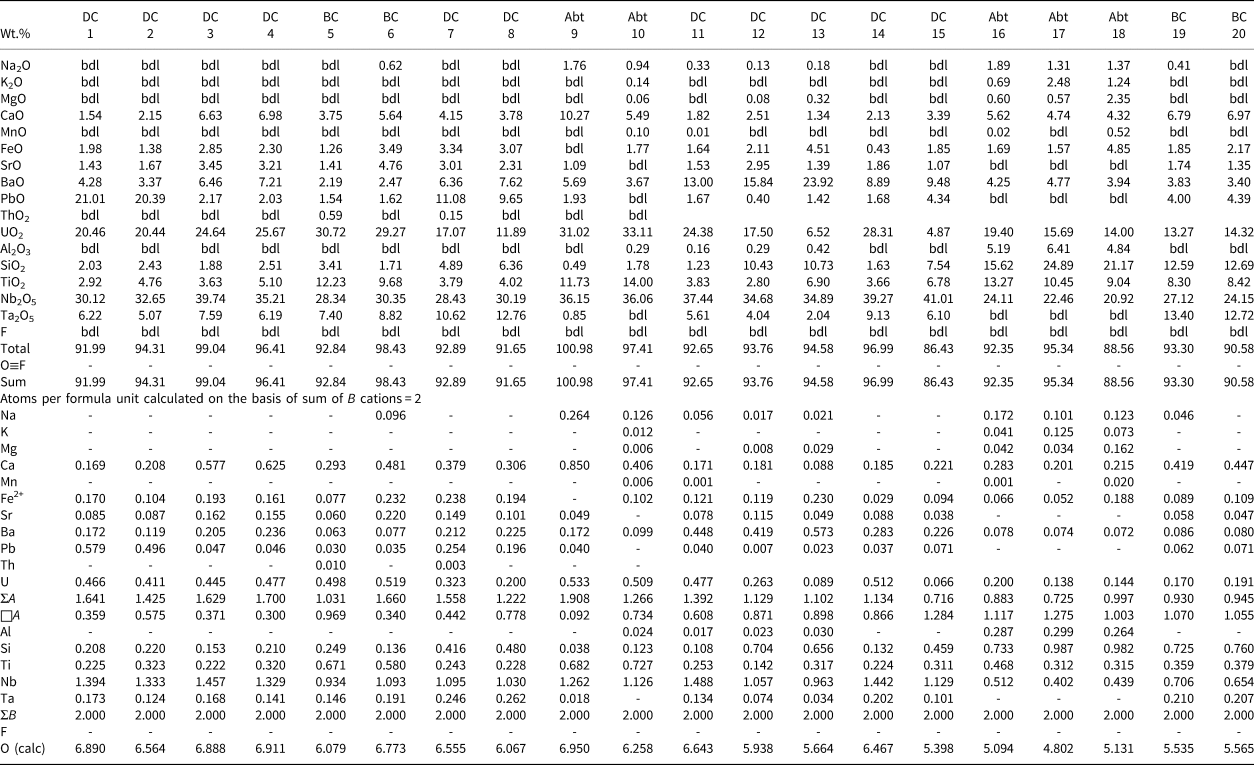
Back-scattered electron images show that Pcl-I occurs exclusively as high-AZ (AZ: average atomic number) inclusions (<5 μm) within Pcl-II (>50 μm) of intermediate-AZ (Fig. 3a,b). Compositionally, Pcl-I is characterised by high Pb [20.4–21.0 wt.% PbO; 0.50–0.58 atoms per formula unit (apfu)], relatively low contents of U (up to 20.5 wt.% UO2), Nb (30.1–32.7 wt.% Nb2O5), Ta (5.1–6.2 wt.% Ta2O5) and Ti (2.9–4.8 wt.% TiO2), with Ca (1.5–2.2 wt.% CaO) (Fig. 4) (Table 2). Pcl-I have minor Ba, Fe and Sr contents.
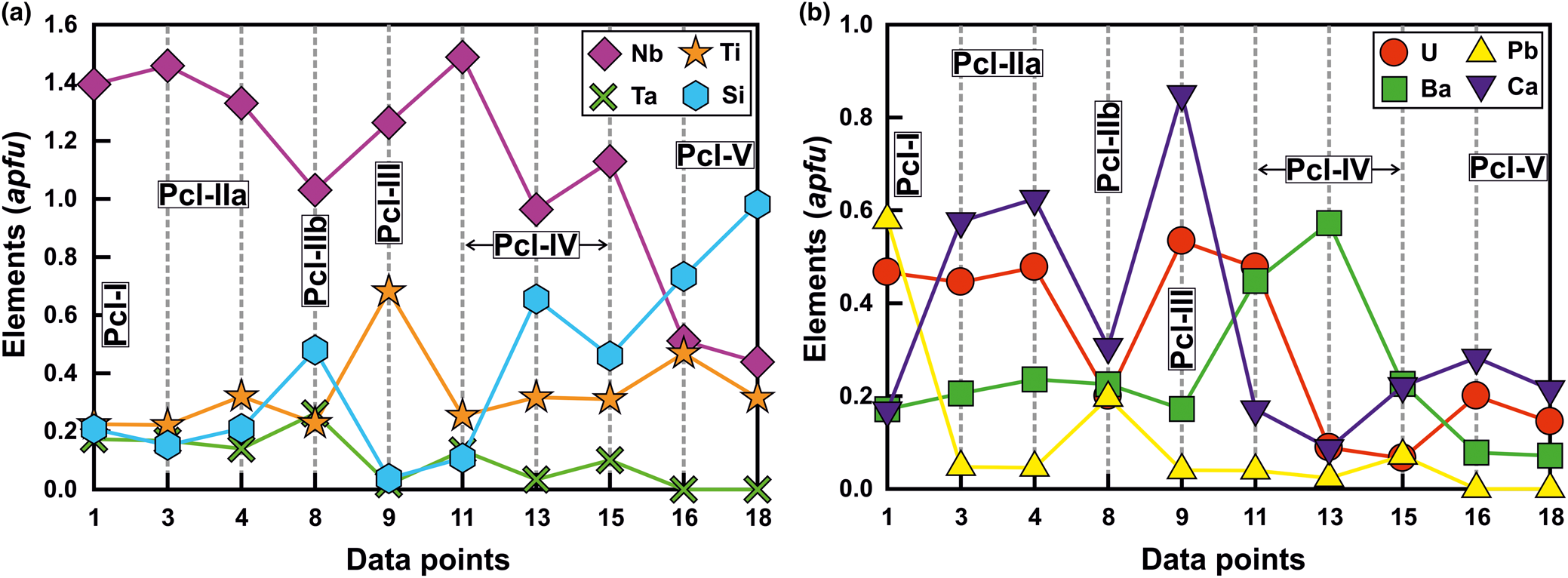
Fig. 4. The compositional variation (apfu) of different pyrochlore types (Pcl-I to Pcl-V) from the Sevattur carbonatite complex illustrating elemental concentrations of B-site (a) and A-site cations (b) respectively. Note that the relatively unaltered pyrochlore (Pcl-IIa and Pcl-III) are rich in Nb, Ca and U compared to the late-formed Pcl-IIb, -IV and -V. The later pyrochlore are essentially Ba- or Ba-Si-enriched.
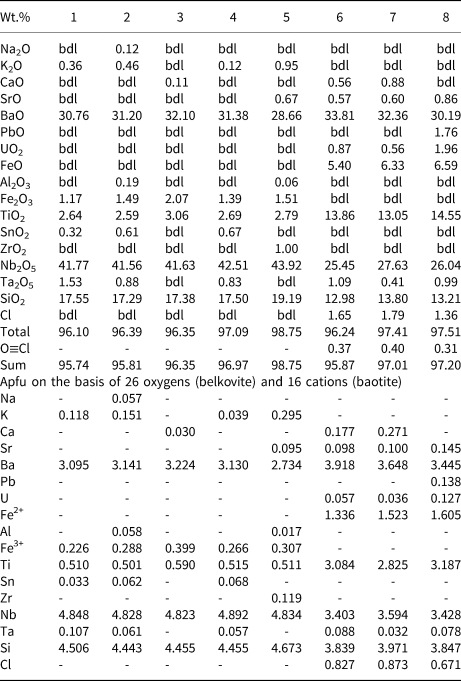
Table 2. Representative pyrochlore compositions.
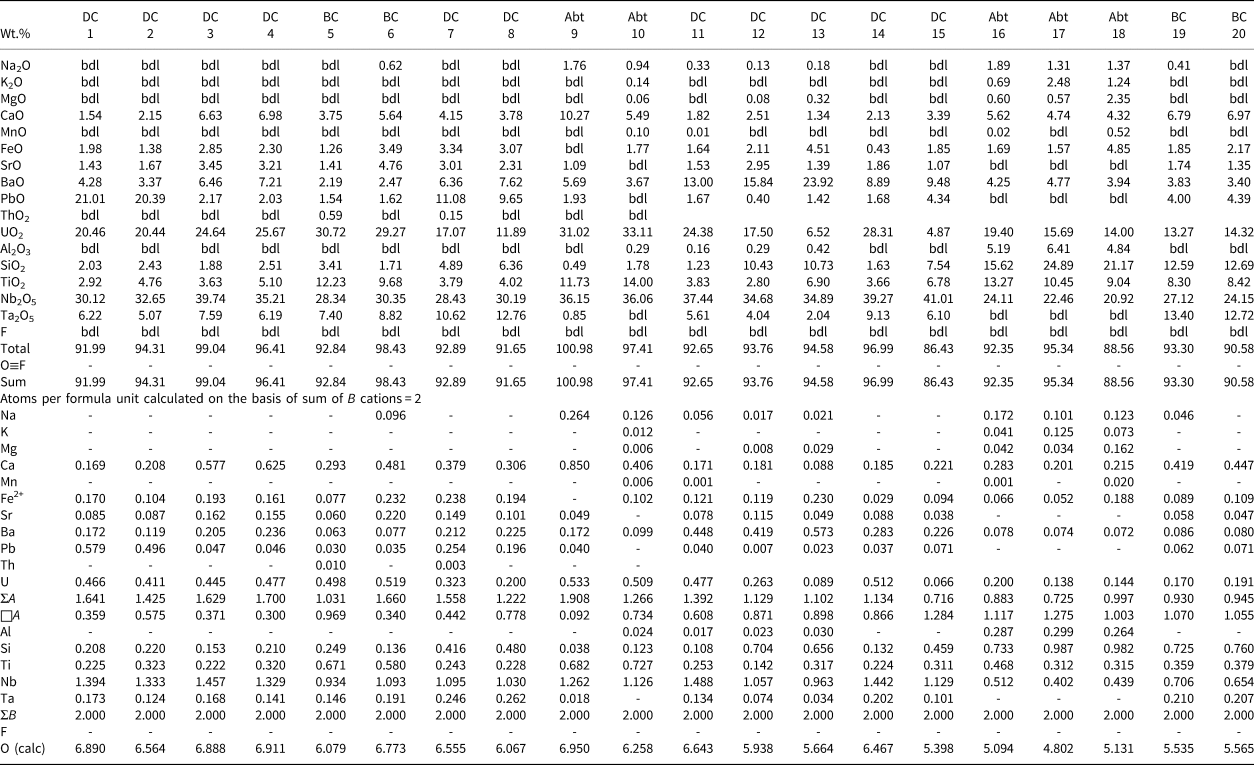
1–2: pyrochlore-I; 3–6: pyrochlore-IIa; 7–8: pyrochlore-IIb; 9–10: pyrochlore-III; 11–15: pyrochlore-IV; 16–20: pyrochlore-V. Compositions 1–9, 14, 15, 19, 20: ED X-ray spectrometry (Lakehead University) and 10–13, 16–18: WD electron microprobe (IIT Roorkee)
bdl: below detection limit
DC: dolomite carbonatite; BC: banded carbonatite; Abt: albitite
Pyrochlore-II
Pcl-II occurs either as hosts to Pcl-I or associated with later generation Pcl-IV and Pcl-V. The Pcl-II is devoid of any BSE-detectable primary zoning and characterised by patchy zoning together with numerous fractures extending from the rim towards the core (Fig. 3a,b). In general, Pcl-II are anhedral and characterised by extensive post-formational alteration resulting in diverse low- and intermediate-AZ zones (Fig. 3a,b). On the basis of compositional variation, Pcl-II is further subdivided into Pcl-IIa and Pcl-IIb (Fig. 3a). The Pcl-IIa is present in dolomite and banded carbonatite, whereas Pcl-IIb is restricted to dolomite carbonatite as low-AZ areas in marginal parts of Pcl-IIa (Fig. 3a). Pcl-IIa, unlike Pcl-I, is characterised by low Pb contents (1.5–2.2 wt.% PbO; 0.03–0.05 apfu) and a higher concentration of U (up to 30.7 wt.% UO2), Nb (28.3–39.7 wt.% Nb2O5), Ta (6.2–8.8 wt.% Ta2O5), Ti (3.6–12.2 wt.% TiO2) and Ca (3.8–7.2 wt.% CaO) (Table 2) (Fig. 4). Pcl-IIa also have minor Ba, Fe and Sr contents. In contrast, Pcl-IIb is relatively poorer in U (11.7–17.1 wt.% UO2), Nb (28.4–30.2 wt.% Nb2O5), Ca (3.8–4.1 wt.% CaO) and Ti (3.8–4.0 wt.% TiO2) with high Ta (up to 12.8 wt.% Ta2O5) and Si (4.9–6.4 wt.% SiO2) contents (Table 2) (Fig. 4).
