Introduction
Endocrine disrupting chemicals (EDCs) are a class of compounds that are capable of interfering with hormone activity. Exposure to EDCs during development has been linked to increased risk of developing disease in adulthood, following the Developmental Origins of Health and Disease paradigm.Reference Haugen, Schug, Collman and Heindel 1 Phthalates are one such example. Phthalates are chemicals added to plastics and personal care products and are present in a wide variety of consumer products, resulting in ubiquitous human exposure.Reference Schettler 2 , Reference Heudorf, Mersch-Sundermann and Angerer 3 Exposure to high levels of phthalates during the period of reproductive tract development leads to malformations in the reproductive tract, including hypospadias and cryptorchidism in males as demonstrated by both animal and human studies,Reference Foster 4 , Reference Hsieh, Breyer, Eisenberg and Baskin 5 and dysregulated ovarian function in females in rodent studies.Reference Hannon and Flaws 6 More recently, human and animal studies have suggested that developmental exposure to lower levels of phthalates may increase the risk of obesity and other features of metabolic syndrome, such as insulin resistance.Reference Hao, Cheng, Guo, Xia and Ma 7 – Reference Strakovsky, Lezmi and Shkoda 10 However, epidemiological studies have presented conflicting evidence with some studies demonstrating associations between early-life phthalate exposure and increased risk for obesity and insulin resistance, whereas other studies found no associations or negative associations.Reference Buckley, Engel and Braun 11 , Reference Shoaff, Papandonatos and Calafat 12 One factor that may contribute to inconsistencies in epidemiological literature is difficulty in interpreting effects of exposures to phthalate mixtures.Reference Braun, Gennings, Hauser and Webster 13 , Reference Braun, Just and Williams 14 Humans are exposed to mixtures of several phthalates, as evidenced by data from the National Health and Nutrition Examination Survey indicating that metabolites from 13 different phthalates were detected in urine samples from a representative study population from the United States.Reference Carlson and Garland 15 Thus, animal experiments utilizing a mixtures approach to characterize metabolic outcomes resulting from developmental phthalate exposures will aid in the interpretation of human epidemiological studies.
Phthalate exposure during development may influence risk of reproductive and metabolic effects by altering the epigenome. The epigenome consists of heritable factors that influence chromatin structure and gene expression without a change in the underlying DNA sequence. DNA methylation at CpG sites is the most widely studied type of epigenetic factor, and consists of a methyl group attached to the 5′ position of a cytosine adjacent to a guanine (C-G dinucleotide). DNA methylation marks are inherited across mitotic cellular divisions and are relatively stable in somatic cells. However, during development, the epigenome is extensively reprogrammed, undergoing waves of DNA de-methylation and re-methylation during pre-implantation and during gametogenesis.Reference Reik, Dean and Walter 16 , Reference Jirtle and Skinner 17 Thus, exposure to environmental chemicals that interfere with this process may result in an altered epigenome that persists throughout the life course.Reference Dolinoy, Das, Weidman and Jirtle 18 , Reference Faulk and Dolinoy 19 Developmental phthalate exposures have been shown to impact DNA methylation levels at loci near genes that are relevant to hormone signaling, including reproductive and metabolic signaling, in germ cells and offspring in both animal and human studies.Reference Zhang, Zhang and Li 20 – Reference Wu, Estill and Shershebnev 24 For example, a recently published birth cohort study identified 27 differentially methylated regions (DMRs) in cord blood from newborns that were associated with gestational exposure to phthalates; many of these DMRs were associated with exposure to multiple phthalates.Reference Solomon, Yousefi and Huen 23 However, no studies to date have examined the mixture effects of developmental phthalate exposures on DNA methylation.
Repetitive elements are particularly susceptible to epigenetic re-programming via developmental exposures, including intracisternal A-particle (IAP) retrotransposons in mice.Reference Dolinoy, Das, Weidman and Jirtle 18 , Reference Waterland and Jirtle 25 IAP retrotransposon repetitive elements are present throughout the mouse genome (several thousand IAPs have been detected in the mouse genome),Reference Qin, Wang and Shang 26 , Reference Faulk, Barks and Dolinoy 27 and contain long terminal repeats (LTRs) carrying CpG sites. DNA methylation at these CpG sites influences expression and regulation of nearby genes, which could have implications for disease risk. One such IAP is found in the cryptic promoter region of the A vy allele in the viable yellow agouti mouse model (A vy ).Reference Dolinoy 28 The A vy mouse model and its use in environmental epigenetics have been thoroughly described in previous studies and literature reviews.Reference Dolinoy, Huang and Jirtle 29 – Reference Dolinoy, Weinhouse, Jones, Rozek and Jirtle 31 In brief, DNA methylation levels at CpG sites within the IAP LTR in the promoter region of the A vy locus are highly correlated with A vy expression and therefore with the coat color of the mouse; yellow fur corresponds to low DNA methylation and high ectopic agouti expression, and brown fur corresponds to high DNA methylation and low agouti expression. DNA methylation at the A vy locus is stochastic and set during early development. Therefore, the coat colors of mice in a colony are approximately normally distributed. However, coat color distributions can be shifted toward brown or yellow in mice exposed to toxicants or nutrients during early development, acting as a biosensor for altered DNA methylation.Reference Dolinoy 28 , Reference Dolinoy, Huang and Jirtle 29
Here, we utilized an established mouse model of developmental exposure to evaluate effects of three individual phthalates and two phthalate mixtures on weaning body weight, organ weight, coat color shift and tail DNA methylation at a specific IAP (A vy ) and IAPs on a global scale. We chose to examine dietary exposure of two high molecular weight (HMW) phthalates, diethylhexyl phthalate (DEHP) and diisononyl phthalate (DINP), and one low molecular weight (LMW) phthalate, dibutyl phthalate (DBP), as well as a mixture of the two HMW phthalates (DEHP+DINP) and a mixture of all three phthalates (DEHP+DINP+DBP). We evaluated offspring at postnatal day (PND) 21, which is roughly equivalent to early childhood, in order to provide comparisons with human birth cohort studies that examine metabolic outcomes and DNA methylation in early childhood. To our knowledge, this is the first study to characterize the effects of developmental exposure to phthalate mixtures on weaning body weight, organ weights and DNA methylation in a mouse model.
