Introduction
The mechanisms modulating the electrical events of sperm–egg interaction (Whitaker, Reference Whitaker2006), fertilization and cytoskeletal dynamics (Whitaker and Larman, Reference Whitaker and Larman2001) have been intensively investigated by use of the sea urchin model. Harrison et al. (Reference Harrison, Falugi, Angelini and Whitaker2002) found at about halfway through the first cell cycle (c. 40 min after fertilization) high spikes of calcium released from the inner stores due to excitation of muscarinic acetylcholine receptors (mAChRs) in two sea urchin species. A relationship between muscarinic receptors and the activation of ryanodine receptors (RyR) has been thoroughly demonstrated in different models and tissues (Galeotti et al., Reference Galeotti, Quattrone, Vivoli, Bartolini and Ghelardini2008; Liu et al., Reference Liu, Zheng, Korde, Yadav, Rathore, Wess and Wang2009). The presence of RyRs in sea urchin unfertilized eggs has been previously demonstrated (Galione et al., Reference Galione, Galione, McDougall, Busa, Willmott, Gillot and Whitaker1993b), possibly localized in the vesicles of the smooth endoplasmic reticulum (SER) (McPherson et al., Reference McPherson, McPherson, Mathews, Campbell and Longo1992, Lokuta et al., Reference Lokuta, Darszon, Beltrán and Valdivia1998). In fact, similar to excitable cells, the cytoplasm of sea urchin eggs, zygotes, and early embryos contains a reticulum of both rough and smooth endoplasmic organelles. The smooth organelles have the same functions as the vertebrate ones, including the presence of channels for intracellular [Ca2+] release. Here, the time-dependent presence and functionality of RyRs during the sea urchin first cell cycles was assessed by the method suggested by Lokuta et al. (Reference Lokuta, Darszon, Beltrán and Valdivia1998) to understand the function of RyRs in sea urchin early developmental events.
Materials and methods
Adult specimens of the sea urchin, Paracentrotus lividus were taken from the Portofino marine park in different year periods, along all the fertile season from October to the beginning of June. Spawning and fertilization were performed in standard sea water (SSW) pasteurized, oxygenated and ultra-filtered through a 0.22-µm Whatman filter. Samples were collected at different stages from unfertilized eggs to four-blastomere embryos.
Part of the sample was fixed for immunostaining of molecules immunologically related to RyRs and to α-tubulin, while the major part was processed for microsome preparation and incorporated into artificial membranes for electrophysiological recording. All the reagents were obtained from Sigma Chemical Co. (Milan, Italy) except calmodulin (Boehringer-Mannheim GMBH) and anti-RyR antibody (ABCAM) and anti-alpha-tubulin (Proteintech).
Immunocytochemical staining
After fixation with cold paraformaldehyde (PFA) 4% in seawater, whole cells were incubated in the cold overnight in primary antibody 1/100 PBS. For molecules immunologically related to RyR, the antibody that gave the best result was the anti-ryanodine receptor antibody [34C] (ab2868) abcam, with a large spectrum of predicted activity (including amphibians and fish). For α-tubulins, the antibody used was polyclonal rabbit IgG, with a wide range of reactivity (mammalians, fish and beetles; Proteintech). After incubation, the materials were rinsed and incubated in the secondary antibody (Alexafluor).
Cortical microsomes preparation
Samples formed by 50 µl of concentrated eggs/embryos (c. 95,000–100,000 eggs/embryos) were stored at −80°C. Cortical membranes were prepared according to the protocol suggested by McPherson et al. (Reference McPherson, McPherson, Mathews, Campbell and Longo1992), modified by Lokuta et al. (Reference Lokuta, Darszon, Beltrán and Valdivia1998) to be adapted for sea urchin eggs. All the procedure was performed on ice. The eggs, zygotes and early morulae were homogenized and centrifuged for 2 min at 2000 rpm (refrigerated Beckman high speed centrifuge, 64xG). The pellet was resuspended in 1 ml of modified SW (MSW), in which NaOH is substituted by 30 mM TRIS pH 8.0 in 1 mM EGTA, and described by Mueller and colleagues (Reference Mueller, Rudin, Tien and Wescott1962) and incorporated in the phospholipid bilayer, as described by Mueller and colleagues (Reference Mueller, Rudin, Tien and Wescott1962), for single-channel recording. The signal from the single-channel current was recorded using a 200 A axopatch amplifier (Axon instruments) at 20 mV holding potential and is shown as upwards deflections.