Pyrochlore-III
Albitite-hosted U-rich pyrochlore (Pcl-III) with Ba-potassium feldspar mantles has been described in detail by Dey et al. (Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021), and only a summary of this paragenesis is included in this work for comparison with pyrochlore occurring in the associated carbonatites. These pyrochlore are restricted to albitite and present in two textural modes: either surrounded by an orbicular mantle of Ba-bearing potassium feldspar; or as an inclusion within the amphibole (Figs 2f, 3d). In places, this mantle is partially disrupted leading to extensive metamictisation together with alteration resulting in the formation of U-poor Si-rich pyrochlore (Pcl-V). Compositionally, Pcl-III (comparable to ‘Pcl-II’ of Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021) is characterised by a very high U content (31–33.1 wt.% UO2; 0.49–0.53 apfu) compared to other pyrochlore (Table 2). Pcl-III is also enriched in Ti, Ca and Na (11.7–14.0 wt.% TiO2; 5.5–10.3 wt.% CaO; 0.9–1.8 wt.% Na2O) (Table 2) and depleted in Ba (3.7–5.7 wt.% BaO) compared to Pcl-I and Pcl-II, whereas the Nb content is comparable to that of Pcl-II. Minor amounts of Si, Pb, Sr and Fe are also present in Pcl-III (Table 2). It is noteworthy that the Pcl-III is relatively less-altered compared to other pyrochlore types and the effects of metamictisation appear to be minimal as evidenced in BSE images (Fig. 3b).
Pyrochlore-IV
Pcl-IV are present in the dolomite carbonatite in association with belkovite (Fig. 3c,e). BSE imagery reveals that majority of the Pcl-IV are partially altered and characterised by patchy zoning with variable high- and low-AZ areas resulting from variations in the A-site cation abundances of Ba and U (Fig. 3e) (Table 2; compositions 11 and 14). In some cases, the Pcl-IV grains are extensively metamictised with profuse development of fractures making them prone to alteration (Fig. 3f). Such grains are characterised by a U-rich (high-AZ) relict core and U contents gradually decrease towards the rim (low-AZ), coupled with progressive enrichment in Ba and Si (Fig. 3f) (Table 2; compositions 12, 13). Extreme compositional variation of these pyrochlore is evident from the variable Ta (2.0–9.1 wt.% Ta2O5), Si (1.2–10.7 wt.% SiO2), U (28.3–4.9 wt.% UO2) and Pb (0.4–4.3 wt.% PbO) (Table 2) contents together with only minor Ca, Sr and Fe. Moreover, Pcl-IV has relatively higher A-site vacancies (0.6–1.3 pfu) compared to Pcl-I, -II and -III. Pyrochlore-IV is enriched in Ba and Nb (8.9–23.9 wt.% BaO; 34.9–41.0 wt.% Nb2O5) (Table 2) relative to all other Sevattur pyrochlore.
Pyrochlore-V
The silica-rich Pcl-V is found in the banded carbonatite and albitite. In the banded carbonatite it occurs as low-AZ patches in association with Pcl-IIa (Fig. 3g) and discrete grains are absent. In contrast, Pcl-V in albitite is present either as a discrete grain or in association with Pcl-III, where the orbicular mantle is partially or completely disrupted (Fig. 3h). Texturally, pyrochlore-V are characterised by numerous fractures related to extensive metamictisation resulting in patchy zoning similar to that exhibited by Pcl-IV (Fig. 3g,h). These pyrochlore are characterised by a high Si content (12.6–24.9 wt.% SiO2; 0.72–1 apfu) together with low Nb and U (20.9–27.1 wt.% Nb2O5; 13.3–19.4 wt.% UO2) (Table 2) contents compared to Pcl-I to Pcl-III; with a variable Ca (4.3–7.0 wt.% CaO) content. It is noteworthy that the Pcl-IIa and Pcl-III associated with Pcl-V in the banded carbonatite and albitite, are essentially U and Nb rich and Si poor (Table 2). However, all Pcl-V are essentially Si rich and U-Nb poor suggesting a significant loss in Nb and U during the alteration of the precursor pyrochlore. Depending on the host lithology, Pcl-V can be further subdivided into Si-Ti-rich (Table 2; compositions 16–18) and Si-Ta-rich varieties (Table 2; compositions 19, 20) present in the albitite and carbonatite, respectively. Pcl-V in albitite are relatively Ba, Al, K and Na rich compared to those present in the banded carbonatite. In general, Pcl-V are characterised by more A-site vacancies (>1 pfu, Table 2) relative to other pyrochlore.
Minerals formed by alteration of pyrochlore
Belkovite
Belkovite [Ba3Nb6(Si2O7)2O12] is a rare sorosilicate which has been reported from the Vuorijärvi and Seblyavr carbonatite complexes (Kola Peninsula, Russia) as a late-stage alteration product of Ba-rich pyrochlore (Voloshin et al., Reference Voloshin, Subbotin, Pakhomovsky, Bakhchisaraitsev and Yamnova1991; Sorokhtina et al., Reference Sorokhtina, Voloshin and Pakhomovsky1998). In common with the Vuorijärvi occurrence, belkovite found at Sevattur is present only within an assemblage consisting of U-rich Pcl-IIa (high-AZ), Ba-rich Pcl-IV (low-AZ), baryte and Ba-Sr carbonates in dolomite carbonatite (Figs 3c, 5a–e). BSE imagery shows that belkovite is restricted mainly to intensely metamictised areas of Pcl-IV containing numerous fractures (Fig. 5b–e). The associated U-rich Pcl-IIa are present as relict patches within this assemblage (Fig. 5c–e). The association of belkovite with Si-rich Pcl-IV suggests it forms as an alteration product of these pyrochlores.
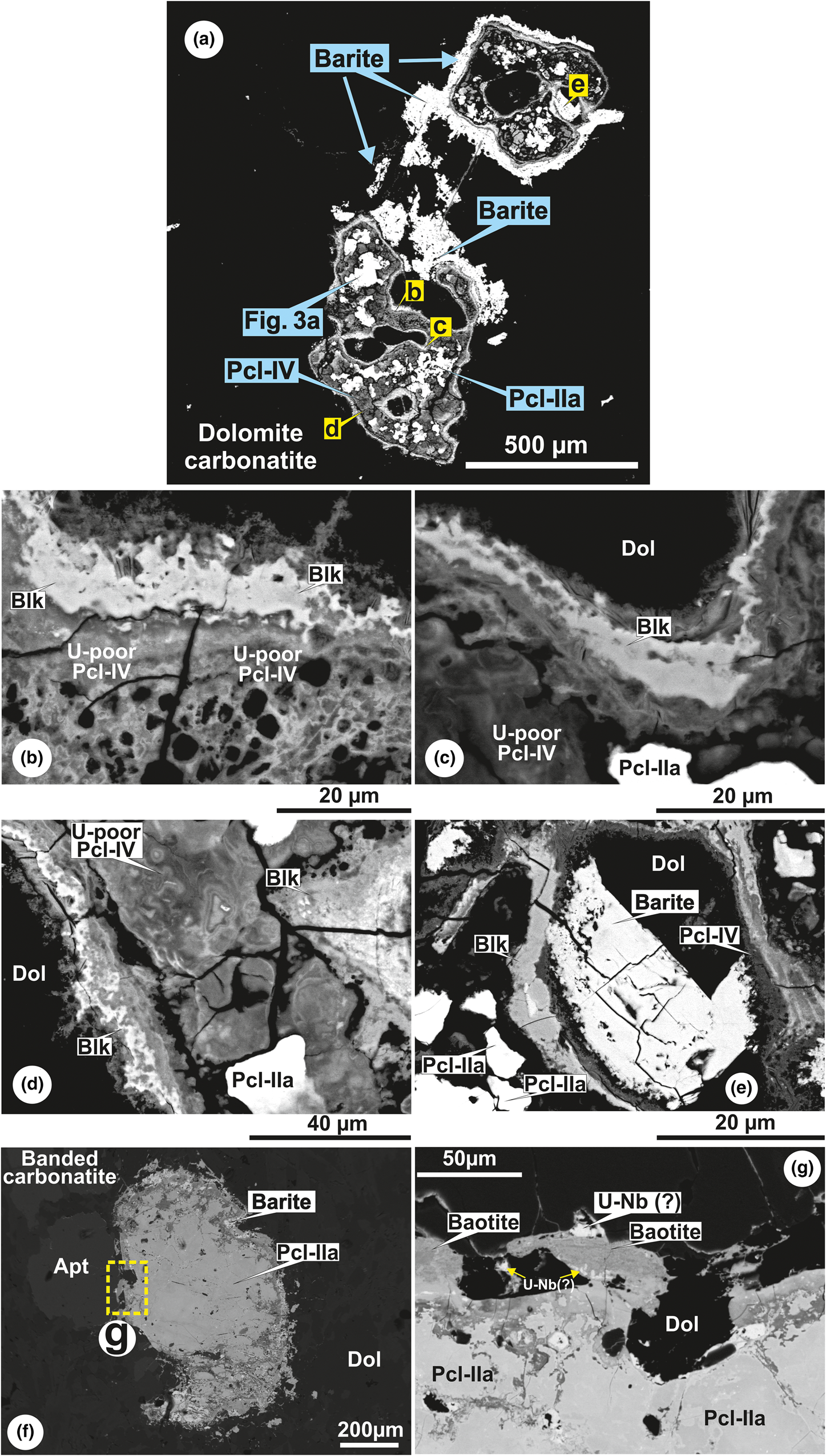
Fig. 5. BSE images showing the lithology specific alteration assemblages in dolomite (a–e) and banded (f–g) carbonatite. (a) A complex association of Pcl-IIa, Pcl-IV, baryte and belkovite (Blk) in dolomite carbonatite. (b) Enlarged view of U-poor Ba-rich Pcl-IV and belkovite along the radiation damaged grain margin. Note the porous nature of the Pcl-IV due to extensive metamictisation. (c, d) Association of late-formed Pcl-IV and belkovite with relict U-rich Pcl-IIa illustrating depletion in U content and subsequent Ba enrichment along the grain margin. (e) Alteration assemblage of belkovite and Pcl-IV formed after Pcl-IIa in dolomite carbonatite. Note a large baryte crystal is also present together with the belkovite. (f) Pyrochlore grain (Pcl-IIa) associated with baryte and apatite (Apt), within the dolomite matrix in banded carbonatite. (g) Alteration assemblage of baotite and an unidentified U-Nb oxide formed after U-rich Pcl-IIa along the grain margin in banded carbonatite.
Sevattur belkovite is similar in composition to that of the Vuorijärvi occurrence (Voloshin et al., Reference Voloshin, Subbotin, Pakhomovsky, Bakhchisaraitsev and Yamnova1991), being enriched in Nb (41.6–42.5 wt.% Nb2O5), Ba (30.8–32.1 wt.% BaO) and Si (17.3–17.4 wt.% SiO2) together with, in order of decreasing abundance, minor amounts of Ti, Ta, Fe, Sn, K and Na (Table 3; compositions 1–4). Because of the high degree of metamictisation, Pcl-IV has low 4.87 wt.% UO2 (Table 2; composition 15) contents compared to those of less-altered Pcl-IIa (up to 25.7 wt.% UO2; Table 2; composition 4) (Fig. 4). Thus, alteration of Pcl-IIa to Pcl-IV and belkovite is indicative of significant U mobilisation.
Table 3. Representative compositions of belkovite and baotite.
