Introduction
Acanthamoeba spp. are free-living amoebae protozoa present in a wide variety of habitats, being isolated from soil, air and water environments. These amoebae have become clinically important, since they are the causative agents of granulomatous amoebic encephalitis (GAE) and pneumonitis, chronic lung, skin lesions and a corneal infection known as Acanthamoeba keratitis (AK) (Visvesvara et al., Reference Visvesvara, Moura and Schuster2007; Carvalho et al., Reference Carvalho, Foronda, Mannis, Höfling-Lima, Belfort and De Freitas2009; Rusciano et al., Reference Rusciano, Capriglione, Pesce, Del Prete, Cennamo, Di Cave, Cerulli and Sasso2013). In contrast to infections caused by other amoebae, Acanthamoeba spp. can form cysts within the tissue. The corneal disease is a progressive sight-threatening infection which can affect both immunocompromised and healthy individuals, and it is particularly prevalent among contact lens users. In developed countries, about 83% of the cases are diagnosed in contact lens users (Carvalho et al., Reference Carvalho, Foronda, Mannis, Höfling-Lima, Belfort and De Freitas2009). In addition, Acanthamoeba spp. can act as a reservoir for pathogenic bacteria, such as Legionella pneumophila, and other pathogenic microorganisms, such as fungi and viruses (Greub and Raoult, Reference Greub and Raoult2004).
The treatment of Acanthamoeba infections remains a challenge. In addition to their toxicity, anti-amoebic agents are not as effective as expected due to their variable efficacy on strains, species and amoeba stages. At present, there is no recommended treatment for GAE and most cases are identified at the post-mortem stage. Drug resistance against AK treatment (combination of a diamidine and a biguanide) has been also reported (Bang et al., Reference Bang, Edell, Eghrari and Gottsch2010; Ferrari et al., Reference Ferrari, Matuska and Rama2011).
In the present study, the effectiveness of 13 flavonoid glycosides isolated from Delphinium gracile, D. staphisagria, Consolida oliveriana and from Aconitum napellus subsp. Lusitanicum is evaluated against trophozoites and cysts of Acanthamoeba castellanii. Flavonoids, widely distributed in the plant kingdom, have a broad spectrum of biological properties, such as antiatherosclerotic, antiviral, anti-inflammatory, antithrombogenic, antiosteoporotic and antitumor effects (Nijveldt et al., Reference Nijveldt, Van Nood, Van Hoorn, Boelens, Van Norren and Van Leeuwen2001). In particular, flavonoids have shown antiparasitic action against neglected protozoan diseases such as those caused by Plasmodium spp., Leishmania spp., Trypanosoma cruzi and T. brucei (Marín et al., Reference Marín, Ramírez-Macías, López-Céspedes, Olmo, Villegas, Díaz, Rosales, Gutiérrez-Sánchez and Sánchez-Moreno2011, Reference Marín, Díaz, Irure Maiques, Ramírez-Macías, Rosales, Guitierrez-Sánchez, Cañas and Sánchez-Moreno2017; Ramírez-Macías et al., Reference Ramírez-Macías, Marín, Díaz, Rosales, Gutiérrez-Sánchez and Sánchez-Moreno2012; Schmidt et al., Reference Schmidt, Khalid, Romanha, Alves, Biavatti, Brun, Da Costa, de Castro, Ferreira, de Lacerda, Lago, Leon, Lopes, das Neves Amorim, Niehues and Ogungbe2012).
Materials and methods
Culture of A. castellanii trophozoites and encystment
Trophozoites of A. castellanii Neff (ATCC 30010) were axenically cultured at 28°C in CGV medium supplemented with 10% heat-inactivated fetal calf serum (FCS) (Willaert, Reference Willaert1971).
Cyst forms were obtained as previously described by Cordingley et al. (Reference Cordingley, Wills and Villemez1996). Briefly, CGV medium was removed from trophozoite cultures, 8% glucose in RPMI medium was added, and trophozoites were incubated at 30°C for up to 48 h. Finally, SDS was added (0.5% final conc.). Trophozoites are SDS-sensitive and are immediately lysed upon its addition of SDS, while cysts are SDS-resistant and remain intact.
Tested compounds
The tested compounds were the flavonoid glycosides shown in Fig. 1. Flavonol glycoside acetates 1 and 2 were isolated from D. gracile as previously described (Marín et al., Reference Marín, Díaz, Irure Maiques, Ramírez-Macías, Rosales, Guitierrez-Sánchez, Cañas and Sánchez-Moreno2017). Compounds 3–8 were acylated flavonol tetraglycosides from D. gracile (Diaz and Herz, Reference Diaz and Herz2010), compound 9 was a Rutin decaacetate from D. staphisagria, compounds 10–12 were flavonoid glycoside acetates from Consolida oliveriana (Carmona et al., Reference Carmona, Perez, Paz, Herz and Dı2008; Paper, Reference Paper2008) and compound 13 was isolated from A. napellus subsp. Lusitanicum (Díaz et al., Reference Díaz, García Ruiz, Rachid Días, Gavín Sazatornil and Werner2005). The compounds were dissolved in 0.1% (v/v) DMSO (Panreac, Barcelona, Spain).