Data were acquired at 200 μs/point and filtered at 1 kHz. Single-channel analysis was carried out according to Colquhoun and Sakmann (Reference Colquhoun and Sakmann1985). Channel open probability was determined from the areas of Gaussian distributions that best fitted the peaks of amplitude histograms from recordings of 30 s duration. Drugs activating/blocking single-channel currents were added to the cis compartment.
The planar bilayer measurements and data analyses were performed according to the experimental procedures described by Buratti et al. (Reference Buratti, Prestipino, Menegazzi, Treves and Zorzato1995).
Characterization of the receptors
Characterization of the receptor was carried out by analyzing the pharmacological properties of the RyR Ca2+-releasing channel reconstituted into bilayers by adding in cis compartment compounds that specifically modulated the mammalian skeletal muscle RyR (Galione et al., Reference Galione, Lee and Busa1991). Specific blockers, such as 2 mM MgCl2 and 25 nM ruthenium red (RuRed), and specific activators, such as 25 mM caffeine, were used to check the activation of cADPR-induced calcium channels, and inhibition of the IP3-induced calcium release (Lokuta et al., Reference Lokuta, Darszon, Beltrán and Valdivia1998; Galione et al., Reference Galione, Lee and Busa1991). Here, 50 nM calmodulin (CaM) was used to activate the RyR channel at a calcium concentration corresponding to the SR one (from 390 μM, reduced by RyR excitation to 8 µM; according to Ziman et al., Reference Ziman, Ward, Rodney, Lederer and Bloch2010); 5 mM ATP, followed by 25 nM ryanodine and 10 µM of ruthenium red was used to check the subsequent reversibility of activation.