Materials and methods
Animals and exposures
This study utilized A vy mice from a colony maintained for >220 generations with sibling mating and forced heterozygosity for the A vy allele that is passed paternally, resulting in a genetically invariant background, 93% similar to C57BL/6.Reference Waterland and Jirtle 32 , Reference Weinhouse, Anderson and Bergin 33 A vy /a mice range in coat color from yellow to brown based on epigenetic modifications present at the A vy locus.Reference Dolinoy 28 , Reference Dolinoy, Weinhouse, Jones, Rozek and Jirtle 31 , Reference Waterland and Jirtle 32 , Reference Miltenberger, Mynatt, Wilkinson and Woychik 34 Mice were exposed to phthalates perinatally through maternal dietary consumption, with an exposure period that spanned pre-conception to weaning; Fig. 1 contains a schematic describing the experimental design of the mouse perinatal exposure study. Two weeks before mating, virgin a/a dams at 6–8 weeks of age were randomly assigned to one of six diets: (1) 7% corn oil control (phytoestrogen-free Teklad diet TD-95092; ENVIGO, Madison, WI), (2) 25 mg DEHP/kg chow, (3) 25 mg DBP/kg chow, (4) 75 mg DINP/kg chow, (5) 25 mg DEHP+75 mg DINP/kg chow or (6) 25 mg DEHP+75 mg DINP+25 mg DBP/kg chow. Dams (a/a) were mated with A vy /a males 2 weeks after being placed on the experimental diets. Dams remained on experimental diets throughout gestation and lactation until weaning. The birth was designated PND0, and pups were weaned 3 weeks after birth at PND21.

Fig. 1 Experimental design. Virgin a/a dams were given one of six types of chow containing either corn oil or phthalates dissolved in corn oil 2 weeks before mating with an A vy /a male. They remained on this chow throughout gestation and lactation until weaning at postnatal day (PND) 21. Body weights at PND21 and tail global intracisternal A-particles (IAP) DNA methylation were analyzed from both A vy /a and a/a offspring. Coat color distribution and A vy DNA methylation from tail tips were analyzed in only A vy /a offspring, and organ and tissue weights were analyzed from a subset of a/a mice. A schematic of the A vy and global IAP assays can be found in Faulk et al.Reference Faulk, Barks, Liu, Goodrich and Dolinoy 61 and Montrose et al.Reference Montrose, Faulk, Francis and Dolinoy 63 , respectively. DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate; A vy , viable yellow agouti.
Exposure diets were based on ENVIGO Teklad diet TD-95092, with phthalate diesters (Sigma) mixed into the corn oil used for the chow. Concentrations in chow were intended to result in estimated maternal doses of 5 mg/kg/day for DEHP and DBP and 15 mg/kg/day for DINP, based on the assumption that pregnant and nursing female mice weigh ~25 g and eat ~5 g of chow per day.Reference Vandenberg, Welshons, Vom Saal, Toutain and Myers 35 These target doses were selected based on studies in the published literature that observed obesogenic effects, including increased body weight, in animals perinatally exposed to phthalates.Reference Schmidt, Schaedlich, Fiandanese, Pocar and Fischer 36 – Reference de Jesus, Negrin, Taboga, Pinto-Fochi and Góes 38 At the beginning of the study, there were no such studies that found metabolic changes consistent with obesogenic effects for DINP; therefore, a three-fold higher target dose of DINP was selected based on its three-fold lower anti-androgenic potency relative to DBP and DEHP.Reference Hannas, Lambright and Furr 39 Although fetal exposure was not directly assessed in this study, a previously published studyReference Calafat, Brock and Silva 40 administered 11 mg/kg/day of DEHP to pregnant rats and found mean levels of DEHP metabolites of 68 ng/ml in amniotic fluid. Amniotic fluid phthalate concentrations were likely lower than 68 ng/ml for the present study (estimated daily intake of 5 mg/kg/day), and would, therefore, be within the range of measured amniotic fluid levels in human populations,Reference Lorber and Calafat 41 – Reference Huang, Kuo, Chou, Lin and Lee 47 albeit on the higher end (Table 1).
Table 1 Comparison of diethylhexyl phthalate (DEHP) daily intakes and DEHP metabolites in amniotic fluid in human and rodent studies

LOD, Limit of Detection.This table was adapted from the recent National Academies of Sciences, Engineering and Mathematics (NASEM) report on implementing systematic reviews to evaluate low-dose effects of endocrine disrupting chemicals. 64 Data from human studies includes a variety of populations.Reference Lorber and Calafat 41 – Reference Huang, Kuo, Chou, Lin and Lee 47 One rat studyReference Calafat, Brock and Silva 40 examined concentrations of DEHP metabolites in amniotic fluid following oral doses of 11 and 100 mg/kg/day and was used to estimate potential amniotic fluid concentrations in the present mouse study.
A total of 98 litters were generated, with 17 control litters, 16 DEHP litters, 15 DBP litters, 15 DINP litters, 16 DEHP+DINP litters and 19 DEHP+DINP+DBP litters. Approximately half of the total offspring were A vy /a (50.5%, n=312) and half were a/a (49.5%, n=296). One DEHP litter (eight pups) and one DEHP+DINP+DBP litter (12 pups) were removed from further analyses based on high pup mortality between PND14 and PND21, which was determined to be due to poor maternal care after animal handlers examined pups. Maternal body weights were recorded at the start of exposure (2 weeks before mating), at mating and at the time of weaning.