Fig. 1. Tested flavonoid glycosides. TPC, trans-p-coumaroyl; TPCF, trans-p-caffeoyl; Rha, α-rhamnopyranosyl; Glc, β-glucopyranosyl; Gal, β-galactopyranosyl; Rutinoside, 6-α-rhamnopyranosyl-β-glucopyranoside; Sophoroside, 2-β-glucopyranosyl-β-glucopyranoside; Robinoside, 6-α-rhamnopyranosyl-β-galactopyranoside.
Flavonoids were prepared in 2-fold dilutions at a concentration range from 800 to 0.78 μ m in CGV medium with 10% heat-inactivated FCS for amoebicidal activity assays, from 200 to 10 μ m in RPMI medium with 8% glucose for cysticidal activity assays, and from 400 to 25 μ m in RPMI medium with 10% heat-inactivated FCS for toxicity tests on Vero cells. The reference drug chlorhexidine digluconate (Sigma, Aldrich Ltd, Barcelona, Spain) was used as a control against A. castellanii and was prepared in the same way as described above. All compounds are stable in these media and can be stored long-term at −20°C.
Controls containing drugs prepared from 800 to 10 μ m in CGV medium with 10% inactivated FCS were analysed using a test wavelength of 570 nm and a reference wavelength of 630 nm with a microplate absorbance reader (SunriseTM, Tecan). Fluorescence of the compounds at different concentrations was measured with a fluorescence microscopy Moticam Pro 2858 (Motic, Asia), no detecting fluorescence from any of the compounds.
In vitro amoebicidal activity assays
The A. castellanii anti-trophozoite activity of compounds was evaluated using the AlamarBlue Cell Viability Reagent® as previously described (Mcbride et al., Reference Mcbride, Ingram, Henriquez, Roberts and Icrobiol2005; Martín-Navarro et al., Reference Martín-Navarro, Lorenzo-Morales, Cabrera-Serra, Rancel, Coronado-Álvarez, Piñero and Valladares2008), with some modifications.
Briefly, 1 × 104 trophozoites well−1 were seeded in 96-well microtiter plates (Sigma, Aldrich Ltd) in 100 μL of CGV medium with 10% heat-inactivated FCS, and plates were incubated for 1 h at 28°C to allow for trophozoites adhesion. After that, 100 μL of the flavonoids prepared in 2-fold dilutions were added to each well. Untreated controls and controls treated with chlorhexidine digluconate (Sigma, Aldrich Ltd) were also included. In total, 20 μL AlamarBlue Reagent® (Thermo Fisher Scientific) was then placed into each well. The plates were incubated for 120 h at 28°C in complete darkness to avoid the oxidation of the resazurin sodium salt reagent. Finally, the plates were analysed using a test wavelength of 570 nm and a reference wavelength of 630 nm with a microplate absorbance reader (SunriseTM, Tecan). All experiments were performed in quadruplicate. The absorbance of controls without trophozoites was deducted from the values of absorbance of the treated trophozoites, and the trophozoital activity was expressed as the IC50 (inhibitory concentration 50) using GraphPad Prism 5 software. Selective indexes (IC50 Vero cells toxicity/IC50 activity on trophozoite forms of the parasite) were also calculated.
Dose-response growth curves with chlorhexidine digluconate and the potential compounds during the first 120 h of incubation were also performed.
Cytotoxicity tests on Vero cells
The cytotoxicity induced by each compound was tested with Vero cells (ATCC CCL-81) growing in RPMI medium with 10% heat-inactivated FCS in a humidified 95% air 5% CO2 atmosphere at 37°C. 1 × 104 Vero cells well−1 were seeded in 96-well microtiter plates in a volume of 100 μL of the medium, and the plates were incubated for 24 h. After that, Vero cells were treated by adding the compounds at a concentration ranging from 400 to 25 μ m. Untreated controls and controls treated with chlorhexidine digluconate (Sigma, Aldrich Ltd) were also included. In total, 20 μL AlamarBlue Reagent® (Thermo Fisher Scientific) was then placed into each well. The plates were then incubated for 120 h at 37°C and subsequently analysed using a test wavelength of 570 nm and a reference wavelength of 630 nm with a microplate absorbance reader (SunriseTM, Tecan). All experiments were performed in quadruplicate. The absorbance of the controls without Vero cells was deducted from the values of absorbance of the treated Vero cells, and the toxicity was expressed as the IC50 (inhibitory concentration 50) using GraphPad Prism 5 software. Selective indexes (SI, IC50 Vero cells/IC50 trophozoite forms) were also calculated.
In vitro cysticidal activity assays
Acanthamoeba castellanii cysts were obtained according to Cordingley et al. (Reference Cordingley, Wills and Villemez1996). Cysts were then washed twice with RPMI medium containing 0.5% SDS that caused the death of trophozoites but without effect over the viability of the cysts.