Results
Frequency of incorporated RyR channels
The presence of RyR channels was identified at the unfertilized egg and 10 min zygote stage with almost the same frequency (frequency = percentage of responding membranes/number of tested membranes). The maximum frequency of reactive receptors was found at c. 40 min after fertilization, this decreased at the two-blastomere stage and disappeared at the four-blastomere stage (Figure 1).
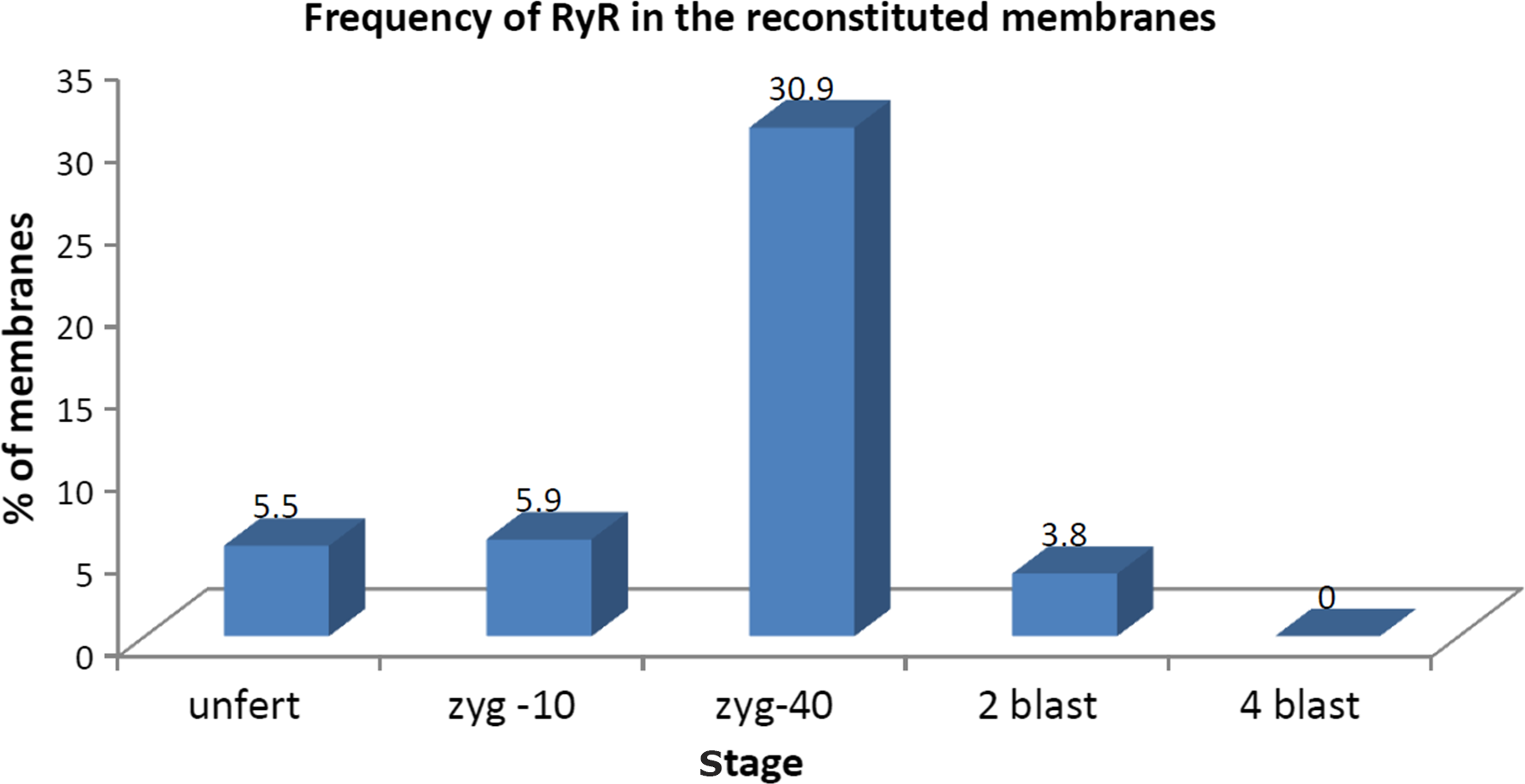
Figure 1. Percentage of membranes presenting RyR responses obtained from eggs and embryos at different stages. unfert, unfertilized eggs; zyg-10, zygotes at 10 min after fertilization; zyg-40, zygotes at 40 min after fertilization; 2 blast, two-blastomere stage; 4 blast, four-blastomere stage.
Positive membranes showed traces of a Ca2+-conducting channel elicited by a pulse of 20 mV, with a rather intense activity with opening times longer than closures (Fig. 2A). The related current amplitudes showed a peak at 4.75 pA corresponding with the open state (Fig. 2B), with a conductance level of 240 pS. The open probability was 0.84. These recordings were obtained from microsomes of sea urchin zygotes at 40 min after fertilization, synchronous with the calcium-induced retraction of the spindle fibres (shown in Fig. 3B) by an α-tubulin immunoreaction (Fig. 3).