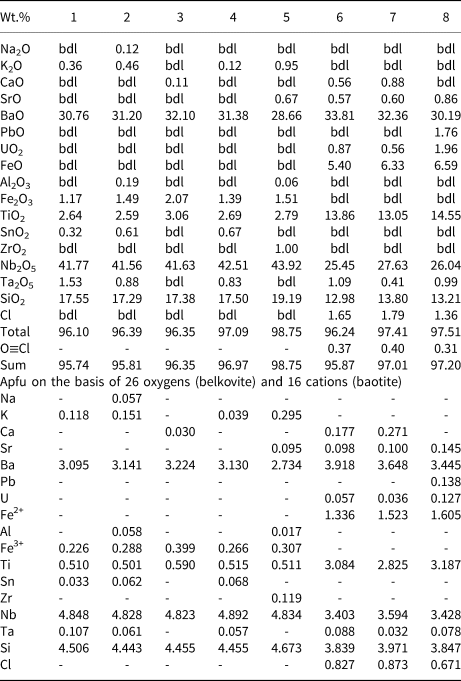
1–5: belkovite (dolomite carbonatite); 1–4: this work; 5: Voloshin et al., Reference Voloshin, Subbotin, Pakhomovsky, Bakhchisaraytsev, Yamnova and Pushcharovsky1990; 6–9: baotite (banded carbonatite)
All compositions were analysed by ED X-ray spectrometry (Lakehead University)
bdl: below detection limit
All Fe is calculated as Fe3+ for belkovite and Fe2+ for baotite
Baotite
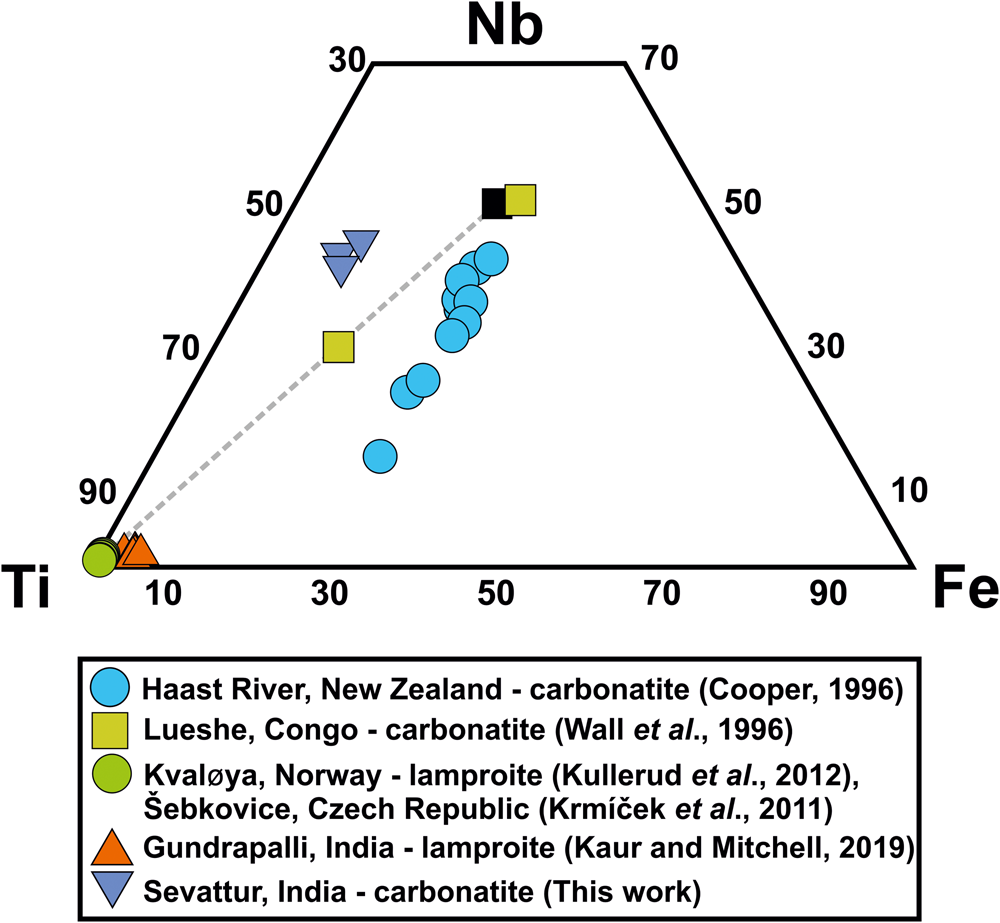
Baotite, is a chlorine-bearing Ba-Ti-Nb cyclosilicate [Ba4(Ti,Nb,Fe)8Si4O28Cl] first reported from hydrothermal quartz veins at Bayan-Obo, Mongolia, China (Peng, Reference Peng1959; Semenov et al., Reference Semenov, Khun and Kapitonova1961). Baotite occurs in alkaline–peralkaline rocks and carbonatites as a late-stage mineral with compositions varying between the end-members Ba4Ti8Si4O28Cl and Ba4Ti2Fe2Nb4Si4O28Cl (Cooper, Reference Cooper1996; Potter and Mitchell, Reference Potter and Mitchell2005; Kullerud et al., Reference Kullerud, Zozulya and Ravna2012). Baotite occurs in lamproites, alkali-granite/alkaline pegmatites, and alkaline metasomatites and is usually rich in Ti (Fig. 6) (Kullerud et al., Reference Kullerud, Zozulya and Ravna2012; Kaur and Mitchell, Reference Kaur and Mitchell2019, and references therein). In India, the only reported occurrence is from the Gundrapalli lamproite, Telangana (Kaur and Mitchell, Reference Kaur and Mitchell2019) (Fig. 6). In general, baotite is rare in carbonatites with notable examples being in the Haast River (New Zealand) and Lueshe (Congo) carbonatite complexes (Cooper, Reference Cooper1996; Wall et al., Reference Wall, Williams, Woolley and Nasraoui1996). In these occurrences, baotite is present as an accessory phase in late-stage ferroan dolomite (ankerite)-, siderite- and carbothermal carbonatites. They are rich in Nb and Fe relative to Ti (Fig. 6).
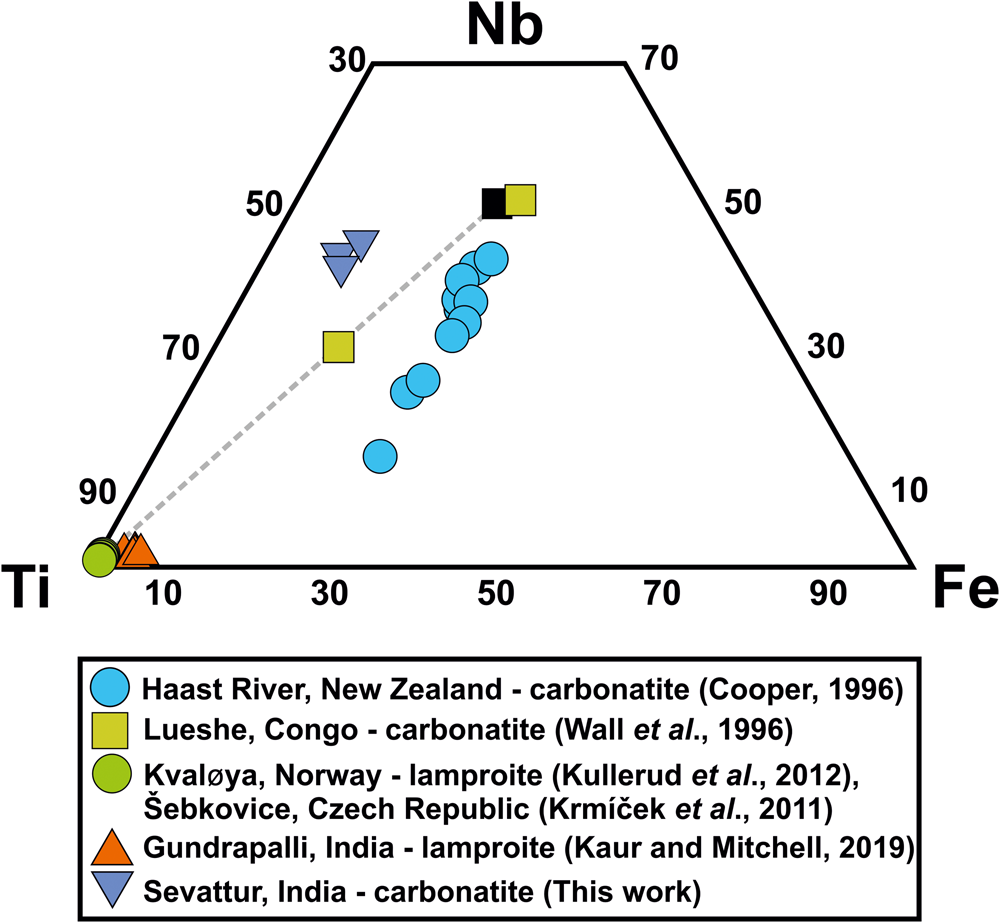
Fig. 6. Ti–Nb–Fe (apfu%) ternary diagram showing the relatively Nb-Ti-rich nature of Sevattur baotite. Data for some other occurrences of baotite from different lithologies are shown for comparison. Note that the only reported occurrence of baotite from the Gundrapalli lamproite, India is essentially Ti-rich. Ideal end-members (Nb- and Ti-rich, respectively) are marked in black squares, and are connected by a dotted line.
At Sevattur, baotite is present in trace amounts as anhedral grains (~50 μm) restricted to the banded carbonatite. BSE imagery shows baotite occurs as low-AZ areas along the metamictised grain margins of Pcl-IIa, associated with an unidentified U-Nb-rich phase (63.7 wt.% UO2, 18.6 wt.% Nb2O5) (Supplementary material 3) (Fig. 5f,g). Sevattur baotite is rich in Ba (30.2–33.8 wt.% BaO), Nb (25.4–27.6 wt.% Nb2O5), Ti (13.0–14.5 wt.% TiO2), Fe (5.4–6.6 wt.% FeO) and Si (13.0–13.8 wt.% SiO2) with Cl contents varying between 0.7 and 0.9 apfu (Table 3; compositions 5–7).
Pyrochlore, belkovite and baotite – classification and nomenclature
Pyrochlore
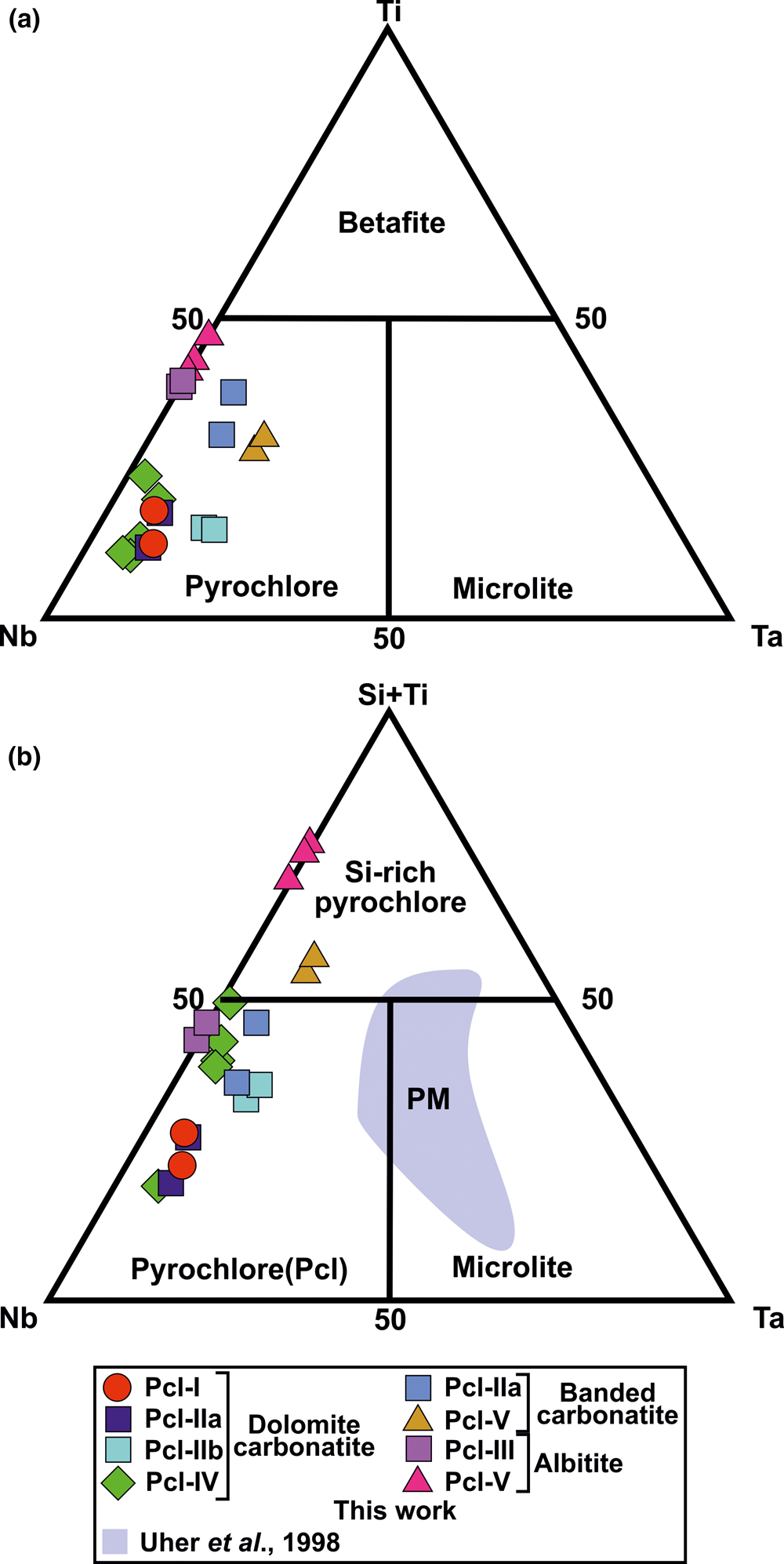
The majority of Sevattur pyrochlore are characterised by Nb > Ti or Ta (apfu) and are classified as pyrochlore sensu stricto in the Nb–Ta–Ti ternary diagram (Hogarth Reference Hogarth1977, Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010) (Fig. 7a). However, Si4+ is the dominant valence cation at the B-site of Pcl-V (Table 2, compositions 16–20) (Fig. 7b). In the IMA-CNMNC pyrochlore classification no element other than Ti4+ (betafite) is assigned to this tetravalent (M 4+) site (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010). Hence, using the IMA nomenclature, the classification of Si-rich Pcl-V pyrochlore (Fig. 7b) might require the establishment of a new member of the pyrochlore supergroup. A similar problem arises in using the IMA nomenclature in classifying Zr-pyrochlore from the Guaniamo kimberlite, Venezuela (Sharygin et al., Reference Sharygin, Sobolev and Channer2009). However, establishing a Si-dominant group of the pyrochlore supergroup is not feasible as the crystal-chemical role of Si in the pyrochlore structure is debatable (Bonazzi et al., Reference Bonazzi, Bindi, Zoppi, Capitani and Olmi2006; Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014). The silicified pyrochlores from the Mariupol massif showed that the Si could not be present in octahedral coordination, and the pyrochlore formula is calculated excluding Si as a B-site cation (Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014). Texturally, these pyrochlores are relatively unaltered, as evidenced by their euhedral habit and relict oscillatory zoning, which was overprinted by patchy zoning. In contrast, Sevattur pyrochlores are subhedral-to-anhedral in shape with intense patchy zonation and characterised by distinctive alteration assemblages. Significantly, Mariupol pyrochlores with a very low U content (0.23–3.14 wt.% UO2) and relatively higher Na and F contents (Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014) are not directly comparable to those found at Sevattur. Thus, it is evident that the effect of metamictisation is far more intense at Sevattur compared to the silicified pyrochlores from the Mariupol massif. However, following the pyrochlore formula calculation of Dumańska-Słowik et al. (Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014) we find that some of our analyses with a low SiO2 result in an overestimation of total oxygens (>7 apfu). In several instances, complications arise for altered pyrochlores (Pcl-IV and Pcl-V) with low analytical totals, leading to a low A-site vacancy with near ideal total oxygen. These anomalies cannot be justified as progressive alterations of pyrochlores usually results in significant A- and Y-site vacancies. This probably suggests that some Si might be present as B-site cation. A similar observation was made by Bonazzi et al. (Reference Bonazzi, Bindi, Zoppi, Capitani and Olmi2006) from the pyrochlores of the Narssârssuk nepheline syenites, Greenland. Consequently, we consider Si as a B-site cation and pyrochlore formula is calculated accordingly as suggested by the IMA (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010).
