Rush skeletonweed is a perennial forb of Eurasian origin that is invasive in Australia, Argentina, Canada, and the United States (Campanella et al. Reference Campanella, McEvoy and Mundt2009; Gaskin et al. Reference Gaskin, Schwarzlander, Kinter, Smith and Novak2013). In Australia and Argentina, the species is a serious pest of both rangeland and wheat production. In Australian wheat crops, rush skeletonweed reduced wheat yields as much as 80% in heavily infested areas (Panetta and Dodd Reference Panetta and Dodd1987). A recent survey of Argentine wheat production areas found rush skeletonweed present in 36% of the surveyed crop area (Scursoni et al. Reference Scursoni, Gigón, Martín, Vigna, Leguizamón, Istilart and López2014). In North America, the species is most prevalent in disturbed rangeland and other noncrop areas (Liao et al. Reference Liao, Monsen, Anderson and Shaw2000), particularly in the US Northwest, where it occupies an estimated 2.5 million hectares of rangeland (Sheley and Hudak Reference Sheley and Hudak1995). Although broadly distributed in noncropland habitats across the inland Pacific Northwest, rush skeletonweed has not moved aggressively into adjacent wheat–fallow cropland despite longstanding concerns that it might (Schirman and Robocker Reference Schirman and Robocker1967; VanVleet and Coombs Reference VanVleet and Coombs2012).
Substantial expansion of rush skeletonweed has, however, occurred into cropland placed in the Conservation Reserve Program (CRP) in eastern Washington, where dense populations have established in many CRP fields. High financial returns from wheat production and changes to program rules have resulted in large numbers of former CRP grass stands being returned to wheat production in the last 5 to 10 years. Dense patches of rush skeletonweed established during the CRP contract period have persisted in wheat crops and are not controlled by the weed management strategies typical of wheat–fallow production in the region.
While yield loss resulting from direct competition with the crop is of concern, moisture use by skeletonweed plants during the fallow period of the rotation is more important (R Dorman, wheat grower, personal communication). Maximizing yield potential in the region depends heavily on early establishment of winter wheat, which requires storage of adequate seed bed moisture over the dry summer of the fallow period (Donaldson et al. Reference Donaldson, Schillinger and Dofing2001). In areas where poorly controlled skeletonweed depletes seed zone moisture during the fallow period, wheat stands either fail to establish or have markedly delayed or reduced emergence and stand establishment. Both cases result in reduced yield potential.
Despite the importance of controlling rush skeletonweed in the fallow phase of the rotation, growers are often reluctant or unable to incorporate additional herbicide inputs in fallow. Crop share lease agreements with absentee landowners who don’t recognize the need for such measures and/or are unwilling or unable to invest in them are a frequent barrier. Financial and logistic considerations can pose additional obstacles. In contrast, in-crop herbicide applications are a standard production practice in the region. Effective chemical control in the crop phase of the rotation would offer an important and easily adopted component of an overall strategy to manage rush skeletonweed in wheat–fallow production. The objective of this study was to identify effective herbicide options for control of rush skeletonweed in dryland winter wheat in eastern Washington.
Materials and Methods
Field trials were conducted near LaCrosse, WA (46.81°N, 117.88°W) in the 2015/2016 and 2016/2017 cropping seasons to test the efficacy of five synthetic auxin herbicides at fall and spring application timings for rush skeletonweed control in winter wheat (Table 1). In both years, fall treatments were applied at wheat tillering (Feekes stage 3), and spring treatments were applied at wheat jointing (Feekes stage 6). Rush skeletonweed plants were early- to mid-rosette stage at both application timings in both years. The 2015/2016 trial was located in a field enrolled in CRP through September 2013, at which point the grass stand was terminated (standing residue burned and tilled with a chisel plow followed by a field cultivator) in early October 2013, and winter wheat was planted immediately thereafter for harvest in July 2014. The field was managed with conventional tillage over the summer fallow period in 2015. Fallow operations consisted of a single pass with heavy double disk in mid-May followed by three separate rod-weeding operations over the course of the summer. The field was fertilized with 90 kg ha−1 nitrogen, 11 kg ha−1 sulfur, and 11 kg ha−1 chloride in mid-August using a straight-shank cultivator/applicator (ripper-shooter, McGregor Company, Colfax, WA). ‘ORCF-102’ winter wheat was seeded at 67 kg ha−1 on September 11, 2015, using a deep-furrow drill (HZ616, John Deere, Moline, IL). The 2016/2017 trial was located in an adjacent field that was also in CRP through September 2013. Management in this field was identical, except that it was fallowed in 2014, planted to winter wheat in 2015 and in fallow over the summer of 2016. Winter wheat was planted in this field as previously described on September 2, 2016.