At weaning on PND21, body weights were recorded for all a/a and A vy /a offspring, and tail tips were collected from all A vy /a offspring. A subset of a/a mice (n=152) were sacrificed for organ and tissue collection, including tail tips, at PND21. Organs were not collected or analyzed from A vy /a mice. For organ and tissue collection, mice were fasted during the light cycle for 4 h beginning in the morning, and then underwent CO2 euthanasia and cardiac puncture in the afternoon, followed by whole-body perfusion with saline (Sigma). Body weights were taken both pre- and post-fasting. Organs were weighed at the time of collection. On PND21, a single observer classified each A vy /a offspring (n=311) into one of five categories based on the proportion of brown fur (yellow, <5% brown; slightly mottled, between 5 and 40% brown; mottled, between 40 and 70% brown; heavily mottled, between 70 and 95% brown; pseudoagouti, >95% brown). Coat color classifications were used as a visual proxy and preliminary assessment of DNA methylation at the A vy allele.
All animals were given food and water ad libitum and kept on a 12-h light–dark cycle. The daily health of the animals was monitored by the University Unit for Laboratory Animal Medicine (ULAM). Mice were treated humanely, and the guidelines for the use and care of laboratory animals were followed throughout the study. The University of Michigan Institutional Animal Care and Use Committee (IACUC) approved all animal procedures used for this project.
Hepatic triglyceride levels
Hepatic triglyceride levels were measured in wildtype non-agouti a/a mice that were sacrificed at PND21. Frozen liver tissue (30–35 mg) was homogenized in lysis buffer containing 10% NP-40 (ThermoFisher Scientific, Waltham, MA), 50 mM Tris-HCl (Sigma) and 100 mM NaCl, and then triglycerides were extracted using chloroform (ThermoFisher Scientific). Triglyceride levels were measured using spectrometry; Infinity Triglycerides reagent (ThermoFisher Scientific) was added to each sample and then read at 550 nm and 660 nm and compared with glycerol standards to estimate triglycerides per milligram of liver tissue.
DNA isolation and methylation analysis
Genomic DNA was isolated from PND21 tail tissue, flash frozen and stored at −80°C from all A vy /a and a/a offspring. Approximately 0.5 cm of thawed distal tail tissue was placed in a tube containing 173.32 µl of Buffer ATL and 26.67 µl of Proteinase K and then incubated overnight at 50°C on a shaker. Samples were then cooled to room temperature before transferring to the Maxwell® 16 Mouse Tail DNA Purification Kit cartridges (Promega Corporation, Madison, WI) using manufacturers’ standard protocol. One modification was made in which only 250 µl of elution buffer was added to the final elution tube to maximize final DNA concentration. DNA concentration was quantified using the NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific); concentrations ranged from 50 to 100 ng/µl.
Genomic DNA was bisulfite converted using the EZ-96 DNA Methylation Kit protocol (Zymo Research Corp., Irvine, CA), which converts all unmethylated cytosines to uracils and methylated cytosines are left unaffected.Reference Grunau, Clark and Rosenthal 48 For A vy /a samples (n=302), 500 ng of DNA was used as input for conversion and 1000 ng was inputted for all a/a samples (n=152); both 500 ng and 1000 ng are considered acceptable according to the EZ-96 DNA Methylation Kit, but 500 ng was used for A vy /a samples due to lower tissue amounts and subsequent DNA concentrations extracted from some samples. After bisulfite conversion, amplification of A vy and global IAP regions was performed by standard polymerase chain reaction (PCR) in order to attain enough DNA to pyrosequence. Each PCR reaction well included 19.4 µl of HotStarTaq DNA Polymerase (Qiagen Inc., Germantown, MD), 0.12 µl forward primer (Invitrogen, Waltham, MA), 0.12 µl biotinylated-reverse primer (Invitrogen) and 13.36 µl H2O. A total of 2 µl of bisulfite converted DNA were added to each well to complete the 35 µl reaction. Each PCR run included a no-template control and laboratory methylation controls (0, 25, 50, 75, 100%). Assay specifications are included in Table 2. PCR amplification lengths were confirmed by the Qiagen QIAxcel Advanced System (Qiagen) and QIAxel ScreenGel software.
Table 2 PCR and pyrosequencing assay conditions

A vy , viable yellow agouti; IAP, intracisternal A-particles.
a Assay adapted from Faulk et al.Reference Faulk, Barks, Liu, Goodrich and Dolinoy 61
b Assay adapted from Montrose et al.Reference Montrose, Faulk, Francis and Dolinoy 63
Pyrosequencing with the PyroMark Q96 ID (Qiagen) was used to assess DNA methylation percent at four CpG sites of interest for A vy and IAP regions on a global scale. The global IAP assay covers all IAPs on the mouse genome with intact LTRs, including the A vy IAP. The protocol followed is outlined in the PyroMark Q96 ID User Manual. PyroMark software determines the percentage of methylation by calculating the fraction of methylated cytosines (read as cytosine after PCR) over the total sum of methylated and unmethylated cytosines (read as thymines after PCR). The amount of input DNA used for both assays was 16 µl. All A vy and global IAP assay specifications and pyrosequencing information are available in Table 2.
Statistical analysis
All statistical analyses were conducted using R version 3.3.1 (www.R-project.org). Exposure-related differences in sex ratio, genotype ratio and survival rate were determined by Fisher’s exact test, comparing each exposure group with the control group. For sex and genotype ratios, exposure groups were also compared with expected values of 50%, since these are the values expected based on standard genetic probabilities. Coat color distribution across exposure groups was compared via the χ2 goodness-of-fit test with the control coat color distribution as the reference. Coat color distribution analyses could not be stratified by sex because doing so yields some coat color categories within exposure groups having a count of 0, making it difficult to make interpretable statistical comparisons. Differences in litter size across the six experimental groups were compared using an ANOVA with a post-hoc Tukey test. Body weight, organ weights and DNA methylation for each exposure group were compared with controls using linear mixed-effects model with the lme4 and lmeTest packages in R. Mixed-effects models were used to account for inherent correlations between littermates, and were also used to account for correlations between DNA methylation levels across nearby CpG sites. Number of pups in each litter was added into models evaluating exposure effects on body weight to control for litter size. Tail A vy methylation levels were bimodally distributed, and we therefore split A vy methylation data into high and low methylation groups, based on the median methylation of the control group (i.e. low methylation=methylation level below the control median, and high methylation=methylation level above the control median) in order to make statistical comparisons.Reference Waterland and Jirtle 32 All analyses were stratified by genotype so that comparisons were made across isogenic mice. Analyses were also stratified by sex if initial analyses indicated that sex modified exposure effects.