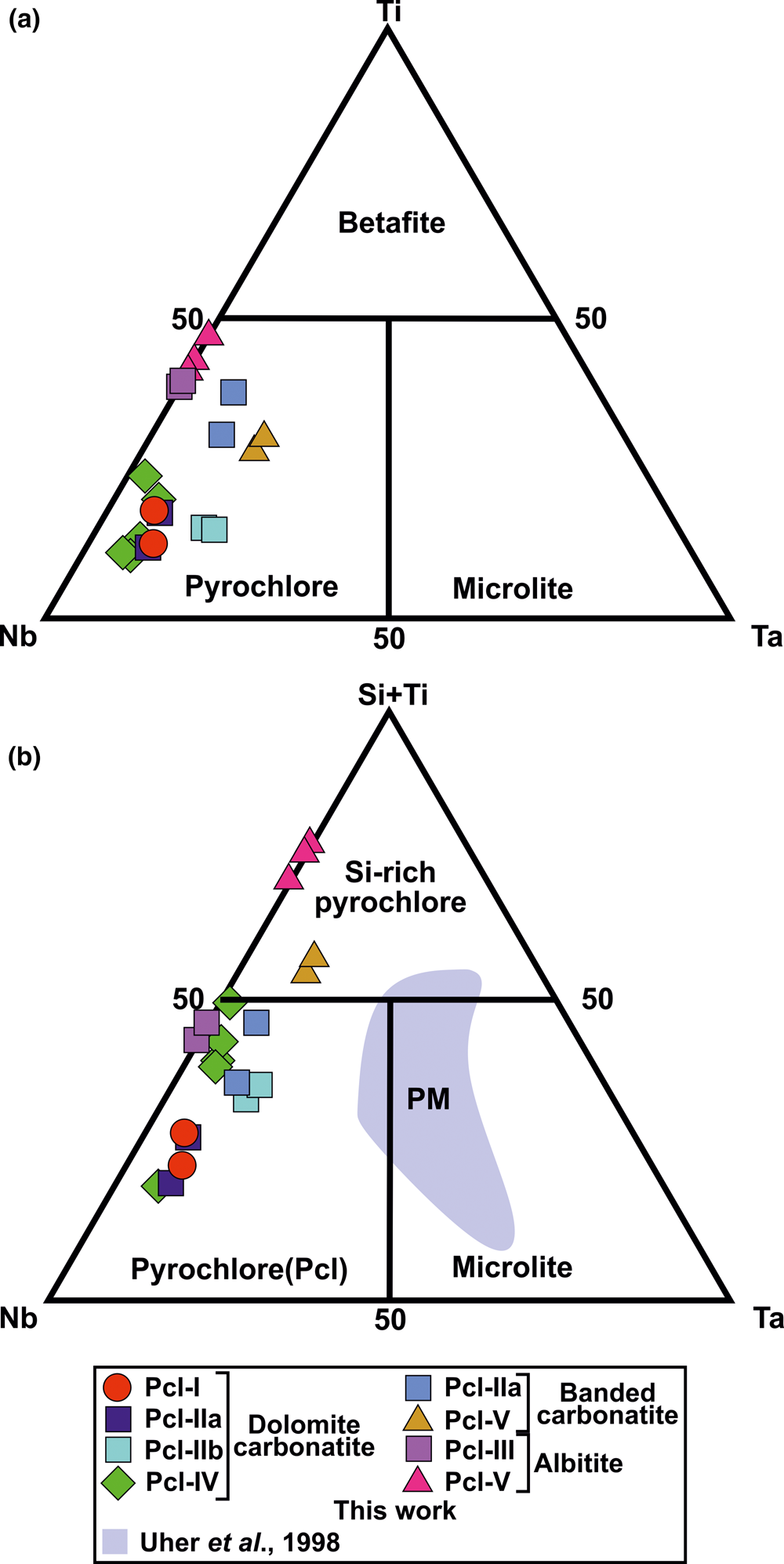
Fig. 7. (a) Compositional variation of the B-site cations (apfu) of different pyrochlore types from the Sevattur carbonatite complex. (b) Ternary plot of Nb–Ta–(Si + Ti) (apfu) illustrating the progressive Si enrichment from early- to late-generation pyrochlore. Si-rich pyrochlore plot in the (Si + Ti) field. Similar Si enrichment is seen at the Prašiviá massif (PM), Slovakia. Note that the Pcl-V in banded carbonatite and albite are extremely Si-rich and comparable with some of the Prašiviá massif pyrochlores.
The IMA pyrochlore nomenclature is based on the ‘dominant-valency rule’, whereas the previous classification (Hogarth, Reference Hogarth1977) was based on the ‘dominant constituent’ at the A-site excepting Ca and Na. The IMA scheme leads to many problems (discussed below) when used for petrogenetic purposes, and in particular for the non-stoichiometric pyrochlore found at Sevattur. To illustrate the nomenclatural problems, we have used both the Hogarth (Reference Hogarth1977) and the IMA pyrochlore nomenclature (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010) (Table 4).
Table 4. Classification and end-member formula of pyrochlore-group minerals from Sevattur.

1–2: Pcl-I; 3–6: Pcl-IIa; 7–8: Pcl-IIb; 9–10: Pcl-III; 11–15: Pcl-IV; 16–20: Pcl-V; #end-member formula in accordance with the dominant-valency rule; *end-member formula calculated by the STC method (Bosi et al., Reference Bosi, Biagioni and Oberti2019a, Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Millsb); $possible news species; §end-member formula with Si-dominant at the B-site (Ca2Si2O6□) is not possible so is discredited; unnamed: no group name exists at present, having Si4+ as the dominant cation of the dominant valency at the B-site.
At Sevattur all Pb-rich Pcl-I are plumbopyrochlore or oxyplumbopyrochlore (Table 4). Regardless of the high U content in Pcl-II and Pcl-III, it is disappointing to note that the IMA nomenclature results in classification of the majority of these either as oxycalciopyrochlore or kenopyrochlore/hydropyrochlore (Table 4). This nomenclature results in a consequent loss of valuable petrogenetic information. In contrast, following the (Hogarth, Reference Hogarth1977) nomenclature these pyrochlore are classified as uranpyrochlore, a name which illustrates their major compositional characteristics and permits comparisons with previous investigations of pyrochlore in carbonatites.
In general, the majority of the U-, Ba-, Sr-rich pyrochlore have significant A- and Y-site vacancies. This is a common feature of pyrochlore and an increase in cation deficiencies at the A-site is typically associated with the progressive alteration together with introduction of large cations such as Ba, Sr and K. (Lumpkin and Ewing, Reference Lumpkin and Ewing1995, Reference Lumpkin and Ewing1996; Wall et al., Reference Wall, Williams, Woolley and Nasraoui1996; Nasraoui and Bilal, Reference Nasraoui and Bilal2000; Zurevinski and Mitchell, Reference Zurevinski and Mitchell2004). In the Hogarth (Reference Hogarth1977) nomenclature compositional names such as bariopyrochlore, strontiopyrochlore, plumbopyrochlore and uranpyrochlore can be used to indicate changes in the melt/fluid composition or the process(es) that led to the enrichment of these elements in pyrochlore. Unfortunately, in IMA nomenclature all of these pyrochlore are grouped into a single species zero-valence-dominant pyrochlore, regardless of the U, Ba and Sr content at the A-site (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010; Christy and Atencio, Reference Christy and Atencio2013).
At Sevattur, one Ba-rich Pcl-IV (Table 2, comp. 11) can be classified as oxybariopyrochlore (Ba2Nb2O7), this is probably a new species of the pyrochlore group with Ba as the dominant A-site cation (Table 4). All other Pcl-IV can be termed kenopyrochlore/hydropyrochlore, kenobariopyrochlore/hydrobariopyrochlore (either of them could be a new species), and hydroxykenopyrochlore/hydropyrochlore. Some Pcl-IV can be classified as uranpyrochlore or bariopyrochlore (Table 2; compositions 11–15) depending on the relative abundance of Ba and U contents (apfu) following Hogarth (Reference Hogarth1977). All the Pcl-V are Si-rich pyrochlore for which no IMA name exists (Table 4).
In terms of end-member composition, both nomenclatures commonly fail to define a unique end-member formula corresponding to a particular pyrochlore species. However, in a few instances following the dominant-valency rule together with double occupancy at a single site e.g. the A-site, charge-balanced end-members such as fluornatropyrochlore (NaCaNb2O6F), oxycalciopyrochlore (Ca2Nb2O7), or oxyplumbopyrochlore (Pb2Nb2O7) are possible. Problems arise with multiple heterovalent substitutions (including vacancies) which are more prevalent at the A-site and the dominant-valency rule typically fails to yield a charge-balanced end-member (Bosi et al., Reference Bosi, Biagioni and Oberti2019a), such as kenopyrochlore/hydropyrochlore (□2Nb2O6□)Ʃ2– (Table 2; composition 14). This problem is addressed in the recent IMA classification (Atencio et al., Reference Atencio, Andrade, Christy, Gieré and Kartashov2010) by introducing a sub-ordinate charge-balancing component, with the end-member formula re-written as (□,#)2Nb2O6□, where ‘#’ could be Ba or U4+.