1 × 104 cysts well−1 were seeded in 96-well microtiter plates (Sigma, Aldrich Ltd) in 200 μL of the RPMI medium with 8% glucose, and the compounds were added at a range concentration from 200 to 10 μ m. Untreated controls and controls treated with chlorhexidine digluconate (Sigma, Aldrich Ltd) were also included. The plates were then incubated for 120 h at 28°C. After that, cysts were slightly washed, fresh CGV medium with 10% heat-inactivated FCS was added to each well, and plates were incubated at 28°C for further 24, 48, 72, 120 and 192 h. The number of cysts and trophozoites were counted at these different times using a Neubauer chamber, and the presence of trophozoites was considered as an indication of cyst viability. All experiments were performed in quadruplicate.
Results
The in vitro trophozoital activity, toxicity and selectivity index are summarized in Table 1. The absorbance values at the different assayed concentrations (400, 200, 100, 50, 25, 12.5, 5, 2.5, 1.25 and 0.62 μ m) and the alterations on the trophozoites were dose-dependent. The results showed in Table 1 reveal that flavonoids 1, 2, 3 and 4 were the most effective against A. castellanii trophozoites, with IC50 values of 3.5 ± 3.0, 1.4 ± 1.2, 1.4 ± 0.4 and 2.3 ± 0.4 μ m, respectively. Hence, these flavonoids showed higher trophozoital activity than the reference drug (5.1 ± 1.4 μ m). Figure 2 shows the dose-response graphs (end-point 120 h) using GraphPad Prism 5 software, and Fig. 3 shows the dose-response growth curves during the first 120 h of incubation for the most active compounds. In addition, their cytotoxicities against Vero cells were very low, reaching selectivity indexes of 55.8, 71.7, 58.1 and 38.8 vs 28.9 of the reference drug.
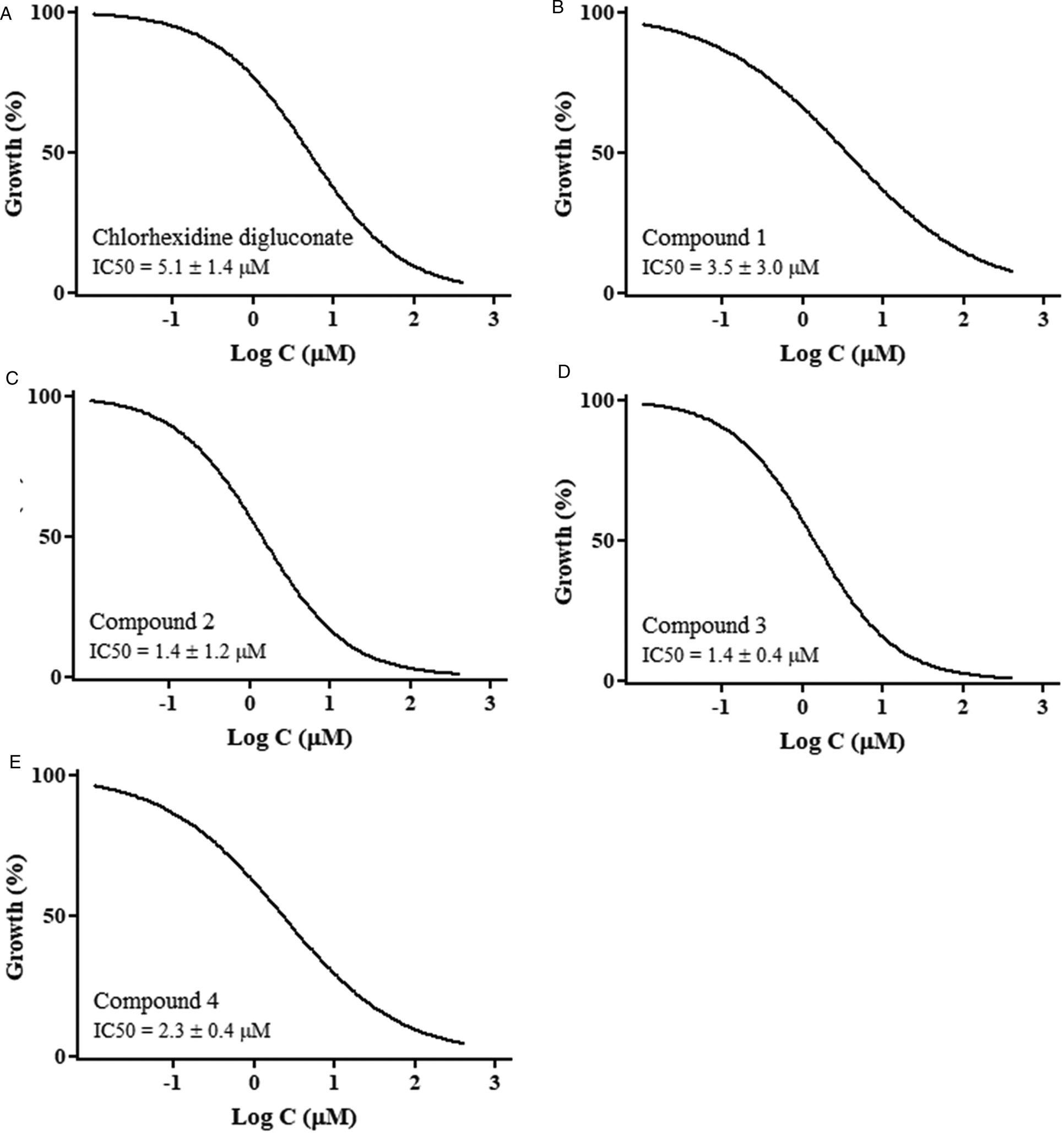
Fig. 2. Dose-response growth curves of Acanthamoeba castellanii trophozoites treated with (A) chlorhexidine digluconate and (B–E) compounds 1–4 for 120 h.