Results
Litter parameters
Perinatal exposure to phthalates did not significantly alter genotype ratio, sex ratio or mean pups per litter compared with controls, but did impact mortality rate in pups through weaning at PND21 (Table 3). Overall mortality rate in controls was 8.3%. Compared with controls, there was a reduced pup mortality rate in the DBP exposure group (mean=1.1%, P=0.02) and increased pup mortality rate in the DEHP exposure group at a level trending towards significance (mean=15.5%, P=0.10). One litter exposed to DEHP and one exposed to a mixture of all three phthalates were removed from subsequent analyses due to high pup mortality between PND14 and PND21 which was attributed to poor maternal care and hyperactive maternal behavior.
Table 3 Litter outcomes: litter size, mortality rates, genotype ratio and sex ratio across exposure groups

DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate.
Genotype and sex ratios were determined for pups that survived until postnatal day 21 (PND21) and do not include pups not surviving until PND21.
*P⩽0.05 v. control, ^P⩽0.10 v. control.
Body weight
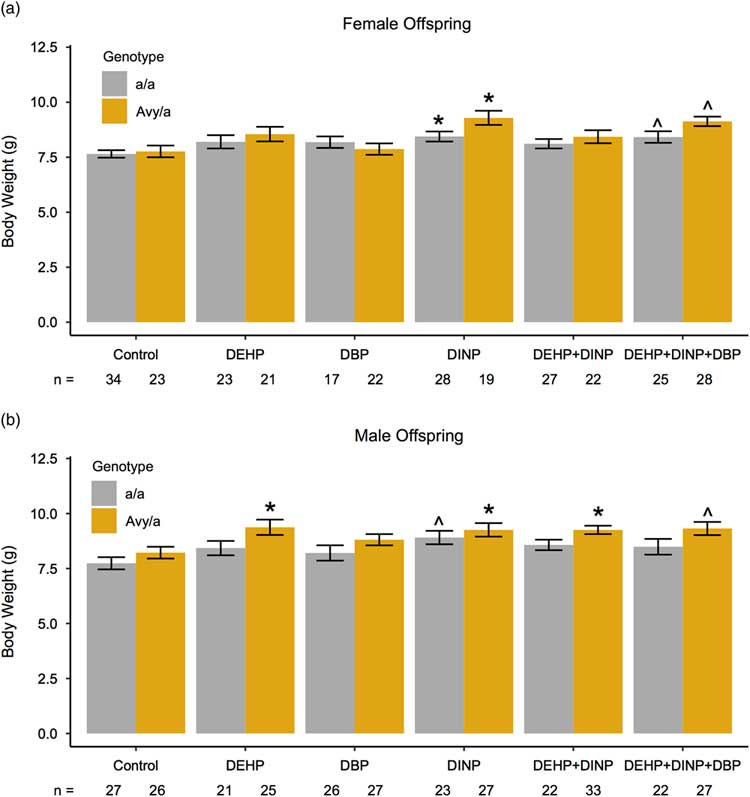
Mice that were perinatally exposed to phthalates and phthalate mixtures had increased body weights at PND21 (Fig. 2). Different phthalate exposures exhibited significant impacts on body weight, depending on sex and genotype. Perinatal exposures to DINP and a mixture of all three phthalates were associated with increased body weights in females (Fig. 2a). Female a/a and A vy /a mice exposed to DINP weighed more than female a/a and A vy /a control mice (P=0.03, P=0.01, respectively). Wild-type a/a and A vy /a females exposed to DINP weighed an average of 8.44 and 9.29 g, respectively, whereas a/a and A vy /a control females weighed an average of 7.65 and 7.77 g, respectively. Females of both genotypes exposed to a mixture of DEHP+DINP+DBP also had increases in body weight at PND21 that approached statistical significance (P=0.08 for both genotypes); a/a females exposed to all three phthalates weighed 8.42 g on average and A vy /a females exposed to all three phthalates weighed 9.13 g on average.

Fig. 2 Postnatal day 21 body weights in females (a) and males (b) developmentally exposed to phthalates and phthalate mixtures. Body weights of mice in each exposure group were compared with those of control mice using linear mixed-effects models controlling for number of pups per litter and within litter effects. Analyses were stratified by sex and genotype. Bars represent the mean for each group and error bars represent standard error (s.e.). ^P<0.10, *P<0.05. DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate.
In males, phthalate-related body weight effects at PND21 were more pronounced in A vy /a mice than in a/a mice, with more exposure groups exhibiting significant differences v. controls in A vy /a mice than in a/a mice (Fig. 2b). Male A vy /a mice that were exposed to DEHP, DINP and DEHP+DINP weighed more than male A vy /a controls (P=0.02, P=0.02 and P=0.03, respectively). A vy /a males exposed to DEHP weighed an average of 9.38 g, and those exposed to DINP and DEHP+DINP weighed an average of 9.26 g, whereas control A vy /a males weighed an average of 8.22 g. In addition, increased body weights observed in male A vy /a mice exposed to DEHP+DINP+DBP (mean=9.32 g) approached statistical significance (P=0.06). On the other hand, the only difference in body weight across exposure groups in a/a males was an increase in body weight in DINP a/a males relative to control a/a males that trended towards significance (mean=8.91 and 7.74 g, respectively; P=0.07).