In such cases, the application of the ‘Site Total Charge’ (STC) method is useful. Here the total charge of a site is calculated and expressed in whole numbers and the most abundant atomic arrangement (complementing the site charge) is given end-member status (Bosi et al., Reference Bosi, Biagioni and Oberti2019a, Reference Bosi, Hatert, Hålenius, Pasero, Miyawaki and Millsb). Thus, the end-member formula using STC for the above composition is (□1.25U0.75)Nb2O6OH (with OH– used for charge balancing) resulting in the species name hydroxykenopyrochlore/hydroxyhydropyrochlore (Table 4; serial number 14) (Supplementary material 1; serial number 14). We also suggest usage of appropriate adjectival modifiers (see Bayliss et al., Reference Bayliss, Kaesz and Nickel2005) to represent the major cations at the A-site, not reflected in the IMA species name, as Ba-bearing, U-rich hydroxykenopyrochlore/hydroxyhydropyrochlore, where U4+ is the dominant cation (0.5 apfu) and there is also a considerable amount of Ba (0.3 apfu) at the A-site (Table 2; composition 14) (for calculation of end-member formula of all pyrochlore species from Sevattur using the STC method; see Supplementary material 1). This method is helpful in understanding the compositional diversity that exists among the pyrochlore-group minerals and certainly a more useful representation than the IMA scheme, especially for practical petrogenetic aspects associated with pyrochlore compositions and their evolution.
Belkovite and baotite
Sevattur belkovite can be represented by an empirical formula of A(Ba3.10K0.12)Ʃ3.22B(${\rm Nb}_{4.85}^{5 + } {\rm Ta}_{0.11}^{5 + } {\rm Ti}_{0.51}^{4 + } {\rm Sn}_{0.03}^{4 + } {\rm Fe}_{0.23}^{3 + }$![]() )Ʃ5.73${^{\rm X}}{\rm Si}_{4.51}^{}$
)Ʃ5.73${^{\rm X}}{\rm Si}_{4.51}^{}$![]() YO26 (Table 3; composition 1) based on 26 oxygens. Simple application of the dominant-valency rule, to the above empirical formula leads to an end-member composition of Ba3Nb6Si4O26, which is close to a synthetic barium silicon tantalum oxide (Ba3Ta6Si4O26) (Choisnet et al., Reference Choisnet, Nguyen, Groult and Raveau1976). However, there is excess of total cations (13.45), which can be justified by the fact that the structure of Ba3Ta6Si4O26 has two sites for larger cations, at (x,0,1/2) and (x,0,0) respectively. When the larger cation is Ba2+ the latter site is empty, when the larger cation is K+ it is fully occupied, giving a total of 16 cations, as in the synthetic compound K6Nb6Si4O26 (Choisnet et al., Reference Choisnet, Nguyen, Groult and Raveau1976). So, it can be argued that the structure of belkovite is able to accommodate >13 cations. But, the presence of excess of Si (>4 apfu) cannot be justified as Si is supposed to be restricted to the tetrahedral X site. Therefore, excess Si could be an analytical error leading to silica (SiO2) overestimation. To eliminate the excess silica, the belkovite formula can also be calculated on the basis of 4 Si cations. However, this results in significantly low total oxygens (~23 apfu), which can only be balanced by considering the presence OH– along with oxygen at the Y site. Moreover, the presence of OH– could be a possibility reflected in low analytical totals, and recalculated H2O (wt.%) obtained by this method makes the analytical total close to one hundred. However, lack of structural data refrains us from assuming the presence of water in belkovite. This suggests a further investigation on the crystallo-chemical aspects of belkovite is needed.
YO26 (Table 3; composition 1) based on 26 oxygens. Simple application of the dominant-valency rule, to the above empirical formula leads to an end-member composition of Ba3Nb6Si4O26, which is close to a synthetic barium silicon tantalum oxide (Ba3Ta6Si4O26) (Choisnet et al., Reference Choisnet, Nguyen, Groult and Raveau1976). However, there is excess of total cations (13.45), which can be justified by the fact that the structure of Ba3Ta6Si4O26 has two sites for larger cations, at (x,0,1/2) and (x,0,0) respectively. When the larger cation is Ba2+ the latter site is empty, when the larger cation is K+ it is fully occupied, giving a total of 16 cations, as in the synthetic compound K6Nb6Si4O26 (Choisnet et al., Reference Choisnet, Nguyen, Groult and Raveau1976). So, it can be argued that the structure of belkovite is able to accommodate >13 cations. But, the presence of excess of Si (>4 apfu) cannot be justified as Si is supposed to be restricted to the tetrahedral X site. Therefore, excess Si could be an analytical error leading to silica (SiO2) overestimation. To eliminate the excess silica, the belkovite formula can also be calculated on the basis of 4 Si cations. However, this results in significantly low total oxygens (~23 apfu), which can only be balanced by considering the presence OH– along with oxygen at the Y site. Moreover, the presence of OH– could be a possibility reflected in low analytical totals, and recalculated H2O (wt.%) obtained by this method makes the analytical total close to one hundred. However, lack of structural data refrains us from assuming the presence of water in belkovite. This suggests a further investigation on the crystallo-chemical aspects of belkovite is needed.
The site-total-charge approach suggests $( {\rm Nb}_4^{5+} {\rm Ti}_2^{4+} ) _{0.27}^{ {\Sigma} 28 + }$![]() as the most abundant charge arrangement at the B-site. Thus, the end-member formula for belkovites from the Sevattur can be written as Ba3(Nb4Ti2)Si4O25 (see Supplementary Material 2).
as the most abundant charge arrangement at the B-site. Thus, the end-member formula for belkovites from the Sevattur can be written as Ba3(Nb4Ti2)Si4O25 (see Supplementary Material 2).
Baotite end-member compositions vary between Ba4Ti8Si4O28Cl and Ba4Ti2Nb4Fe2Si4O28Cl (Krmíček et al., Reference Krmíček, Cempírek, Havlín, Přichystal, Houzar, Krmíčková and Gadas2011; Kullerud et al., Reference Kullerud, Zozulya and Ravna2012; Kaur and Mitchell, Reference Kaur and Mitchell2019 and references therein). The role of chlorine in the baotite structure is enigmatic, commonly it is considered as a neutral species and not required to balance the structure electrostatically (Nekrasov et al., Reference Nekrasov, Ponomarev, Simonov and Kheiker1969; Nemec, Reference Nemec1987; Potter and Mitchell, Reference Potter and Mitchell2005; Kullerud et al., Reference Kullerud, Zozulya and Ravna2012). However, following the STC method, baotites from Sevattur have an ideal composition of Ba4Ti3(Nb3.33Fe1.67)Si4O28Cln, where n varies from 0.7 to 0.9 (see Supplementary material 2).
Discussion