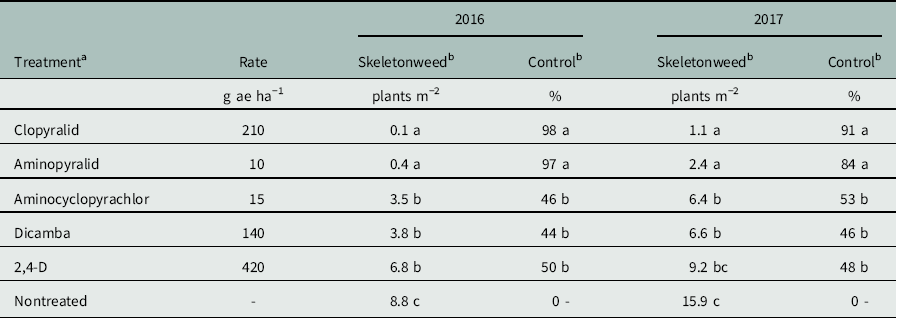
Table 1 Herbicide treatments, mean counts of surviving skeletonweed plants, and mean skeletonweed control ratings at June evaluations, approximately 30 d before wheat harvest.
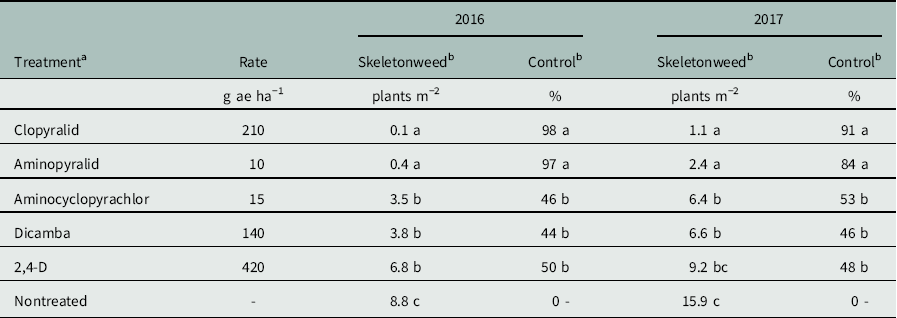
a Treatments were applied independently at both fall and spring application timings; results presented are averaged across timings. All applications included a nonionic surfactant at 0.25% (v/v). Formulations: aminopyralid, Milestone (Dow AgroSciences, Indianapolis, IN); clopyralid, Stinger (Dow AgroSciences); aminocyclopyrachlor, DPX-MAT28-128 (Dow AgroSciences); dicamba, Clarity (BASF, Florham Park, NJ); 2,4-D, 2,4-D LV6 (Albaugh LLC, Ankeny, IA); and nonionic surfactant, R-11 (Wilbur-Ellis Company, Fresno, CA).
b Within a column, means followed by the same letter are not significantly different according to Tukey’s multiple comparison procedure (α=0.05).
Trials were established in a randomized complete block design with four blocks and individual plot size of 3 by 10 m. Treatment structure was a full two-way factorial combination of herbicide (6 levels) by application timing (2 levels: fall and spring). All herbicide treatments (Table 1) were applied using a CO2-powered backpack sprayer with four Teejet XR11003 flat-fan nozzles (Teejet Spraying Systems Co., Wheaton, IL) at 76 cm spacing, calibrated to deliver 140 L ha−1 at 172 kPa. For the 2015/2016 trial, fall treatments were applied November 12, 2015, and spring treatments applied on March 17, 2016. For the 2016/2017 trial, fall treatments were applied October 29, 2016, and spring treatments applied on April 5, 2017.