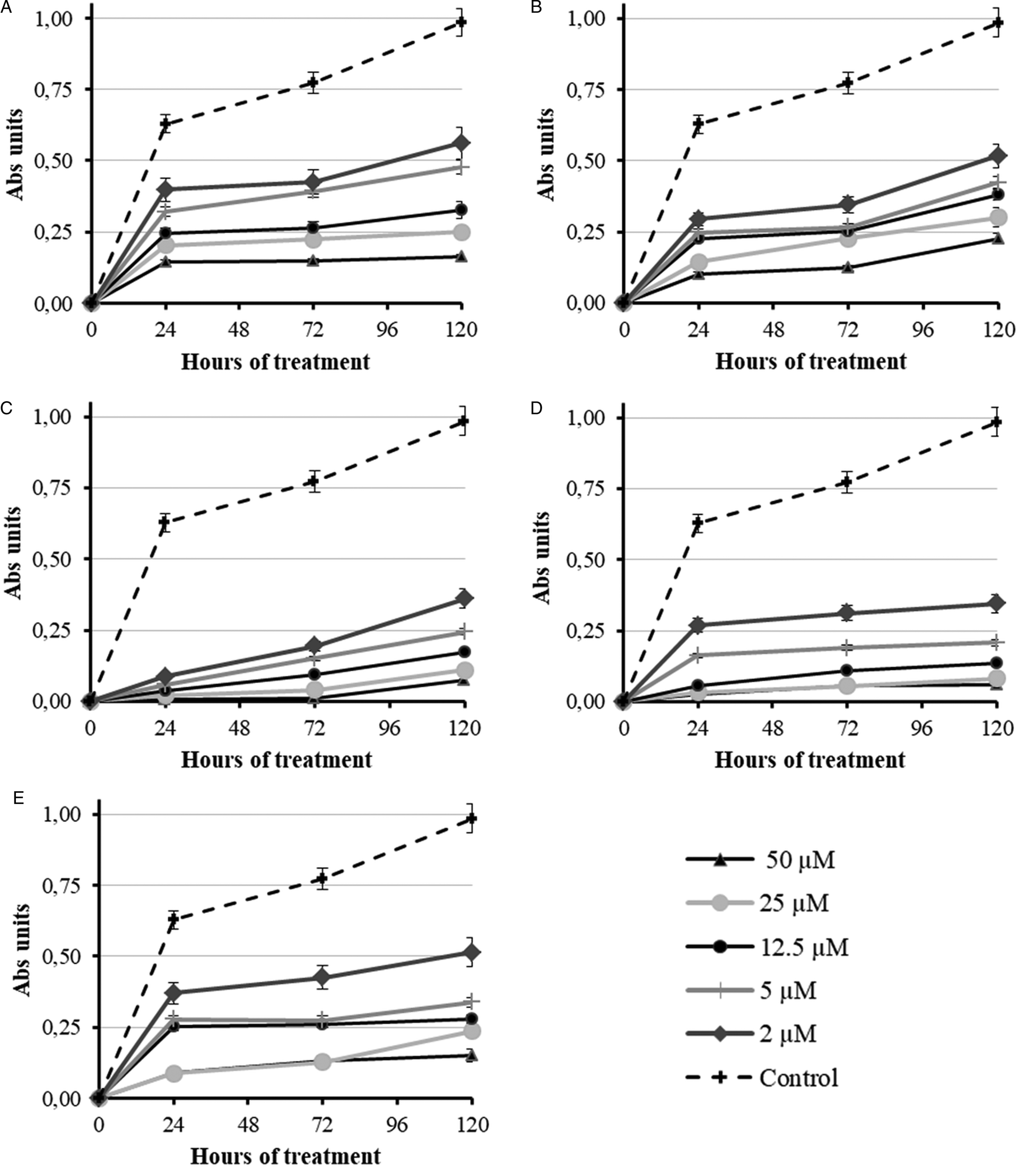
Fig. 3. Dose-response growth curves of Acanthamoeba castellanii trophozoites treated with (A) chlorhexidine digluconate and (B–E) compounds 1–4 during the first 120 h.