Pyrochlore substitutional mechanisms, alteration and related compositional variation
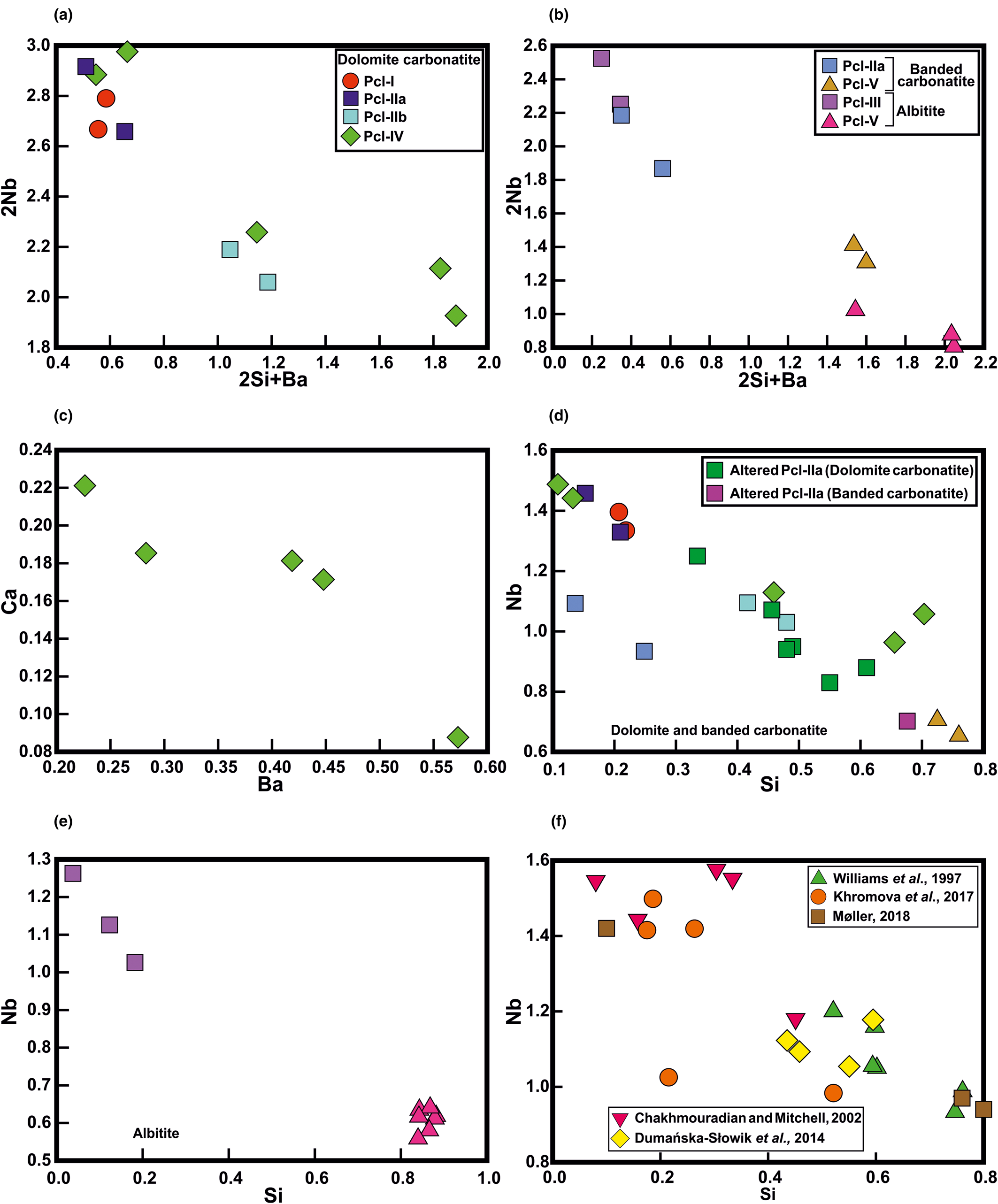
Pyrochlore-group minerals at Sevattur show wide compositional variations. These diverse compositions are useful for discerning the diverse magmatic and post-magmatic processes which led to their formation. In general, U-rich pyrochlores are characterised by extensive metamictisation and damaged crystal structures making them susceptible to moderate-to-intense alteration and U mobility (Lumpkin and Ewing, Reference Lumpkin and Ewing1995, Reference Lumpkin and Ewing1996; Hogarth et al., Reference Hogarth, Williams and Jones2000). Intense alteration of such pyrochlores leads to patchy zoning characterised by several high-AZ, intermediate-AZ, and low-AZ zones as evident in BSE images (Figs 3, 5). Compositionally, these pyrochlores are extremely heterogeneous and therefore no single trend of evolution is evident. Sevattur pyrochlores show a gradual change in composition from U-rich pyrochlore (Pcl-II, -III) to Si-rich pyrochlore (Pcl-V) through Ba-Si-rich pyrochlore (Pcl-IV) with a variable Ta/Ti ratios. A systematic increase in Si and Ba at the expense of Nb from Pcl-I to Pcl-IV (dolomite carbonatite) and Pcl-IIa to Pcl-V (banded carbonatite and albitite) can be explained by the following coupled substitution:
involving both the A- and B-sites with a negative correlation (R = –0.89 and –0.97) (Fig. 8a,b). The Ba enrichment reaches a maximum in the dolomite carbonatite. Ba-rich pyrochlore is typically considered to form during late-stage hydrothermal or supergene alteration (Jager et al., Reference Jäger, Niggli and Van der Veen1959; Harris, Reference Harris1965; Traversa et al., Reference Traversa, Gomes, Brotzu, Buraglini, Morbidelli, Principato, Ronca and Ruberti2001; Melgarejo et al., Reference Melgarejo, Costanzo, Bambi, Gonçalves and Neto2012). Although, primary Ba-pyrochlore with >11 wt.% BaO has been reported from Araxá (Brazil), this was subsequently replaced along fractures by a later generation of Ba-rich pyrochlore (Mitchell, Reference Mitchell2015). At Sevattur, the enrichment of Ba coupled with an increase in A-site vacancy at the expense of Ca is prominent for Pcl-IV (Fig. 8c) and reflects the following substitution with a negative correlation (R = –0.93):
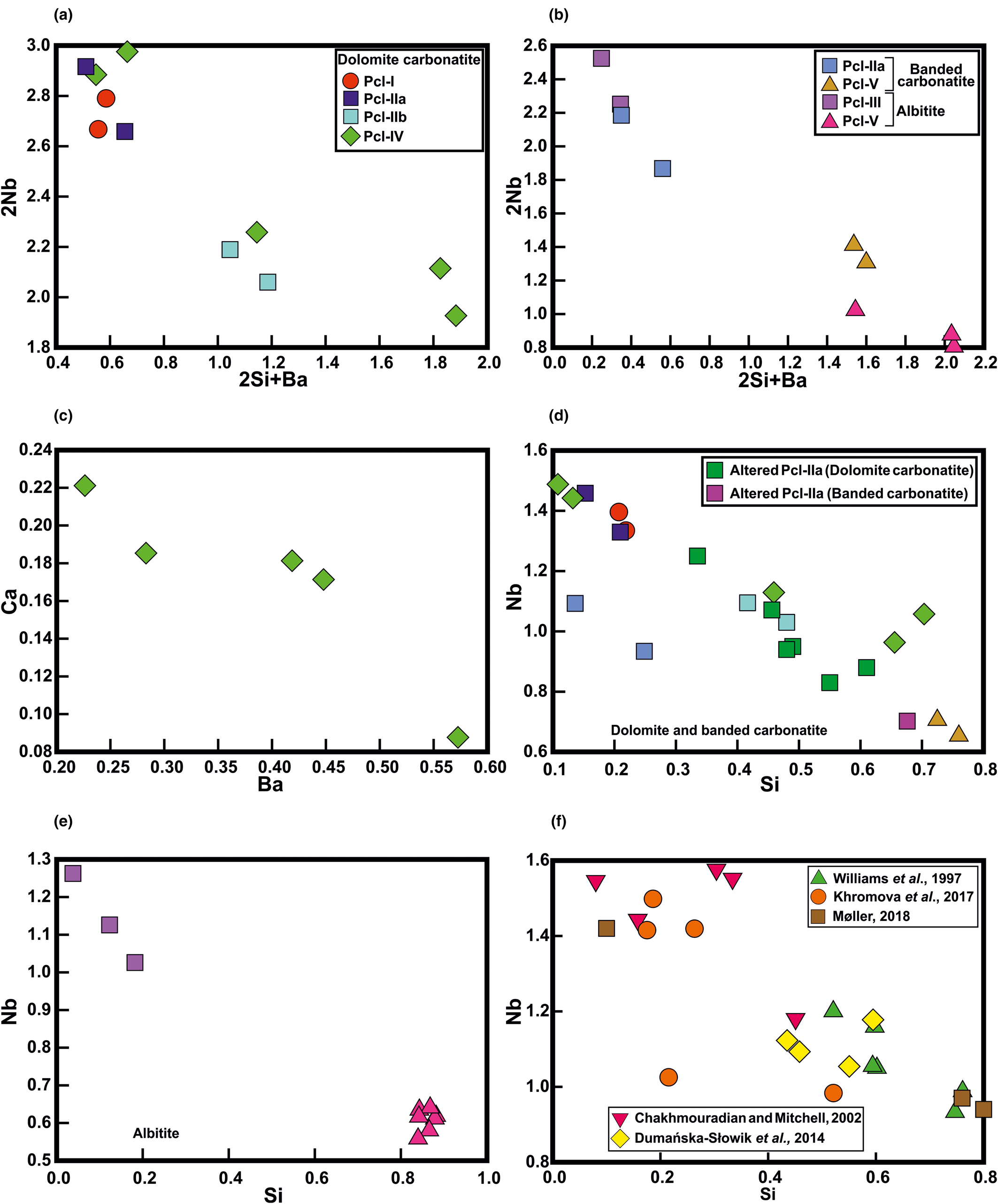
Fig. 8. (a,b) The 2Nb5+ → 2Si4+ + Ba2+ (apfu) substitution for different pyrochlore generations illustrating Ba and Si enrichment in the later generation pyrochlore (Pcl-IIb and Pcl-IV) in dolomite carbonatite. This substitution is more evident in the Pcl-V hosted by banded carbonatite and albitite. (c) Ca2+ → Ba2+ (apfu) substitution showing Ba enrichment in Pcl-IV relative to the U-poor Ca-rich Pcl-IIa in dolomite carbonatite. (d,e) A strong negative correlation is observed in the Nb versus Si plot for all the pyrochlore generations (Pcl-I to Pcl-V) with an R value of –0.93 for dolomite and banded carbonatite; R = –0.96, for albitite. (f) The figure illustrates a similar correlation for Si-rich pyrochlore from: the Bingo carbonatite, Congo (Williams et al., Reference Williams, Wall, Woolley and Phillipo1997); the Lovozero alkaline complex, Russia (Chakhmouradian and Mitchell, Reference Chakhmouradian and Mitchell2002); the Mariupol massif, Ukraine (Dumańska-Słowik et al., Reference Dumańska-Słowik, Pieczka, Tempesta, Olejniczak and Heflik2014); the Belaya Zima pluton, Russia (Khromova et al., Reference Khromova, Doroshkevich, Sharygin and Izbrodin2017); Iímaussaq, South Greenland (Møller, Reference Karup-Møller2018).
The compositional variation of Pcl-IV is the result of extensive metamictisation and development of numerous cracks/fractures which facilitate U mobility with concomitant increase in Ba. The Nb depletion with increasing Si can be explained by a Nb versus Si substitution, which in a bivariate plot shows an excellent correlation for the carbonatites (dolomite and banded; R = –0.93) and albitite (R = –0.96) (Fig. 8d,e) respectively. This correlation is also evident for other occurrences of Si-rich pyrochlore (Fig. 8f).
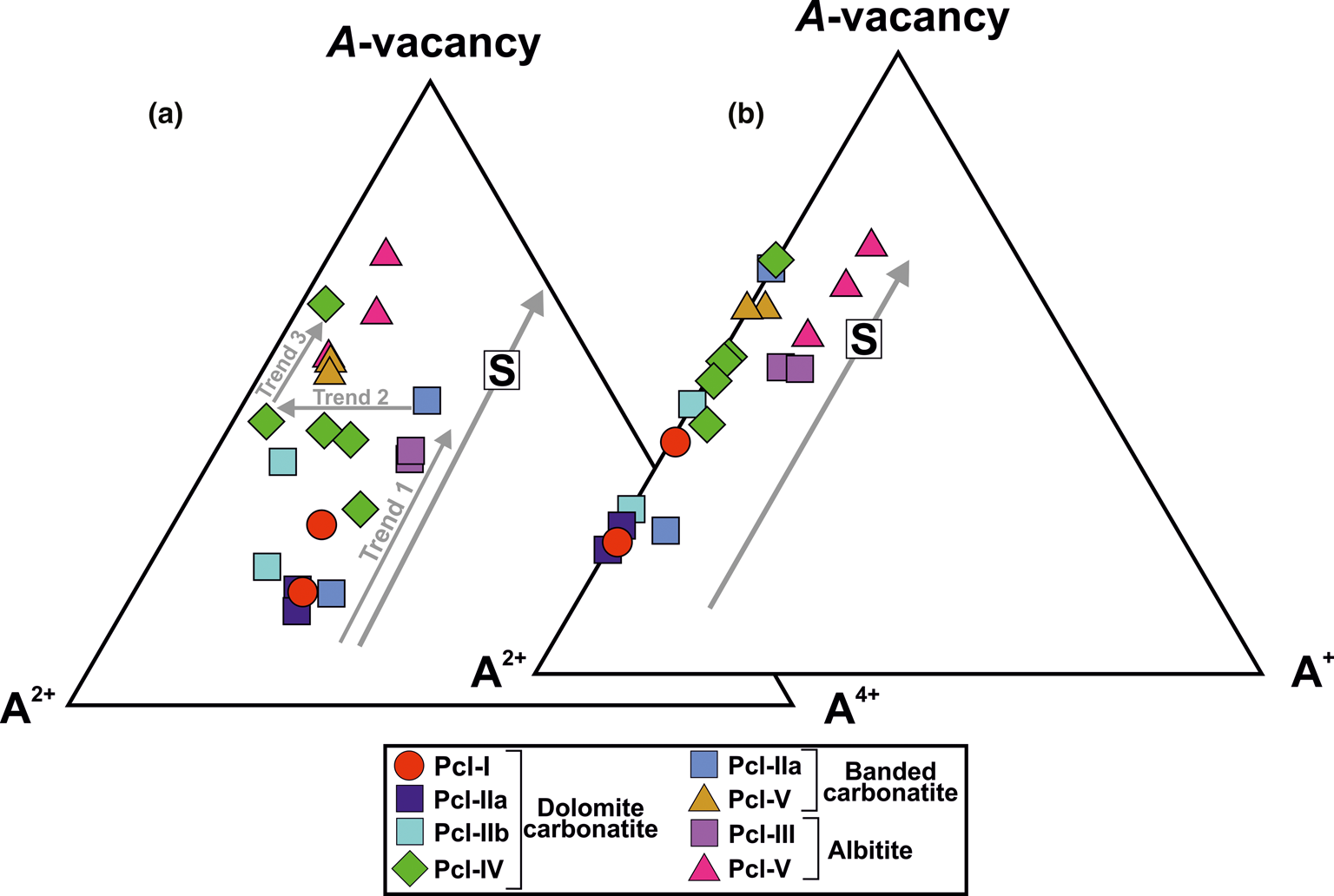
Lumpkin and Ewing (Reference Lumpkin and Ewing1995; Reference Lumpkin and Ewing1996) have defined three different alteration trends: primary; transitional; and secondary, to represent the compositional variation in pyrochlore-group minerals during progressive alteration. Sevattur pyrochlore are characterised by low Na contents and loss of U and Ca, coupled with increase in Ba- and A-site vacancies from early (Pcl-I, -IIa and -III) to late (Pcl-IIb, -IV and -V) pyrochlore generations as shown in Figs 9a and b. Three broad trends are shown in the A4+–A2+–A-vacancy plot (Fig. 9a): (1) increase in A-site vacancies with near-constant values of U4+ from Pcl-I to U-rich Pcl-III (Trend 1); (2) depletion of U4+ with near-constant A-site vacancies in Pcl-IV (from U-rich to U-poor) (Trend 2); and (3) a further increase in A-site vacancies at low U content (Trend 3) in some Pcl-IV and Pcl-V. The compositional variation of Pcl-IV is significantly different compared to that of other pyrochlores (Fig. 9a) and is due mainly to the variation in U and Ba contents. The initial alteration of Pcl-IV resulted in the substantial loss of U at constant Ca (Fig. 8c; Supplementary material 3; HA1–HA4) without significant Ba-enrichment. Although, for some Pcl-IV the effect of Ba, Si-enrichment is more conspicuous with a variable degree of U loss (Fig. 9a; Table 2; compositions 11–15). Simple correlations between U, Ca, Ba and Si are difficult to establish.
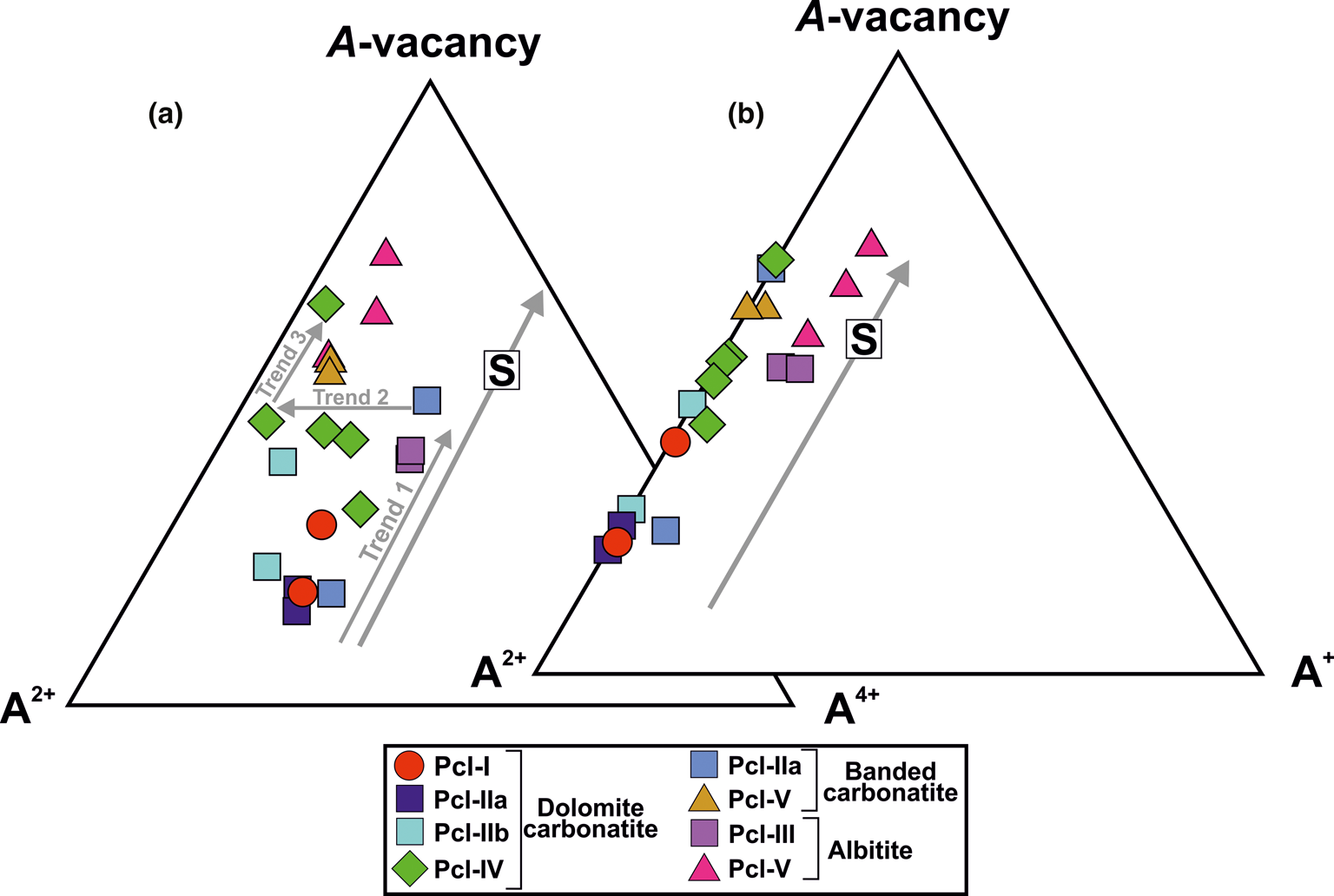
Fig. 9. (a) Ternary plot of A 4+ (A-site tetravalent cations) – A 2+(A-site divalent cations) – A-site vacancy (apfu) (after Lumpkin and Ewing, Reference Lumpkin and Ewing1995) illustrating three different trends (1, 2 and 3) related to progressive alteration from early- to late-stage pyrochlore formation (see discussion for details). Note that the significant variation in A 4+ cations in Pcl-IV in dolomite carbonatite are related to partial replacement of Ca by Ba and subsequent loss of U. (b) Ternary plot of A 2+–A+ (A-site monovalent cations) – A-site vacancy (apfu) (after Lumpkin and Ewing, Reference Lumpkin and Ewing1995) showing the overall loss of divalent cations and subsequent increase in the A-site vacancy during progressive alteration of all pyrochlore-bearing lithologies at Sevattur. Note that the Pcl-III and Pcl-V in albitite are relatively rich in monovalent cations Na+ and K+, respectively. Both ternary plots demonstrate the secondary (S) alteration trend of Lumpkin and Ewing (Reference Lumpkin and Ewing1995).