Maternal (F0) body weights were not significantly different in exposure groups v. the control group at the time of exposure, time of mating or time of weaning, with or without controlling for number of pups given birth to (P>0.10) (Supplemental Table S1). However, dams exposed to DINP gained more body weight between mating and weaning than controls at a level near statistical significance (P=0.06) when controlling for the number of pups per litter; dams exposed to DINP gained 9.77 g whereas control dams gained 8.16 g. Dams exposed to DINP also gained more weight across the duration of the study (from exposure onset to weaning) than controls (P=0.01), and dams exposed to DBP gained more weight than controls between exposure and weaning at levels nearing statistical significance (P=0.08).
Organ and tissue weights
Several organs and tissues were collected from fasted a/a mice at PND21; full details on organ and tissue weights can be found in Supplemental Table S2. The most prevalent effect of developmental phthalate exposures on organ weight was observed in female livers (Fig. 3; Supplemental Table S2). Females exposed to DINP, and DEHP+DINP had increased relative liver weights, expressed as percent of body weight, compared with control females (P=0.02 and P=0.006, respectively). DEHP+DINP+DBP exposed female offspring also exhibited increased relative liver weights v. controls at a level near statistical significance (P=0.08). Mean relative liver weights of DINP, DEHP+DINP and DEHP+DINP+DBP exposed females were an average of 4.17, 4.25 and 3.99%, respectively, whereas control females had a mean relative liver weight of 3.61%. Hepatic triglyceride levels were measured to determine whether differences in relative liver weights were due to an increase in lipid accumulation in the liver; however, we did not observe any significant differences in hepatic triglycerides across exposure groups (data not shown).

Fig. 3 Relative liver weights in a subset of postnatal day 21 a/a mice (n=152) exposed to phthalates and phthalate mixtures. Relative liver weights are liver weight normalized to body weight, expressed as a percentage. Relative liver weights in exposure groups were compared with controls using linear mixed-effects models controlling for within litter effects, and analyses were stratified by sex. Bar height represents mean relative liver weights for each group and error bars represent standard error (s.e.). ^P<0.10, *P<0.05, **P<0.01. DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate.
Relative gonadal fat weight was also significantly higher in females exposed to all three phthalates compared with control females (P=0.02); gonadal fat from females exposed to DEHP+DINP+DBP weighed 0.15% of body weight whereas gonadal fat from control females weighed 0.10% of body weight. Relative brain weights were lower in females exposed to DEHP+DINP+DBP compared with controls (P=0.04) and decreases observed in relative brain weights in DINP and DEHP+DINP+DBP exposed males approached statistical significance (P=0.07 for both). Absolute brain weights, however, were not significantly different across exposure groups, with mean brain weights that ranged from 288.7 to 305.3 grams for all a/a mice. Males exposed to all three phthalates had lower absolute pancreas weights than controls (P=0.05), and females exposed to all three phthalates had lower absolute pancreas weights than controls at nearly statistically significant levels (P=0.07), but alterations observed in relative pancreas weights did not reach statistical significance. An increase observed in absolute spleen weights between females exposed to all three phthalates compared with controls approached statistical significance (P=0.08), but relative spleen weights were not significantly different across exposure groups. Lastly, kidney weights were not impacted by developmental phthalate exposure (P>0.10).
Coat color distribution
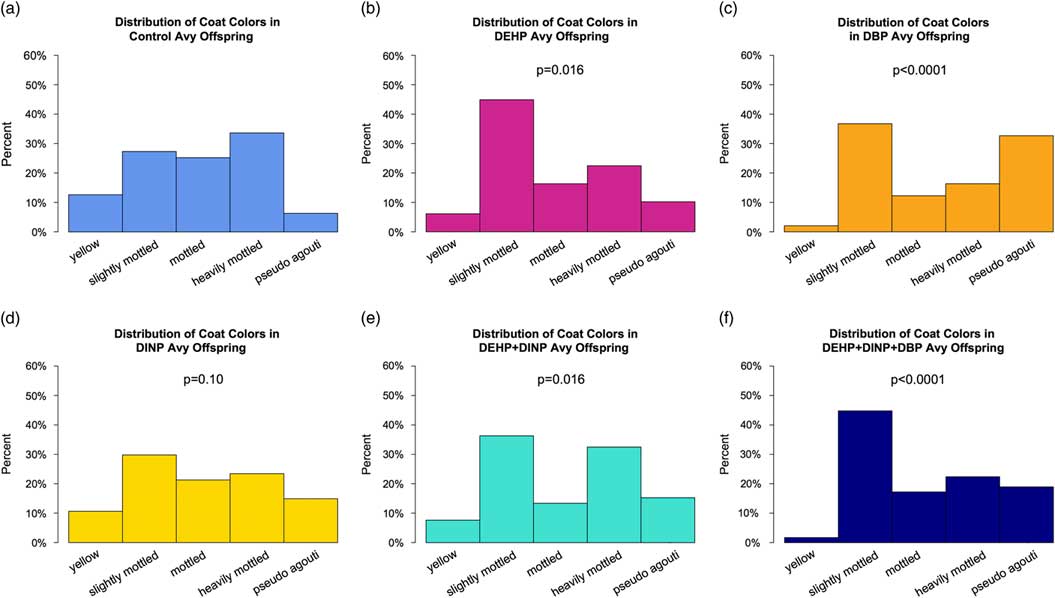
Developmental exposure to phthalates resulted in altered coat color distributions in A vy /a offspring. Exposure to DEHP, DBP, DEHP+DINP and DEHP+DINP+DBP significantly altered coat color distributions (P=0.02, P<0.0001, P=0.02, P<0.0001, respectively; Fig. 4), whereas exposure to DINP resulted in only a modest change in coat color distribution compared with controls (P=0.10). Developmental exposure to DBP alone (Fig. 4c) and in the mixture (DEHP+DINP+DBP, Fig. 4f) exhibited a pronounced increase in the proportion of pseudoagouti offspring; 6.12% of the control offspring were classified as pseudoagouti, compared with 32.7% of the DBP offspring and 17.9% of the DEHP+DINP+DBP offspring. DEHP exposure (Fig. 4b) showed the most pronounced increase in proportion of slightly mottled offspring, represented by 44.9% compared with 26.5% of the control offspring. There was also a relative increase in slightly mottled offspring among the DEHP+DINP exposed group (32.7%), and the DBP exposed group (36.7%).