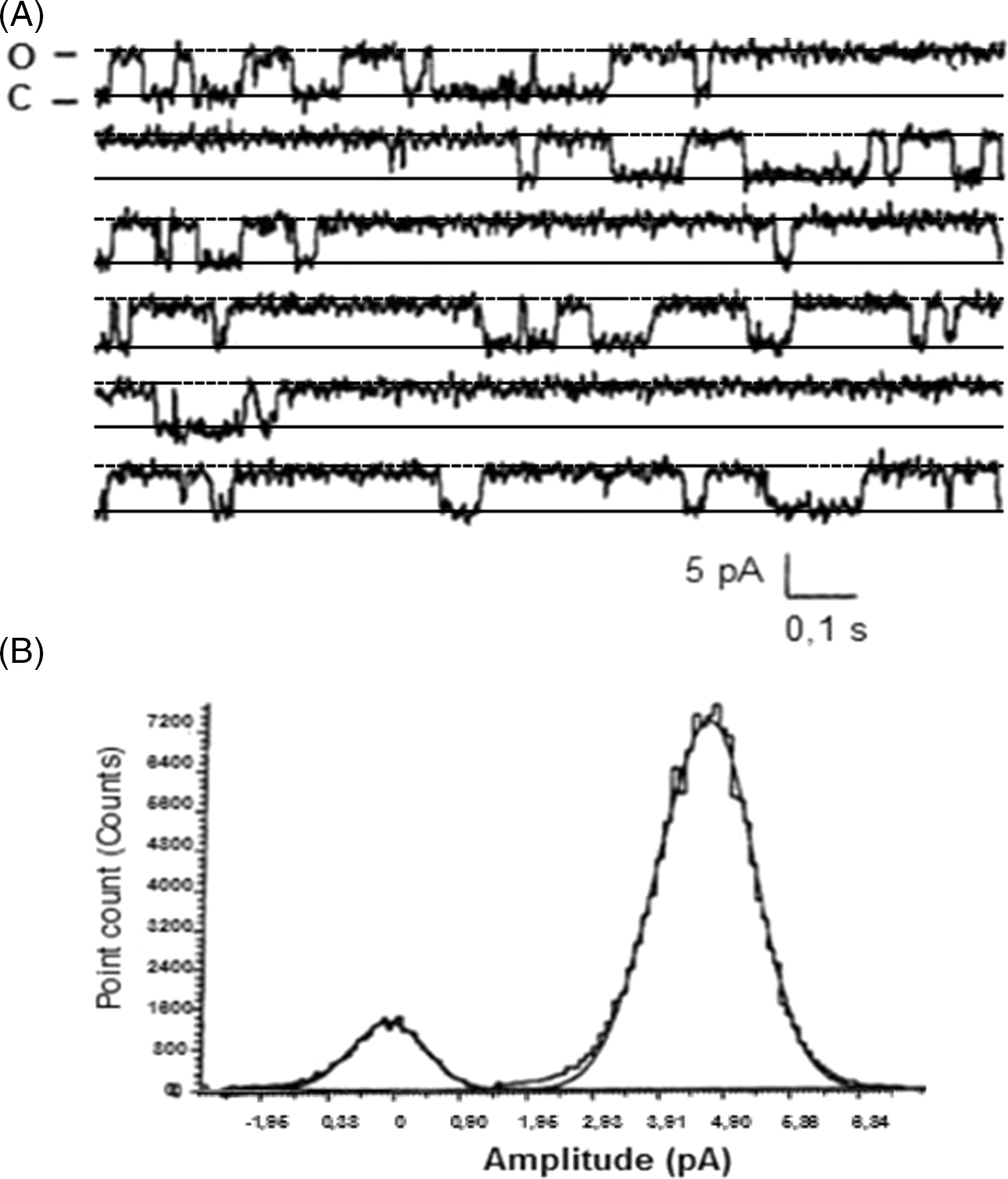
Figure 2. Single-channel recordings after incorporation of microsomes obtained from membranes of Paracentrotus lividus (Pl) zygotes at 40 min after fertilization. (A) Current recordings at +20 mV in the presence of 1:6 cis/trans concentration gradient. Here, 5 µl of microsomes was previously added in cis. (B) Amplitude histograms, showing two spikes at 0 and 4.75 pA corresponding to the closed and open states of the channel, respectively.