It is evident that all the pyrochlores from the carbonatites have exceptionally low contents of monovalent cations and show a gradual increase in A-site vacancies during evolution from Pcl-I to Pcl-V (Fig. 9b). However, the Si-rich Pcl-V and Pcl-III in albitite are distinctly different from those occurring in carbonatite being enriched in Na and K (Fig. 9b).
Uranium and niobium mobilisation in pyrochlore
Uranium-rich pyrochlore might have diverse origins and can be a primary magmatic phase or crystallise subsequently to Na-Ca pyrochlore (Mitchell, Reference Mitchell2015). Uranium is soluble as fluorides and sulfates at low pH (acidic); as phosphates at neutral pH; and as carbonates and hydroxides in alkaline conditions (Boyle, Reference Boyle1982). Experimental studies have shown that a hot, acidic, chloride-rich solution is capable of transporting U4+ as a uranium chloride species (UCl4)0 under reducing conditions (Geisler et al., Reference Geisler, Berndt, Meyer, Pollok and Putnis2004; Migdisov et al., Reference Migdisov, Boukhalfa, Timofeev, Runde, Roback and Williams-Jones2018; Timofeev et al., Reference Timofeev, Migdisov, Williams-Jones, Roback, Nelson and Xu2018).
Considering the overall mineral assemblages present at the Sevattur carbonatite complex (Table 1), ${\rm CO}_3^{2\ndash }$![]() , ${\rm SO}_4^{2\ndash }$
, ${\rm SO}_4^{2\ndash }$![]() , F–, and Cl– are the primary ligands which are capable of mobilising uranium during alteration of U-rich pyrochlore. This hypothesis is supported by the ubiquitous presence of baryte and different carbonates (dolomite and calcite) in association with Pcl-IIa, Pcl-IIb and Pcl-IV, and suggests that ${\rm CO}_3^{2\ndash }$
, F–, and Cl– are the primary ligands which are capable of mobilising uranium during alteration of U-rich pyrochlore. This hypothesis is supported by the ubiquitous presence of baryte and different carbonates (dolomite and calcite) in association with Pcl-IIa, Pcl-IIb and Pcl-IV, and suggests that ${\rm CO}_3^{2\ndash }$![]() and ${\rm SO}_4^{2\ndash }$
and ${\rm SO}_4^{2\ndash }$![]() played an important role in mobilising uranium as uranyl complexes in dolomite carbonatite (Fig. 5a–e). In contrast, the banded carbonatite with an assemblage of baotite, U-rich Pcl-IIa and baryte suggests that ${\rm CO}_3^{2\ndash }$
played an important role in mobilising uranium as uranyl complexes in dolomite carbonatite (Fig. 5a–e). In contrast, the banded carbonatite with an assemblage of baotite, U-rich Pcl-IIa and baryte suggests that ${\rm CO}_3^{2\ndash }$![]() , ${\rm SO}_4^{2\ndash }$
, ${\rm SO}_4^{2\ndash }$![]() , and Cl– facilitated the uranium mobilisation resulting in the formation of an exceptionally uranium-rich phase (>63 wt.% UO2) (Fig. 5f,g). The formation of baotite and the unknown U-rich phase with relict unaltered U-rich Pcl-IIa probably suggests that the uranium mobilisation might have taken place by the interaction with a Cl–-rich hot acidic solution under alkaline and reducing conditions at least on a micrometre scale.
, and Cl– facilitated the uranium mobilisation resulting in the formation of an exceptionally uranium-rich phase (>63 wt.% UO2) (Fig. 5f,g). The formation of baotite and the unknown U-rich phase with relict unaltered U-rich Pcl-IIa probably suggests that the uranium mobilisation might have taken place by the interaction with a Cl–-rich hot acidic solution under alkaline and reducing conditions at least on a micrometre scale.
Albitite-hosted pyrochlores (Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021) are the most U-rich (Pcl-III; >36 wt.% UO2) and the majority of these are enveloped by a Ba-rich potassium feldspar mantle (Fig. 3d). However, there are pyrochlores that either lack mantles or these are partially disrupted (Fig. 3h), resulting in the formation of Si-rich and U-poor pyrochlore (Pcl-V) as evidenced by the strong correlation of the B-site cations (Nb, Ta and Ti) versus Si (Fig. 8d). Thus, the formation of Pcl-V in albitite took place upon interaction with a late-stage Ba-Si-rich aqueous fluid. These features suggest that the Ba-rich potassium feldspar mantle around Pcl-III, restricted U-mobility compared to the pyrochlores in the carbonatites.
In the pyrochlore structure, B-site cations (Nb, Ta and Ti) are relatively immobile and remain unaffected by hydrothermal alteration and weathering processes (Lumpkin and Ewing, Reference Lumpkin and Ewing1995). Although the late-stage alteration of pyrochlore shows that at temperature <150°C niobium is remobilised by diffusion from BO6 octahedra (Lumpkin and Ewing Reference Lumpkin and Ewing1995, Geisler et al., Reference Geisler, Berndt, Meyer, Pollok and Putnis2004). Experimental studies have shown that the mobilisation of B-site cations is possible in acidic fluoride-rich hydrothermal solutions. Increasing HF activity in the fluid makes Nb more soluble than Ta regardless of the temperature (Timofeev et al., Reference Timofeev and Williams-Jones2015; Reference Timofeev, Migdisov and Williams-Jones2017; Akinfiev et al., Reference Akinfiev, Korzhinskaya, Kotova, Redkin and Zotov2020). Consequently, the removal of HF from the hydrothermal solution by precipitation of fluorite and fluorapatite could enhance Nb-mineral precipitation (Timofeev et al., Reference Timofeev and Williams-Jones2015; Reference Timofeev, Migdisov and Williams-Jones2017). Even under low-temperature, alkaline conditions (pH > 8), Nb leaching and transportation are possible as polyoxometalate Lindqvist ions [(HxNb6O19)(8–x)–] which implies that surficial weathering processes are quite effective in mobilising Nb as polyoxometalate ions (Friis and Casey, Reference Friis and Casey2018).
In common with uranium, mobility of Nb is also observed during alteration of Pcl-IIa to Pcl-IIb in dolomite carbonatite, and Pcl-III to Pcl-V in albitite (Fig. 4a; Table 2). In dolomite carbonatite, the association of Pcl-IIa together with Pcl-IV and secondary minerals such as baryte and belkovite suggest that Nb was liberated from the pyrochlore upon interaction with a sulfate-rich solution. The alteration sequence of U-rich pyrochlore to belkovite in the dolomite carbonatite through an intermediate metamict pyrochlore (Pcl-IV) with low analytical totals (~76–86 wt.%) (Supplementary material 3) suggests that the Nb mobilisation was accompanied by Ba, and Si enrichment. The formation of baotite in the banded carbonatite suggests that Nb mobilisation was accompanied by Ba, Ti and Si enrichment in the presence of Cl– during the alteration process. Furthermore, late-stage Ba, Si enrichment resulted in the formation of Si-rich Pcl-V and baryte. Considering the very low modal abundance of baotite relative to baryte; it is probable that baryte was formed late in the paragenetic sequence. In contrast to the carbonatites, albitite also shows significant Nb mobilisation during alteration from U-rich Pcl-III to Si-rich Pcl-V. The absence of apatite or fluorite in albitite probably suggests that the Nb mobilisation might have been triggered by the sulfate-bearing solutions alone.
Genesis of pyrochlore, belkovite and baotite
Sevattur pyrochlore-group minerals exhibit a wide compositional variation similar to those in many carbonatite complexes, e.g. St. Honoré, Prairie Lake, Oka. This could be related to the multiple episodes of carbonatitic magmatism or due to the interactions with hydrothermal fluids. Considering the complex nature of both inter- and intra-grain compositional variation within a given lithological unit, it is difficult to determine a single trend of evolution for the Sevattur pyrochlores.
In dolomite carbonatite, Pcl-I is characterised by high Pb and low Ca contents, is exclusively present as inclusions in Pcl-IIa, and was probably formed from an evolved Pb-rich hydrothermal fluid. Consequently, Pcl-I are considered as unrelated to the other pyrochlore types (Pcl-II to Pcl-IV) and were probably transported to their current host (dolomite carbonatite) by a later carbonatite pulse which crystallised Pcl-IIa on Pcl-I nuclei. The U-Ta-rich Pcl-IIa are characteristic of carbonatite pyrochlore evolution and considered to be early-forming pyrochlore (Mitchell, Reference Mitchell2015). In contrast, Pcl-IIb with significant Pb, lower U, and high Si contents relative to Pcl-IIa, suggests that the Pcl-IIb formed by alteration of both Pcl-I and Pcl-IIa. The alteration assemblage of Pcl-IV + belkovite formed after Pcl-IIa (Fig. 3c) suggests interaction with a Ba-Si-rich hydrothermal fluid by the following reactions:
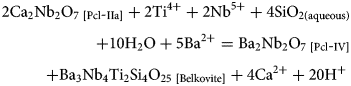




These reactions (3–7) explain the formation of Ba-Si-rich Pcl-IV and belkovite by alteration of Pcl-IIa and subsequent removal of U (reaction 8). It is worthwhile noting that belkovite is present exclusively in dolomite carbonatite and restricted to pyrochlore alteration assemblages.
In contrast, the banded carbonatite is characterised by an alteration assemblage of Si-rich Pcl-V + baotite ± U-Nb-rich phase formed after Pcl-IIa. The formation of baotite can be explained by the following reactions:


These reactions suggest that baotite (9, 10) and Si-rich Pcl-V (11) were formed upon interaction of Pcl-IIa with a Ba-Si-rich and a Si-rich fluid respectively. However, there are significant compositional differences in the Ba-U-Nb-Ti contents between the Pcl-IIa found in the dolomite carbonatite and banded carbonatite, respectively. Consequently, the alteration assemblages are essentially controlled by the primary composition of the pyrochlore (Pcl-IIa). The formation of baotite in the banded carbonatite results mainly from the high Ti content of Pcl-IIa compared to that in the dolomite carbonatite. The high Nb content in Pcl-IIa facilitated belkovite formation in the dolomite carbonatite. This confirms that the pyrochlore-group minerals in the different varieties of carbonatite are not linked genetically by differentiation and probably there is no single trend of evolution for Sevattur pyrochlores.
In albitite, U-Ti-rich Pcl-III were protected initially by the Ba-K-rich potassium feldspar mantle, but subsequently altered to Si-rich Pcl-V with significant remobilisation of U and Nb, where the mantle is disrupted. The formation of Pcl-V in albitite can be explained by the following reactions:
Note that the Si-enrichment in albitite-hosted Pcl-V can be best represented by the non-ideal end-member [(□Ca)Si2(O4OH2)□] obtained by the STC method (Supplementary material 1; serial number 16–18). As mentioned above, establishment of such an end-member formula where Si is the dominant B-site cation is not possible at present due to lack of clarity on the crystal-chemical role of Si in the pyrochlore structure.