Fig. 4 Coat color distributions of A vy /a offspring across exposure groups. Coat color distributions for each exposure group were compared with the control using a χ2 goodness-of-fit test. (a) Control coat color distribution; n=16 litters, 50 animals. (b) Diethylhexyl phthalate (DEHP) coat color distribution; P=0.016; n=14 litters, 49 animals. (c) Dibutyl phthalate (DBP) coat color distribution; P<0.0001; n=14 litters, 49 animals. (d) Diisononyl phthalate (DINP) coat color distribution; P=0.10; n=15 litters, 47 animals. (e) DEHP+DINP coat color distribution; P=0.016; n=16 litters, 55 animals. (f) DEHP+DBP+DINP coat color distribution; P<0.0001; n=17 litters, 61 animals. Avy, viable yellow agouti.
A vy DNA methylation
Molecular analyses of DNA methylation at A vy were used to corroborate the coat color assignments. Mean DNA methylation percent at four CpG sites within the 5′ IAP LTR in the promoter of the A vy allele corresponded appropriately to the coat color phenotypes (Supplemental Fig. S1). Mice classified as having a yellow coat color had methylation levels at or approaching 0% (mean=0.73%), and increasing amounts of brown fur corresponded with increasing amounts of methylation. Pseudoagouti offspring had the highest amount of methylation (mean=74.4%). Mean A vy methylation levels across exposure groups, with and without sex stratification, can be found in Table 4. Distributions of mean methylation for each exposure were not normally distributed, and in many exposure groups followed a bimodal distribution (Supplemental Fig. S2). Thus, methylation for each exposure group was dichotomized at the median methylation value of the control group (⩽29.9=low methylation, >29.9=high methylation) to more appropriately compare mean methylation values. The high-methylation group in DBP exposed females was significantly greater than control females, with a mean A vy methylation of 67.37% in highly methylated DBP exposed females and a mean A vy methylation of 50.03% in highly methylated control females (P=0.006). The high-methylation group in females perinatally exposed to DEHP+DINP+DBP also had modestly increased A vy DNA methylation compared with control females (60.45 v. 50.03%, respectively; P=0.07). There were no significant differences (P<0.10) observed in males, or among the low-methylation categories.
Table 4 Methylation percent for viable yellow agouti (A vy ) across four CpG sites

DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate.
Reported as percent mean methylation (s.d.).
**P<0.01 v. control, *P<0.05 v. control, ^P<0.10 v. control.
Global IAP DNA methylation
After observing phthalate-related differences in DNA methylation patterns at the A vy IAP, we measured global IAP methylation levels in tail tips from A vy /a (n=302) and a/a mice (n=152) to further explore whether developmental phthalate exposures are capable of altering DNA methylation on a global level. Developmental phthalate exposure altered IAP methylation on a global scale, in a sexually dimorphic manner. Developmental phthalate exposure tended to result in decreased IAP DNA methylation in males and increased IAP DNA methylation in females (Fig. 5). Compared with controls, male A vy /a mice exposed to DBP had a 1.4% decrease in mean global IAP methylation and DEHP+DINP had a 1.5% decrease in mean global IAP methylation across four CpG sites assayed by pyrosequencing (P=0.03, P=0.01, respectively). In contrast, female A vy /a mice exposed to DEHP had a 1.9% increase and DEHP+DINP had a 2.2% increase mean DNA methylation relative to controls (P=0.03, P=0.01, respectively). Female A vy /a mice exposed to DINP had increased global IAP DNA methylation at levels approaching statistical significance when compared with controls (P=0.09). Trends in mean and site-specific DNA methylation levels were similar in a/a mice, but were mostly non-significant, possibly due to lower statistical power (Supplemental Fig. S3).

Fig. 5 Mean tail global intracisternal A-particles (IAP) DNA methylation across four CpG sites from PND21 A vy /a mice across exposure groups. Mean methylation in exposure groups was compared with controls via linear mixed-effects models, which take methylation levels at nearby CpG sites into account, as well as within litter effects. Analyses were stratified by sex since initial analyses indicated a significant interaction between exposure and sex. Lines within boxes represent medians and whiskers represent 1.5×IQR. Gray dots are values outside of 1.5×IQR. ^P<0.10, *P<0.05. DEHP, diethylhexyl phthalate; DBP, dibutyl phthalate; DINP, diisononyl phthalate; A vy , viable yellow agouti.
Discussion
Perinatal exposure to phthalates resulted in sex-specific and phthalate-specific effects on weaning body weight, organ weights and tail DNA methylation at IAPs. Exposure to phthalates in mixture form did not appear to have an exaggerated effect for most outcomes tested, with the exception of certain organ and tissue weights. In general, developmental phthalate exposure was associated with an increase in PND21 body weight, especially in mice exposed to DINP. The DINP exposure group showed the most consistent effects on body weight, with both genotypes and both sexes having increased body weight relative to controls; in a/a males, the only group that was notably different compared with controls was the group exposed to only DINP. To our knowledge, this study was the first to report on body weight effects in mice following developmental exposures to DINP. Thus, the consistent increases in PND21 body weight we observed in mice developmentally exposed to DINP is a novel finding. One study in rats reported an association between decreased body weight gain in adult rats that were exposed to DINP from preconception to weaning; however, the exposure levels investigated in that study were much higher than those used in the present study (0.5–1.5% DINP in chow v. 0.075% in our study).Reference Waterman, Keller and Trimmer 49 Another study in rats similarly found that exposure to DINP from gestational day 15 to PND10 resulted in decreased weight gain between PND2 and PND10, but the exposure levels examined were also relatively high (20,000 ppm or 2% in chow).Reference Masutomi, Shibutani and Takagi 50 EDCs have been demonstrated to exhibit non-monotonic responses and low-dose effects.Reference Vandenberg, Colborn and Hayes 51 It is possible that increased body weight is only associated with lower-level developmental exposures of DINP, such as the levels used in this study.