Table 1. In vitro trophozoital activity against A. castellanii, toxicity on mammalian Vero cells and selectivity index for new flavonoid derivatives

Results are averages of four separate determinations.
a Inhibition concentration 50 (IC50) = concentration required to give 50% inhibition, calculated by linear regression analysis from the Kc values at the concentrations employed.
b Towards Vero cells after 120 h of culture.
c Selectivity index = IC50 Vero cells/IC50 activity on trophozoites of the parasite.
The effect of the compounds against A. castellanii trophozoites was observed by inverted microscopy. Figure 4 shows the appearance of untreated trophozoites (Fig. 4A) vs trophozoites treated with chlorhexidine digluconate (Fig. 4B) and with the most effective flavonoid compounds 1–4 (Fig. 2C–F) at the lowest concentration tested (5 μ m) after 120 h of incubation. The alterations were observed to be dose-dependent, but at the lowest concentration (5 μ m) many trophozoites appeared agglutinated and floating in the supernatant (Fig. 4B) after treatment with the reference drug chlorhexidine digluconate. Flavonoids 2, 3 and 4 (Fig. 4D–F) caused the detachment and death of many trophozoites, and others showed significant structural alterations such as reduction in size, loss of acanthopodia and/or roundness compared to untreated trophozoites (Fig. 4A). Compound 1 was less effective (Fig. 4C), although it also produced round trophozoites without acanthopodia and filled with large vacuoles.
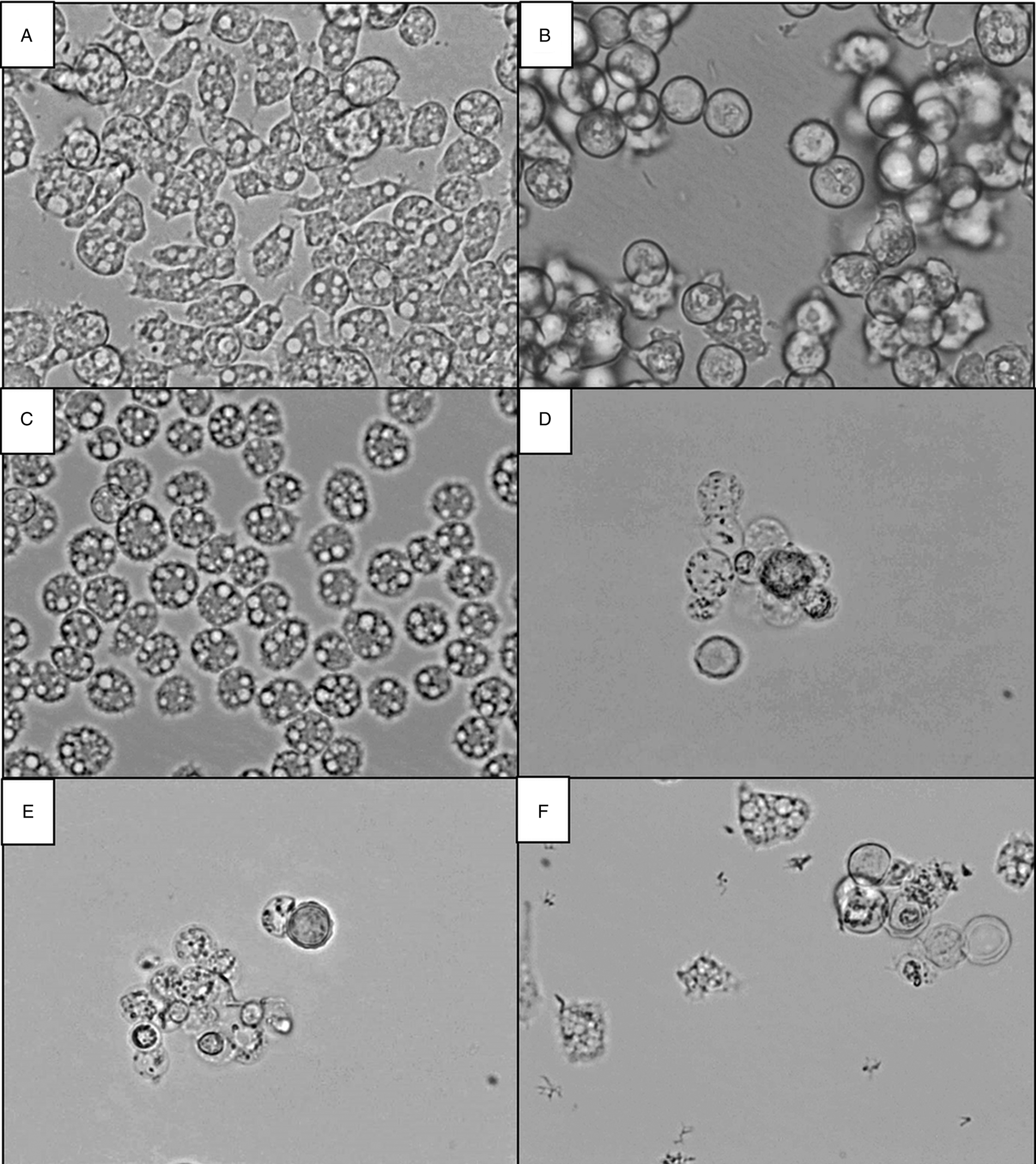
Fig. 4. Inverted microscopy pictures × 400 of Acanthamoeba castellanii treated with compounds for 120 h at 5 μ m. (A) Untreated trophozoites, (B) trophozoites treated with chlorhexidine digluconate, and trophozoites treated with flavonoids (C) 1, (D) 2, (E) 3 and (F) 4.