Rush skeletonweed density was variable across plot sites, with patchy and nonuniform distribution on a subplot scale (ie, patchiness of plant presence/absence at distances of less than 1 m), making objective assessment of herbicide efficacy on the basis of whole-plot plant density infeasible. To facilitate assessment of plant density, two 1-m2 quadrats were placed in each plot at the first evaluation date in each year, and the same quadrats were monitored for the remainder of the trial period. Quadrats were placed over areas with relatively high skeletonweed density (range 4 to 46 plants, median 17) in a uniformly established wheat stand. In 2016, plant density was quantified on April 6 and June 2. In 2017, plant density was quantified on April 4 and June 12. Herbicide control of skeletonweed was also estimated visually on a whole-plot basis relative to the nontreated check using a percent scale ranging from 0 (no observed injury) to 100 (complete plant death). In 2016, visual assessments of control were made on March 8, March 31, and June 2. In 2017, visual assessments were made on April 4, April 20, and June 12.
Plots were combine harvested on July 20, 2016, or July 19, 2017. Moisture content of 2016 grain samples was determined with an Infratec 1241 Grain Analyzer (Foss North America, Eden Prairie, MN). In 2017, grain samples were oven dried at 60 C for 72 h and moisture content calculated on a wet-weight basis. All yields were adjusted to 12% moisture basis for analysis.
Skeletonweed counts and estimates of control made at the June evaluation date were highly reflective of earlier evaluations (data not shown), and for brevity only analyses of data from the June evaluation are presented. All statistical analyses were conducted in R 3.4.1 (R Core Team, 2017). Biological count data generally follow a negative binomial distribution (Stroup Reference Stroup2015), and counts were analyzed with generalized linear mixed models using a negative binomial distribution and log link function in lme4 version 1.1-13 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). Herbicide, application timing, and year were modeled as fixed factors and block was modeled as a random factor. Yield data were analyzed with linear mixed models in lme4, with herbicide and application timing as fixed factors and block as a random factor. Consistency with model assumptions of normal distribution of errors and homoscedasticity was confirmed via quantile-quantile plots and plots of Pearson residuals vs. fitted values, respectively. Percent control data are a type of continuous proportion best described by a beta distribution (Stroup Reference Stroup2015), so estimates of percent control were analyzed using generalized linear models with beta regression and the logit link function in betareg version 3.1-0 (Cribari-Neto and Zeileis Reference Cribari-Neto and Zeileis2010). As betareg does not support mixed modeling, herbicide, application time, and block were all modeled as fixed factors. A Breusch-Pagan test was used to confirm consistency with model assumption of homoscedasticity with lmtest version 0.9-35 (Zeileis and Hothorn Reference Zeileis and Hothorn2002). Significance of fixed effects for all models was evaluated with Wald tests in car version 2.1-4 (Fox and Weisburg Reference Fox and Weisberg2011), and mean separations were conducted using Tukey’s multiple comparison procedure in lsmeans version 2.26-3 (Lenth Reference Lenth2016). Initial analyses of combined data indicated a significant three-way interaction between herbicide, time of application, and trial for both yield (P<0.01) and percent control data (P<0.01). Accordingly, data were analyzed separately by trial.
Results and Discussion
Clopyralid and aminopyralid provided good to excellent control of rush skeletonweed, while the remaining herbicides were ineffectual (Table 1). These findings are consistent with previously published studies. Wallace and Prather (Reference Wallace and Prather2010a, Reference Wallace and Prather2010b) obtained 88% to 100% control of rush skeletonweed in Idaho with aminopyralid at a slightly higher rate (30 g ae ha−1). High efficacy of clopyralid at comparable rates was also reported by Heap (Reference Heap1993) on several Australian rush skeletonweed populations and by Cheney et al. (Reference Cheney, Belles and Lee1980) on Idaho populations; both studies reported poor efficacy of 2,4-D and dicamba. Dicamba and 2,4-D are widely used, economical options for general broadleaf weed control in eastern Washington in both wheat and CRP fields, and were included in the present study for comparison to common grower practice.