At Sevattur, the most important feature of the pyrochlore is the lithology specific variation in composition and their unique alteration assemblages. Belkovite and baotite in dolomite and banded carbonatites are restricted exclusively to pyrochlore alteration assemblages. This lithology specific alteration assemblage indicates a very complex evolution for these pyrochlores and suggests that all have undergone several stages of alteration leading to formation of belkovite/baotite in an unknown host prior to their incorporation to the current host as altered antecrysts (a hypothesis also proposed for albitite-hosted pyrochlores at Sevattur; Dey et al., Reference Dey, Mitchell, Bhattacharjee, Chakarabarty, Pal, Pal and Sen2021). Therefore, they exhibit different styles of alteration and also different alteration assemblages, regardless of lithology (Fig. 2).
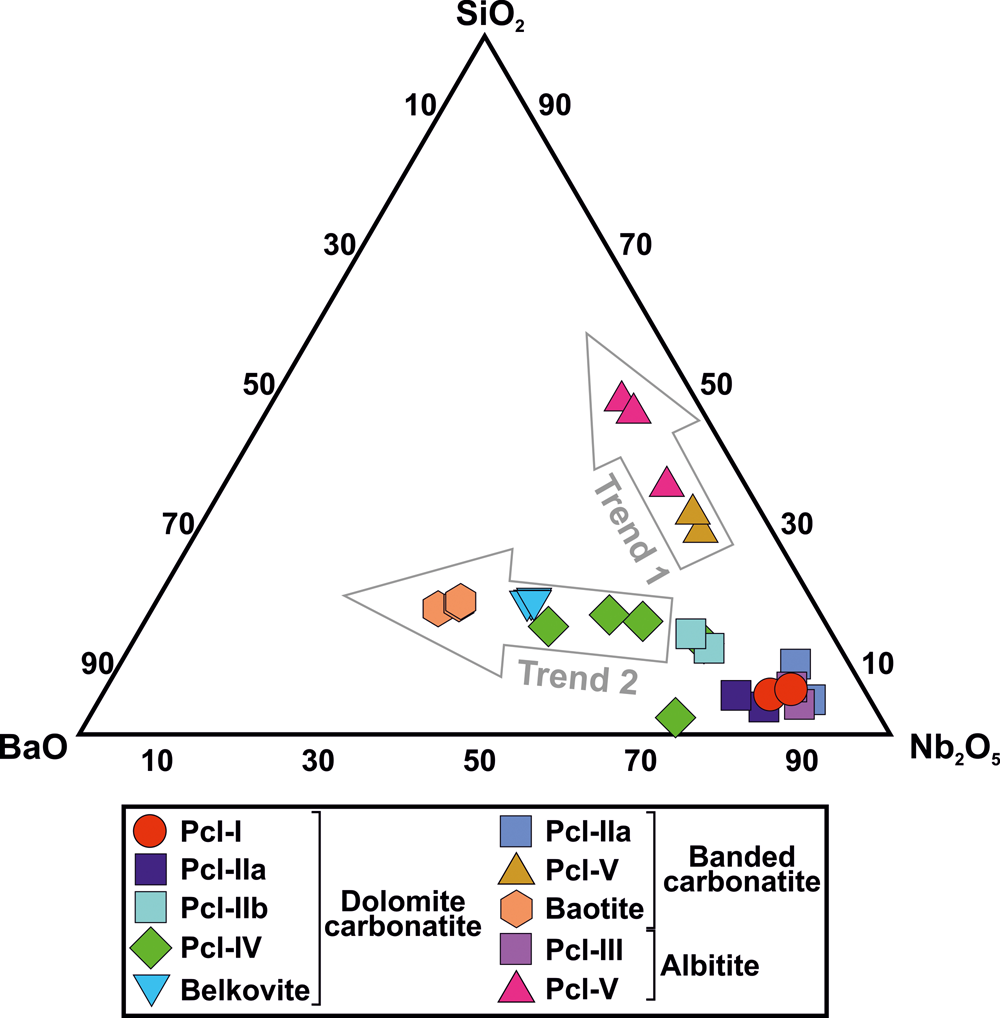
The ubiquitous presence of baryte suggests that a Ba-rich fluid affected all the lithological units at Sevattur with baryte forming very late in the paragenetic sequence. This event has resulted in Ba-Si-enrichment in all the pyrochlore types and baryte precipitation within the fractures formed by metamictisation. This is evident from the complete absence of Si-rich Pcl-V in the dolomite carbonatite as this unit is devoid of silicates compared to other Sevattur lithologies. Consequently, Ba-rich and U-poor Pcl-IV and baryte dominated the alteration assemblages formed after Pcl-IIa in the dolomite carbonatite. In contrast, the banded carbonatite and albitite with higher modal abundances of silicates facilitated the supply of Si during interaction with late-stage Ba-rich fluids (probably acidic) and formation of Pcl-V. Such Si-enrichment in all the pyrochlores and related alteration assemblages is shown in the BaO–Nb2O5–SiO2 ternary diagram (Fig. 10) which shows two trends: one with increasing Si content leading to the formation of Pcl-V (Trend 1) and another with both Ba and Si enrichment resulting in the formation of Pcl-IV, belkovite and baotite (Trend 2).

Fig. 10. Ternary plot of major oxides (Nb2O5–BaO–SiO2) (wt.%) for pyrochlore, belkovite and baotite showing two different trends. Trend 1 shows an extreme Si-enrichment that led to the formation of Si-rich Pcl-V. Trend 2 illustrates a progressive Ba-enrichment in Ba-rich Pcl-IV, belkovite and baotite within different Sevattur lithologies.
Conclusion
On the basis of texture and composition, five different pyrochlores (Pcl-I to Pcl-V) are recognised at Sevattur. Relatively unaltered, early pyrochlore generations (Pcl-I, -IIa and III) have elevated U–Ca–Nb contents compared to altered late-pyrochlores (Pcl-IIb, -IV and -V) (Fig. 11). The later-formed pyrochlores are characterised by higher Ba-, Si- and A-site vacancies. The compositional variations in different pyrochlore generations (Pcl-I to Pcl-V) suggest an overall transitional to secondary alteration.
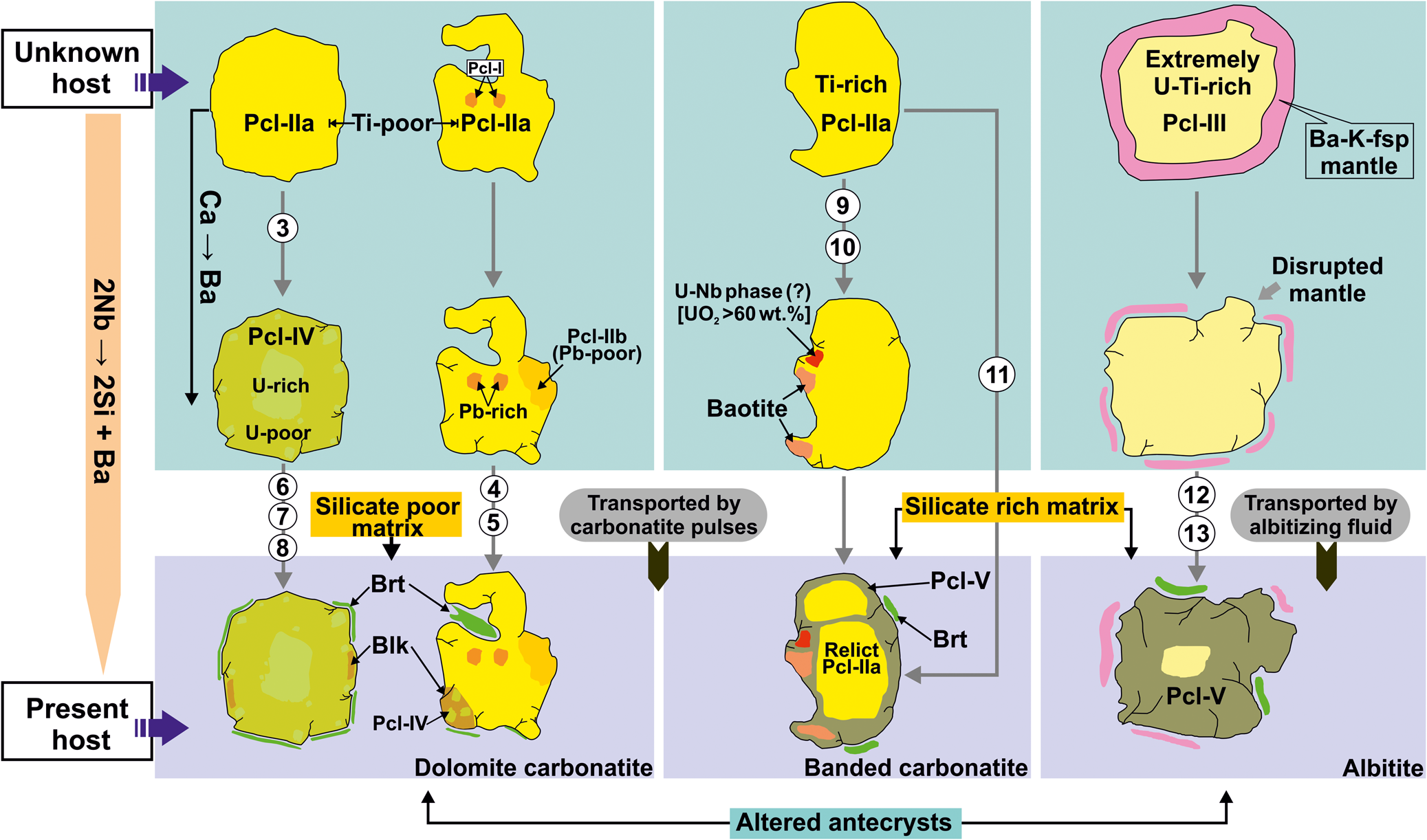
Fig. 11. Schematic diagram illustrating the lithology specific compositional evolution of pyrochlore and their alteration assemblages. The initial stage is the formation of early pyrochlore generations and their alteration assemblages in their respective source lithologies (unknown host). In the later stages, all the pyrochlore with their alteration assemblages were transported to their present host lithologies as altered antecrysts. Interaction with a late-stage Ba-Si-rich fluid produces Si-rich Pcl-V in banded carbonatite and albitite. Baryte (Brt) is present in all the Sevattur lithologies. Numbers marked in Arabic numerals indicate possible reaction(s) of formation of the individual pyrochlore species and related alteration assemblage(s). (see text for discussion).
The lithology specific alteration assemblage of Pcl-IIb + belkovite + Pcl-IV and baotite + Pcl-V ± U-Nb-rich phase in the dolomite and banded carbonatite formed after Pcl-IIa is a characteristic feature at Sevattur. These lithology specific alteration assemblages are suggestive of initial alteration within their respective sources before being carried by later carbonatite pulses as altered antecrysts to the present host (Fig. 11).
The presence of baryte in all Sevattur lithologies suggests that baryte formed late in the paragenetic sequence compared to the Pcl-I to Pcl-IV (Fig. 11). The association of baryte and Pcl-V in the banded carbonatite and albitite suggests that all the Sevattur rocks were affected by a late-stage Ba-rich acidic hydrothermal fluid. This fluid scavenged silica from the associated silicates to an extent dependent upon their relative modal abundances. This observation is in accordance with the presence of Si-rich Pcl-V in albitite and banded carbonatite as a consequence of the high modal abundance of silicate phases. This conclusion is further substantiated by the complete absence of Pcl-V in the dolomite carbonatite, which has a very low modal abundance of silicates (Fig. 11). Thus, the compositional diversity of pyrochlore-group minerals at Sevattur is complex and related to the superposition of multiple events spanning from magmatic to hydrothermal processes.
Acknowledgements
This work is supported by the Ministry of Mines (MOM), India, through an Extra Mural Project (F.No. 8/4/2009-Met.IV Dt. 27.11.2014) awarded to AKS and AC. MD, SB and AC also acknowledge Prof. K. N. Ganesh, Director, IISER Tirupati for providing financial assistance through an Institute Travel Grant to carry out necessary field work. R. H. Mitchell's work on alkaline rocks is supported by the Natural Sciences and Engineering Research Council of Canada, Lakehead University and Almaz Petrology Inc. We are thankful to Ferdinando Bosi and Kåre Kullerud for extending their help to understand the intricacies of the STC method and baotite structure. We acknowledge Jan Cempírek and an anonymous reviewer for their valuable suggestions which helped to improve the manuscript significantly. We acknowledge Stuart Mills and Helen Kerbey for the Editorial and production aspects of the publication of this work.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.37