Flavonoids 1, 2, 3 and 4 were then tested against A. castellanii cysts, resistant forms that are less susceptible to treatment than trophozoites. Contrary to trophozoites, cysts are highly resistant to chemical injury and can be present in the eyes, brain, lungs and skin of patients; the development of new treatments is focused on compounds with cysticidal activity. Figure 5 shows the cysticidal activity of the reference drug chlorhexidine digluconate (Fig. 5A) and flavonoids 1–4 (Fig. 5B–E, respectively) vs untreated control cysts. Cyst viability was studied by assessing the excystment of untreated and treated cysts with the reference drug and flavonoids during 192 h. Trophozoites obtained from treated cysts were certainly viable, showed acanthopodia, and proliferated to establishing new cultures. Similar cysticidal activity was obtained with the reference drug chlorhexidine digluconate and compound 3, and no cysts reverted to trophozoites at 200 and 100 μ m during the 192 h test (Fig. 5A and D, respectively). Treatment with flavonoid 2 was the most effective as the cysts did not revert to trophozoites at 200, 100 and even 50 μ m (Fig. 5C), presuming they were not viable.

Fig. 5. Percentage of cysts after treatment with (A) the reference drug and flavonoids (B) 1, (C) 2, (D) 3 and (E) 4 at concentrations ranged from 200 to 10 μ m for 120 h in encystment medium and subsequent incubation during 192 h in culture medium CGV with 10% heat-inactivated fetal calf serum. Non-viable cysts did not revert to trophozoites.
Finally, the morphology of Acanthamoeba cysts was observed by light microscopy and compared with untreated controls after 120 h of treatment with the reference drug and the compound 1–4 at 10 μ m (Fig. 6). Flavonoids 2 and 3 were the most effective against the cysts (Fig. 6D and E, respectively); these treatments killed most of the cysts, caused a large amount of cyst debris, and those not destroyed were small and their walls appeared thinner than those of the untreated cysts (Fig. 6A). Cysts treated with flavonoids 1 and 4 (Fig. 6C and F, respectively) were morphologically similar to those treated with the reference drug (Fig. 6B), with a dry appearance and many vacuoles compared to untreated cysts (Fig. 6A).