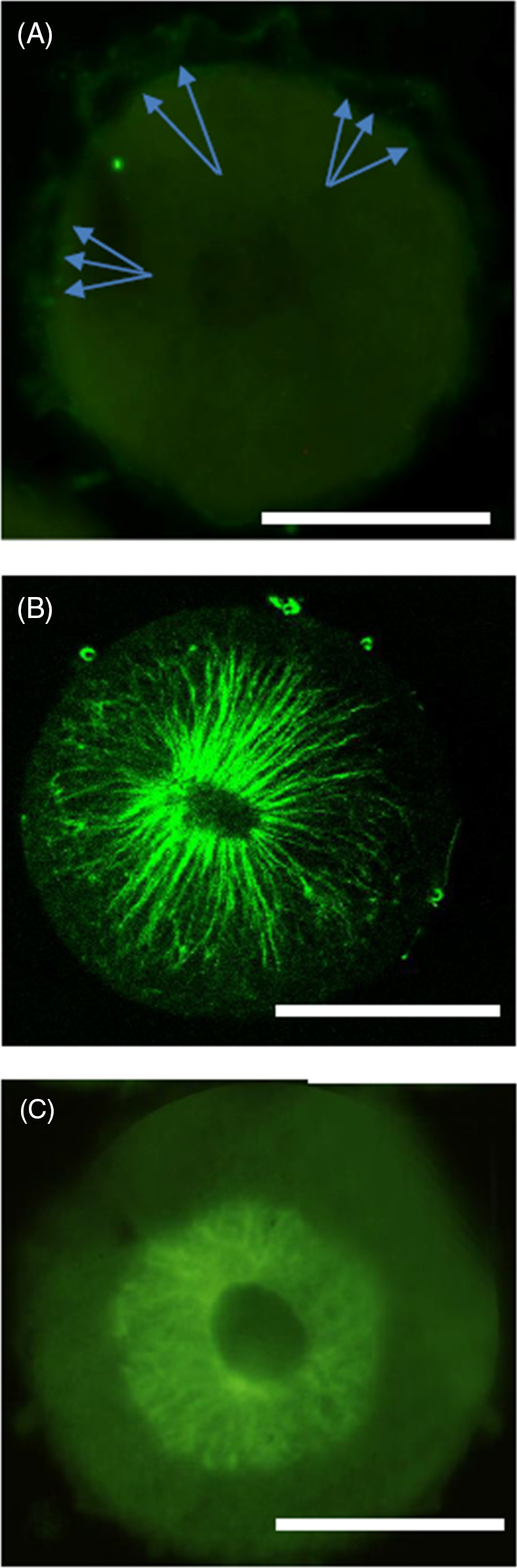
Figure 3. Whole mount immunoreactivity of 40 min zygote. (A) RyR-like staining of cortical vesicles. Arrows point to some vesicles, just beneath the plasmalemma. (B) α-Tubulin immunostaining showing the spindle fibres 40 min after fertilization. (C) Disorganization of microtubules after calcium release inhibition. Nucleus breakdown also seems slower. Scale bars, 50 µm.
Characterization of the receptor
Evidence for these results is supplied in Supporting Information Figures S1–S3:
-
2 mM MgCl 2 in the cis compartment caused channel closure at 0 pA (Fig. S1A).
-
Ruthenium red (RuRed) blocked all the current fluctuations and maintained the channel in a long-lasting close state (Fig S1B).
-
25 mM caffeine caused a strong increase in current fluctuations increasing the probability of channel opening to 0.73 (Fig S2A).
-
50 nM calmodulin in the presence of Ca2+–EGTA in the cis compartment activated the RyR channel at a Ca2+ concentration corresponding to the SR one, increasing the probability of the channel opening to 0.81.
-
ATP and ryanodine (Fig. S3B) caused a marked increase in channel activity, rapidly fluctuating between the open and closed states; the subsequent addition of ryanodine blocked the channel in the open state (Fig. S3C), causing almost complete inhibition of the fluctuation between the two channel states.