Only A vy /a males had increased body weight following developmental exposure to DEHP alone. Other studies on developmental DEHP exposure in mice have demonstrated mixed effects on body weight, with some studies showing an increase and some a decrease.Reference Schmidt, Schaedlich, Fiandanese, Pocar and Fischer 36 , Reference Pocar, Fiandanese and Secchi 52 Mice that were exposed to DBP alone did not have significantly different body weights from controls for either genotype or sex. This study is the first to examine body weight effects following developmental DBP exposure in mice, but studies in rats have also demonstrated mixed effects on offspring body weight.Reference Mylchreest, Wallace, Cattley and Foster 37 , Reference Jiang, Xu and Zhu 53 , Reference Okayama, Wakui and Wempe 54
This is the first animal study to examine the impact of developmental exposures to mixtures of phthalates on body weight. Exposure to phthalate mixtures did not appear to result in an exaggerated effect on PND21 body weight; females exposed to DEHP+DINP did not weigh significantly more than controls, and those exposed to DEHP+DINP+DBP weighed more than controls to a moderate degree of statistical significance. In addition, male A vy /a mice exposed to DEHP+DINP during development had increased PND21 body weights relative to controls, and those exposed to DEHP+DINP+DBP had a modest trend towards increased body weight compared with controls, but male a/a mice exposed to phthalate mixtures did not have increased body weight relative to controls. It is possible that mixture effects would be more apparent at lower exposures, especially if increased body weight is more prominent at lower exposures of phthalates. Since the mice exposed to mixtures of phthalates had higher total phthalate exposures, it is possible that any additive effects, or lack thereof, could be due to dose effects rather than mixture effects.
Because body composition was not assessed in this analysis, it is unknown whether the observed increases in body weight in phthalate-exposed mice were due to an increase in body fat, lean mass or both. Additional studies are needed with inclusion of different dosing levels to confirm whether developmental exposures to phthalate mixtures have exaggerated effects on body weight, as well as on body composition. A follow-up study of a subset of wildtype non-agouti a/a mice that were aged to 10 months and includes body composition analysis is currently underway in order to assess long-lasting impacts and to determine whether perinatal phthalate exposures impact body fat or lean mass.
Multiple organ and tissue weights were altered by developmental exposures to phthalates in a/a mice, and females appeared to be more sensitive than males in this respect. Relative liver weights were increased in females exposed to DINP and DEHP+DINP compared with controls, and were increased in females exposed to DEHP+DINP+DBP at levels trending towards statistical significance. Phthalates have been demonstrated to activate peroxisome proliferator-activated receptor (PPAR)-α, the dominant PPAR in the liver, in other animal and in vitro studies,Reference Oshida, Vasani and Thomas 55 – Reference Hayashi, Ito and Yamagishi 57 and PPAR-α activation has been associated with increased relative liver weights in investigations of other chemicals.Reference Laughter, Dunn and Swanson 58 The observed increases in relative liver weights of phthalate-exposed female offspring were likely not due to an increase in lipid accumulation in the liver since hepatic triglyceride levels did not differ across exposure groups. Thus, these findings are consistent with the idea that developmental exposure to DINP and DINP mixed with DEHP activate PPAR-α in the liver, and indicate that females may be more sensitive at this early developmental time point. The mixture of DEHP+DINP had a slightly larger impact on relative liver weights, but not at a statistically significant level, and the mixture of DEHP+DINP+DBP had only a modest effect on relative liver weights compared with controls. Thus, phthalate mixtures at the exposure levels tested in this study do not appear to have exaggerated effects on relative liver weights at PND21.
On the other hand, the DEHP+DINP+DBP exposure group was the only group that exhibited an alteration in relative gonadal fat weight at PND21, with females showing an increase relative to controls. This observation is consistent with the theory that phthalate exposure increases the risk of obesity, and indicates that a mixture of HMW and LMW phthalates have the largest impact on body fat at PND21, with females being more sensitive than males. A recent meta-analysis of developmental DEHP exposure in animal studies also indicated that early life phthalate exposures results in increased fat weight.Reference Wassenaar and Legler 59 Absolute pancreas weights were increased in mice perinatally exposed to DEHP+DINP+DBP compared with controls, but this difference was mitigated when comparing pancreas weights relative to body weight, and therefore the increased pancreas weights might have been due to the modest increased body weights observed in DEHP+DINP+DBP-exposed females. Decreased relative brain weights were observed in females exposed to a mixture of all three phthalates, and to a lesser degree of significance in males exposed to all three phthalates. However, these findings are skewed by the significant and non-significant increases in body weights observed in the mice in these exposure groups, since absolute brain weights did not differ by exposure.