Rush skeletonweed is an obligate apomict, with numerous clonal biotypes present across its native and introduced ranges (Gaskin et al. Reference Gaskin, Schwarzlander, Kinter, Smith and Novak2013). In the invasive range, unique, nonoverlapping sets of biotypes are present on each invaded continent. The three biotypes present in Australia have demonstrated differential susceptibility to biocontrol agents (Burdon et al. Reference Burdon, Groves, Kaye and Speer1984) and herbicides, including clopyralid, metsulfuron, and 2,4-D (Black et al. Reference Black, Pederson and Stephenson1998, Heap Reference Heap1993). In North America, differential susceptibility to biocontrol agents has been observed (Campanella et al. Reference Campanella, McEvoy and Mundt2009). Using the nomenclature and methods of Gaskin et al. (Reference Gaskin, Schwarzlander, Kinter, Smith and Novak2013), a sample of 12 individual plants collected at this study site were experimentally confirmed as Genotype 3 (JF Gaskin, personal communication), which is the primary biotype found in Washington (Gaskin et al. Reference Gaskin, Schwarzlander, Kinter, Smith and Novak2013). Genotype 1 is found across most of Idaho, including the study areas of Cheney et al. (Reference Heap1980) and Wallace and Prather (Reference Wallace and Prather2010a, Reference Wallace and Prather2010b), and it is presumed that the plants tested by both were Genotype 1. As the large majority of rush skeletonweed found across the United States is either Genotype 1 or Genotype 3 (Gaskin et al. Reference Gaskin, Schwarzlander, Kinter, Smith and Novak2013), clopyralid and aminopyralid should be similarly effective across the United States.
Rush skeletonweed biotypes may, however, respond differently to aminocyclopyrachlor. Aminocyclopyrachlor at 15 g ae ha−1 did not adequately control rush skeletonweed in this study, providing 46% to 53% control (Table 1). In contrast, Wallace and Prather (Reference Wallace and Prather2010b) reported that aminocyclopyrachlor at 9 or 18 g ae ha−1 provided no less than 90% or 97% control of rush skeletonweed in southern Idaho, respectively. As probable biotype differences between study locations coincide with apparent differences in efficacy, the potential for differential response of US rush skeletonweed biotypes to aminocyclopyrachlor warrants further investigation.
Timing of application (fall or spring) did not have a strong or consistent effect on herbicide efficacy. No interaction was observed between herbicide and time of application on final skeletonweed counts (2016: P=0.13; 2017: P=0.08). Accordingly, no interaction term was included in final models for either count or percent control data. In 2016, there was no effect of application timing on final rush skeletonweed counts (P=0.22), but there was a small effect of application timing on percent control (P=0.04, with mean rating of 65% control for fall applications and 70% control for spring applications, averaged across herbicides). In 2017, there was no effect on percent control ratings (P=0.32), but a marginal effect of timing on final counts (P=0.049, with mean count for fall applied herbicides of 4.3 plants m−2 and mean count for spring applied herbicides of 5.9 plants m−2, averaged across herbicide treatments). While this may provide weak evidence for higher efficacy of herbicides applied in the fall rather than in the spring, any possible effect appears small. Logistic considerations of when an application best fits into typical field operations are likely more important than any potential change in efficacy resulting from application timing.