This experiment shows an apparent long-lasting state of subconductance, similar to the effect shown by Rousseau et al. (Reference Rousseau, Smith and Meissner1987) and Lindsay et al. (Reference Lindsay, Tinker and Williams1994) for skeletal and cardiac muscle. Further addition of 10 µM RuRed also caused strong inhibition of channel activity (Fig. S3D), suggesting that RuRed may antagonize the RyR effect by inducing a long-lasting closed state. These results showed the similarity between the RyRs present in the sea urchin zygote and in vertebrate skeletal and cardiac muscle membranes (Table 1).
Table 1. Comparison between the responses of RyR of sea urchin cortical SER and mammalian skeletal muscle (SR)

CaM = Calmodulin; RuRed = ruthenium red; Rya = Ryanodine.
a Rousseau et al., Reference Rousseau, Smith and Meissner1987; McPherson et al., Reference McPherson, McPherson, Mathews, Campbell and Longo1992; Lindsay et al., 1995; Lokuta et al., Reference Lokuta, Darszon, Beltrán and Valdivia1998; Pérez et al., Reference Pérez, Marengo, Bull and Hidalgo1998.
Whole mount immunostaining of the zygote at 40 min
RyR-like molecules were faintly stained in the membrane of vesicles in the cortical region of the cell, just beneath the plasmalemma (Fig. 3A)
Molecules immunologically related to α-tubulin showed the different aspects of microtubules in normal (Fig. 3B) and inhibited (Fig. 3C) zygotes.
Additional experiments were performed to check the presence of RyR expression along the development from unfertilized egg to pluteus stage. RT-PCR showed the presence of the signal at all the stages, with a peak at the zygote stage (Fig. S4).
Discussion
The reports available in the literature about the presence of RyRs in echinoderms refer to unfertilized eggs (Galione et al., Reference Galione, White, Willmott, Turner, Potter and Watson1993a; Lokuta et al., Reference Lokuta, Darszon, Beltrán and Valdivia1998) evoking calcium waves at fertilization (Galione et al., Reference Galione, Galione, McDougall, Busa, Willmott, Gillot and Whitaker1993b). The presented outcomes suggest that the RyR channels are mainly involved in Ca2+ fluctuations occurring at the first cleavage. In fact, high intracellular [Ca2+] is known to activate nucleus breakdown (Whitaker and Larman, Reference Whitaker and Larman2001) and microtubule depolymerization involved in chromosome transport (Wolniak et al., Reference Wolniak, Hepler and Jackson1983). The absence of responsiveness in the membranes obtained from the four-blastomere stages agrees with the finding that, at these stages, the cleavages presented Ca2+ waves of lower amplitude and were due to L-type channels (Dale et al., Reference Dale, Yazaki and Tosti1997), while the high frequency found at the 40 min zygote stage seemed to correspond rather well with the Ca2+ spikes evoked by muscarinic drugs at the beginning of the first prophase stage (Harrison et al., Reference Harrison, Falugi, Angelini and Whitaker2002).
The RyRs present in sea urchin zygote and in skeletal and cardiac muscle seem to be similar, with small differences. (Pérez et al., Reference Pérez, Marengo, Bull and Hidalgo1998) (Table 1). These results suggested the pre-eminent role of RyR function during the cleavage of the first blastomeres (Dale et al., Reference Dale, Yazaki and Tosti1997; Galione and Churchill, Reference Galione and Churchill2002; Harrison et al., Reference Harrison, Falugi, Angelini and Whitaker2002; Whitaker, Reference Whitaker2006), while other calcium-dependent events, such as egg activation or cleavage at the four-blastomere stage may depend on alternative or parallel pathways for intracellular calcium dynamics that could alternate or cooperate in the different phases of sea urchin development.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0967199421000514
Acknowledgements
We thank Marco Millio (IBF) for skilled help in figure acquisition.
Conflicts of interest
All the authors declare no competing financial interests.
Financial support
This work was supported by endowment funds of CNR Biophysics Institute and Laboratory of Developmental Neurobiology of University of Genoa.
Ethical standards
Not applicable
Supporting information
Additional Supporting information is available with the online version of the article.