This study was the first to utilize the A vy mouse model as a biosensor to determine whether developmental exposures to phthalates and phthalate mixtures are capable of altering epigenetic marks at repetitive elements in the genome, such as the IAP located in the A vy promoter region. Coat color distributions were significantly altered in all groups exposed to phthalates, except for the group exposed to only DINP. Mixtures did not appear to have an exaggerated effect on offspring coat color. Coat color distributions for DEHP and DEHP+DINP were extremely similar to one another, with marked increases in slightly mottled mice in both exposure groups. Coat color distributions for DBP and DEHP+DINP+DBP were also similar to one another, with prominent increases in pseudoagouti mice in both groups. The shift towards pseudoagouti coat color may indicate a protective effect against the yellow obese phenotype in mice perinatally exposed to DBP and DEHP+DINP+DBP; however, we did not follow the A vy /a mice into adulthood, when coat color-associated metabolic phenotypes emerge, to confirm this. In addition, developmental exposures to phthalates may influence metabolism through other mechanisms distinctive from altered DNA methylation at the A vy locus. It should be noted, however, that while A vy hypermethylation in the viable yellow agouti mouse model is likely protective against obesity, hypermethylation in humans is context dependent, and could result in protective, adaptive or null effects depending on which regulatory or genic region is affected. These data suggested that DEHP and DBP both influenced shifts in coat color, and that DEHP, a HMW phthalate, had different effects from DBP, a LMW phthalate. Consistent with previous studies utilizing the A vy mouse model to investigate environmental exposures,Reference Dolinoy, Huang and Jirtle 29 , Reference Anderson, Nahar and Faulk 60 , Reference Faulk, Barks, Liu, Goodrich and Dolinoy 61 we confirmed that coat colors were representative of methylation at the A vy locus via tail DNA. Our analyses of DNA methylation at the A vy locus indicated that there was an increase in methylation in the DBP exposure group, which was consistent with the observed increase in pseudoagouti mice.
Tail DNA methylation levels at IAPs were also altered by developmental phthalate exposures on a global level, and in a sexually dimorphic manner. We observed a general trend towards increased global IAP methylation in females exposed to phthalates, but in males exposed to phthalates we observed a general trend towards decreased global IAP methylation. In addition, different individual phthalates had more pronounced effects on global IAP methylation in females v. males. In A vy /a mice, developmental exposure to DEHP, and DINP to a lesser degree of significance, resulted in altered global IAP DNA methylation in females, whereas developmental exposure to DBP resulted in altered DNA methylation in males. Phthalate mixtures effects were complex. The DEHP+DINP mixture group had altered DNA methylation in both sexes, whereas the DEHP+DINP+DBP mixture group did not exhibit significant differences in either sex. Effect sizes for exposure-related changes in global IAP methylation were between 1.4 and 2.2%, and while small, are comparable to effect sizes for DNA methylation at repetitive elements frequently associated with developmental exposures to other environmental chemicals.Reference Breton, Marsit and Faustman 62 Similar trends in tail global IAP DNA methylation was observed in a/a mice, but we observed less statistically significant differences across exposure groups, likely due to decreased power resulting from lower sample size. It should also be noted that phthalate-mediated alterations in DNA methylation at repetitive elements are unlikely to be the sole drivers of phthalate-mediated metabolic impacts. Because phthalate metabolites have been demonstrated to activate PPARs,Reference Oshida, Vasani and Thomas 55 , Reference Sarath Josh, Pradeep and Vijayalekshmi Amma 56 additional studies investigating DNA methylation at the promoter region of PPAR-α and PPAR-γ target genes in liver and adipose, respectively, would provide crucial mechanistic insights.
A vy methylation levels were not predictive of global IAP methylation levels in this study (R 2=0.001, P=0.22 via Pearson correlation test). However, this is not unexpected. The A vy locus is just one IAP out of thousands of IAPs that are present in the mouse genome, and previous studies that have demonstrated high variation in DNA methylation across different IAPs.Reference Faulk, Barks and Dolinoy 27 Another study examining perinatal lead (Pb) exposure in mice found different impacts of Pb on individual IAPs;Reference Montrose, Faulk, Francis and Dolinoy 63 thus phthalates may also have IAP-specific effects that are not fully detected by examining A vy and global IAP methylation levels alone. Based on the findings of this study, evaluation of methylation at additional specific IAPs may be warranted, especially those that are near promoter regions of genes that are known to play a role in metabolism.
Phthalates are ubiquitous chemicals that present a significant risk for human exposure, and recent research has suggested that exposure to physiologically relevant levels may influence risk of obesity and metabolic syndrome. Further, phthalates are known EDCs, and high exposures during development have been linked to permanent reproductive tract abnormalities. However, little is known regarding metabolic health outcomes resulting from developmental exposures to phthalates, and even less is known about risks from developmental exposures to phthalate mixtures. In this study, developmental exposures to phthalates in the perinatal window influenced body weight, liver weight and gonadal fat weight in mice at weaning. Interestingly, females appeared to be more sensitive than males. Tail IAP DNA methylation at the A vy locus and on a global level was altered by developmental phthalate exposures, presenting a possible mechanistic link between early-life exposures and potential later-life health outcomes; research focused on characterizing long-term metabolic impacts on a subset of a/a mice is currently underway in order to evaluate this potential. The results from this study are the first to describe metabolic and epigenetic effects of developmental exposure to phthalate mixtures. Mixture effects were complex in that they did not necessarily result in exaggerated effects, and in some cases, phthalate mixtures exhibited attenuated effects. However, this may be attributed to dose effects rather than mixture effects, since the groups receiving mixtures of phthalates received higher exposures to total phthalates. Future work investigating phthalate mixtures at multiple dose levels is needed to better understand these effects, which will aid in the interpretation of human epidemiological studies and further our understanding of molecular mechanisms.
Acknowledgments
The authors would like to sincerely thank Christine Rygiel and Nicole Urdahl for their assistance with carrying out the global IAP pyrosequencing assays, as well as Elizabeth H. Marchlewicz for her guidance in measuring hepatic triglycerides.
Financial Support
This work was supported by the University of Michigan (UM) NIEHS/EPA Children’s Environmental Health and Disease Prevention Center P01 ES022844/RD83543601, the Michigan Lifestage Environmental Exposures and Disease (M-LEEaD) NIEHS Core Center (P30 ES017885), as well as the UM NIEHS Institutional Training Grant T32 ES007062 and NICHD Institutional Training Grant T32 079342 (K.N.).
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Guide for the Care and Use of Laboratory Animals and has been approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418000430