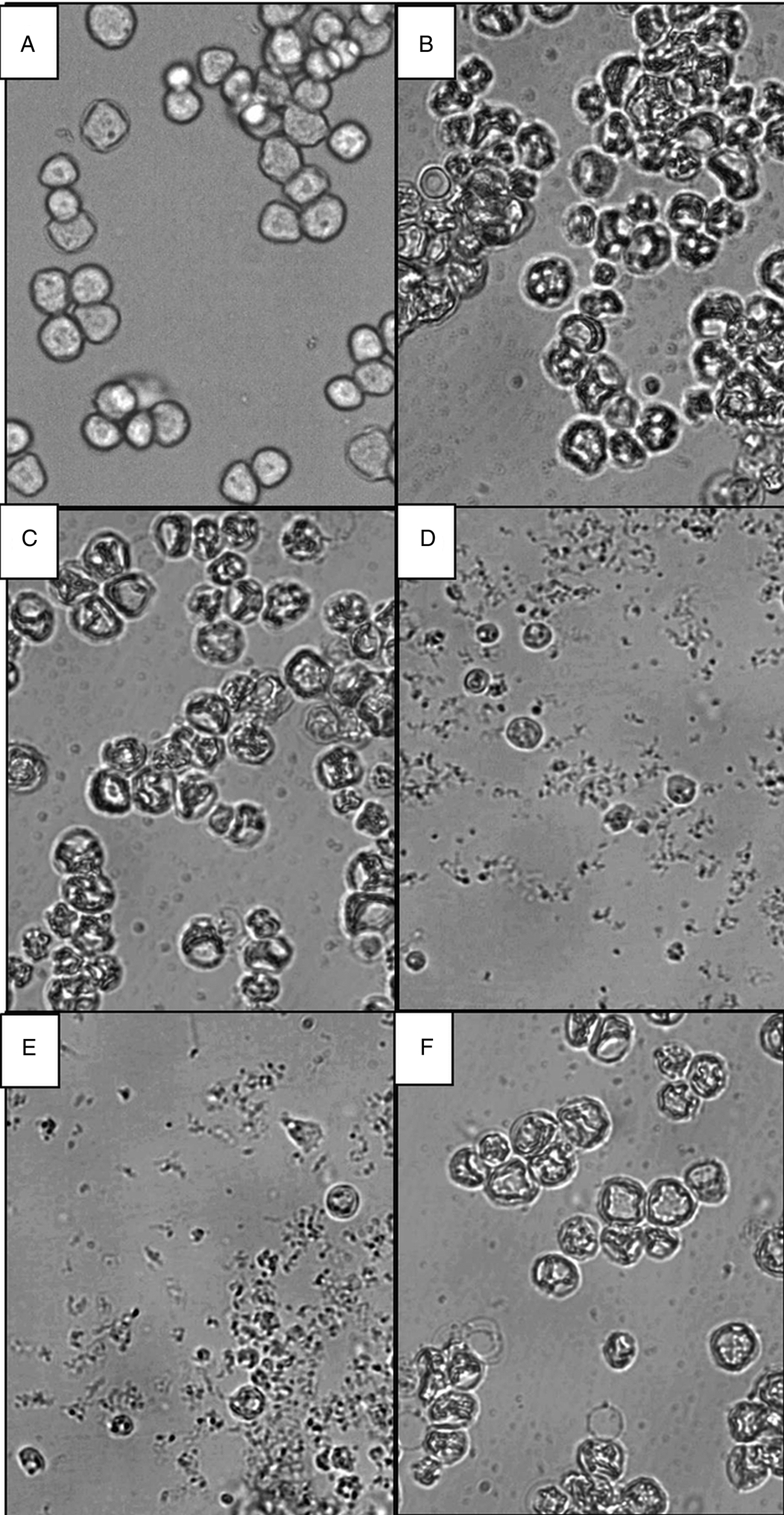
Fig. 6. Inverted microscopy pictures × 400 of Acanthamoeba castellanii cysts treated with compounds for 120 h at 10 μ m. (A) Untreated cysts, (B) cysts treated with chlorhexidine digluconate, and cysts treated with flavonoids (C) 1, (D) 2, (E) 3 and (F) 4.
Discussion
Although many studies have been conducted to find new amoebicidal drugs, treatment of these infections is frequently unsuccessful because of the limited drug efficacy, frequent side-effects and increasing drug resistance. Therefore, the development of new molecules is urgently needed (Niyyati et al., Reference Niyyati, Dodangeh and Lorenzo-Morales2016; Hajaji et al., Reference Hajaji, Sifaoui, López-Arencibia, Reyes-Batlle, Valladares, Pinero, Lorenzo-Morales and Akkari2017). Investigations for potential and effective treatments against amoeba are gaining a wide interest in public health research. Recently, research has turned towards natural products to find alternative drugs. Medicinal plants could be considered as a potential source for the development of more efficient compounds against Acanthamoeba spp. as shown in previous studies (Polat et al., Reference Polat, Tepe and Vural2007; Ródio et al., Reference Ródio, Da Rocha Vianna, Kowalski, Panatieri, Von Poser and Rott2008; Sauter et al., Reference Sauter, Dos Santos, Apel, Cibulski, Roehe, Von Poser and Rott2011, Reference Sauter, Rossa, Lucas, Cibulski, Roehe, da Silva, Rott, Vargas, Cassel and von Poser2012; Degerli et al., Reference Degerli, Tepe, Celiksoz, Berk and Malatyali2012; Derda et al., Reference Derda, Thiem, Budzianowski, Hadaś, Wojt and Wojtkowiak-Giera2013; Sifaoui et al., Reference Sifaoui, López-Arencibia, Ticona, Martín-Navarro, Reyes-Batlle, Mejri, Lorenzo-Morales, Jiménez, Valladares, Lopez-Bazzocchi, Abderabba and Piñero2014; Ghazouani et al., Reference Ghazouani, Sifaoui, Bachrouch, Abderrabba, Pinero and Lorenzo-Morales2017; Saoudi et al., Reference Saoudi, Sifaoui, Chammem, Reyes-Batlle, López-Arencibia, Pacheco-Fernández, Pino, Hamdi, Jiménez, Bazzocchi, Piñero and Lorenzo-Morales2017).