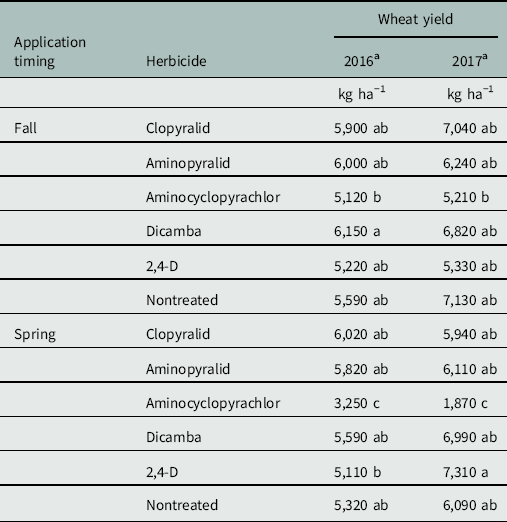
Wheat yields were greatly reduced by spring applications of aminocyclopyrachlor (Table 2), which sterilized wheat florets in these treatments in both years. This effect was readily visible in the field in fall treatments also, but not significantly so in final grain yield. Kniss and Lyon (Reference Kniss and Lyon2011) reported substantial to severe yield loss in winter wheat from PRE applications of aminocyclopyrachlor made 2 to 6 months before planting, indicating probable yield loss from aminocyclopyrachlor applied to winter wheat at any timing. No meaningful differences were observed between other treatments—including nontreated checks—in either year (Table 2). Rush skeletonweed and wheat stand densities were highly variable within plots, and it is suspected that this variability may have inflated plot error to a degree that even meaningful treatment effects could have been obscured. Furthermore, overall yields were exceptional in both years, exceeding long-term averages by nearly 2,500 kg ha−1 (R Dorman, personal communication). Unusually high yields may have further obscured treatment differences by minimizing the competitive effect of rush skeletonweed. Beyond the severe yield loss caused by spring-applied aminocyclopyrachlor, no conclusions could be made on the basis of observed yields.
Table 2 Mean wheat yield for each combination of herbicide by application timing.
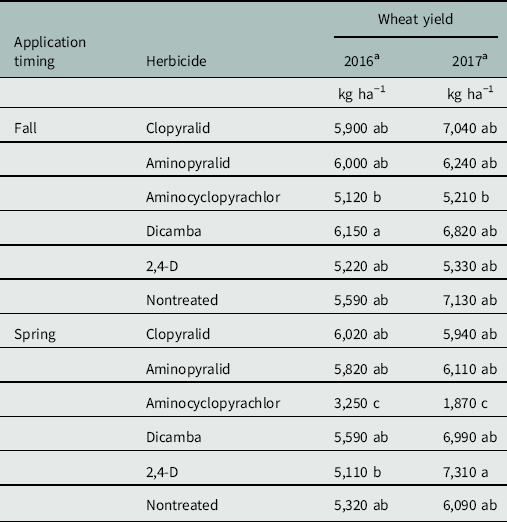
a Within a column, means followed by the same number are not significantly different according to Tukey’s multiple comparison procedure (α=0.05).
Clopyralid is an effective option for control of rush skeletonweed in winter wheat when applied to plants in the rosette stage in either fall or spring. Clopyralid is also registered for use in canola, one of the best alternative crop options for the wheat–fallow zone of Washington (Young et al. Reference Young, Whaley, Pan, Roe and Alldredge2014). Aminopyralid controls rush skeletonweed as well, but is not currently labeled for use in wheat. At one time it was available at 10 g ae ha−1 in the formulated product Cleanwave (Dow AgroSciences, Indianapolis, IN), but national product registration was retracted and the formulation is no longer available (JP Yenish, Dow AgroSciences, personal communication). Aminopyralid is registered for use in CRP and rangeland at up to 122 g ae ha−1 (as Milestone, Dow AgroSciences) and could be an effective treatment for rush skeletonweed–infested CRP fields prior to grass stand termination. Depending on the timing of such applications relative to wheat planting date, carry-over injury could be a concern with higher rates of aminopyralid; this would require further investigation. Future research is also needed regarding effective, integrated control options for use in the fallow phase of the wheat–fallow rotation.
Acknowledgements
The authors thank Ryan Dorman of RR Dorman Farms for collaboration and use of trial sites, Henry Wetzel and Tara Burke for assistance with trial implementation, John Gaskin of the US Department of Agriculture Agricultural Research Service for confirming the genotype of rush skeletonweed samples from the trial site, and the Washington Grain Commission for partial funding of the research. Partial support of this work was provided by the US Department of Agriculture National Institute of Food and Agriculture, Hatch Project 23507. No conflicts of interest have been declared.