In this study, we tested the amoebicidal and cysticidal activity of 13 natural flavonoids against A. castellanii vs the reference drug chlorhexidine digluconate. This reference drug is a standard antiseptic used for the treatment of AK with proven cysticidal activity (Schuster and Visvesvara, Reference Schuster and Visvesvara2004; Khan, Reference Khan2006). It should be highlighted that these flavonoids were obtained from D. gracile, and flavonoids from this plant species showed effectiveness against other parasites such as Plasmodium spp., Leishmania spp., as well as T. cruzi and T. brucei (Marín et al., Reference Marín, Ramírez-Macías, López-Céspedes, Olmo, Villegas, Díaz, Rosales, Gutiérrez-Sánchez and Sánchez-Moreno2011, Reference Marín, Díaz, Irure Maiques, Ramírez-Macías, Rosales, Guitierrez-Sánchez, Cañas and Sánchez-Moreno2017; Ramírez-Macías et al., Reference Ramírez-Macías, Marín, Díaz, Rosales, Gutiérrez-Sánchez and Sánchez-Moreno2012; Schmidt et al., Reference Schmidt, Khalid, Romanha, Alves, Biavatti, Brun, Da Costa, de Castro, Ferreira, de Lacerda, Lago, Leon, Lopes, das Neves Amorim, Niehues and Ogungbe2012). Metabolite excretion and ultrastructural alterations were observed in treated Leishmania spp. and T. cruzi parasites, causing severe modifications in organelles such as glycosomes and mitochondria, which could be the ultimate reasons for the alterations observed in all these species (Ramírez-Macías et al., Reference Ramírez-Macías, Marín, Díaz, Rosales, Gutiérrez-Sánchez and Sánchez-Moreno2012; Marín et al., Reference Marín, Díaz, Irure Maiques, Ramírez-Macías, Rosales, Guitierrez-Sánchez, Cañas and Sánchez-Moreno2017).
We used the reference drug chlorhexidine digluconate because topical biguanide, chlorhexidine or polyhexamethylene biguanide (0.02%) are the chosen drug for AK (Gupta and Miescke, Reference Gupta and Miescke213AD). Chlorhexidine digluconate is used at 0.02% concentration in the initial therapy of AK. It acts damaging the membrane of the amoebas, with irreversible loss of calcium and cell electrolytes from the cytoplasm causing cell lysis and death (Radford et al., Reference Radford, Minassian and Dart2002). Being a chemical capable of destroying the membrane of the amoeba, a similar negative effect can be expected on the plasma membranes of the patient iris and lens cells due to long-term treatment leading to the development of cataracts (Ehlers and Hjortdal, Reference Ehlers and Hjortdal2004; El-Sayed et al., Reference El-Sayed, Ismail, Ahmed and Hetta2012). In any case, the cysticidal activity of chlorhexidine digluconate at 0.02% is only 80% (El-Sayed et al., Reference El-Sayed, Ismail, Ahmed and Hetta2012). In addition, chlorhexidine digluconate has shown poor corneal penetration into the anterior chamber after topical administration (Banich et al., Reference Banich, Bu, Jacob, Fox, Zhang, Perlman and Bouchar2003). Here, flavonoids 1, 2, 3 and 4 were more effective against trophozoites than the reference drug chlorhexidine digluconate, and flavonoid 3 exhibited similar cysticidal activity to that of the reference drug. Flavonoid 2 was the most effective, with higher amoebicidal activity than the reference drug (IC50 value of 1.4 vs 5.1 μ m, respectively), and better cysticidal activity. Indeed, only a concentration of 50 μ m was needed to kill 100% of the cysts. In summary, flavonoids 2 and 3 may be alternatives for the treatment of A. castellanii infections (AK, GAE, EP).
As features to highlight, quercetin derivatives are much more active than kaempferol analogues. All active compounds have a trans-caffeoyl or trans-p-coumaroyl ester group at position C-4 of the inner rhamnopyranosyl unit. Our results indicate that the acetylated compounds performed better than the phenolic analogues. It appears that kaempferol derivatives possessing a monosubstituted B-ring are less active than the quercetin analogues. For example, compounds 1 and 2 have IC50 values of 3.5 and 1.4 μ m, respectively, in contrast to 689.5 and 876.9 μ m for their kaempferol analogues 7 and 8.
From all these data, it can be concluded that the flavonoids isolated from D. gracile (1, 2, 3 and 4) are potential candidates for the development of efficient drug treatments against A. castellanii. Flavonoid 2 is noteworthy because its efficacy against trophozoites and cysts was higher than that of the reference drug chlorhexidine digluconate. The activity, stability, low cost of starting materials and straightforward synthesis make flavonoid derivatives appropriate molecules for the development of an affordable therapy against A. castellanii. Further chemical modifications, tests with other strain and in vivo experiment are necessary to improve their activity, as well as to assess their tolerance and effectiveness in animal models.
Author contribution
M.J.R. conceived and designed the study. J.G.D. isolated the flavonoids. S.M.R., C.M. and M.S.-M. performed the experiments. R.M.-E. and M.J.R. conducted data gathering. R.M.-E. and M.J.R. wrote the article.
Financial support
This work was supported by the Spanish MINECO, FEDER funds of the EU (grant number CTQ2013-14892); CONSOLIDER INGENIO (grant number 2010-CSD2010-00065); Unidad de Excelencia MDM (grant number 2015-0038); Generalitat Valenciana (grant number PROMETEO II 2015-002); and Ministerio de Ciencia e Innovación (MICIN) (grant number RTI2018-094356-B-C21). R.M.-E. is grateful for the fellowship from the Alfonso Martín Escudero Foundation.
Conflict of interest
None.
Ethical standards
Not applicable.