Introduction
Dysphagia is common and costly. Nearly 20 per cent of the general population experiences intermittent to weekly episodes of dysphagia,Reference Cho, Choung, Saito, Schleck, Zinsmeister and Locke 1 and as many as 50 per cent of in-patient elderly patients report swallowing difficulty.Reference Leder and Suiter 2 Common causes of minor to moderate swallowing dysfunction include acid reflux, cricopharyngeus muscle dysfunction, oesophageal webs and rings, and advancing age.Reference Hoy, Domer, Plowman, Loch and Belafsky 3 These disorders are frequently treatable with conservative medical and/or endoscopic intervention. When swallowing dysfunction becomes severe, however, treatment options are limited and invasive. Common causes of profound oropharyngeal dysphagia include head and neck cancer, stroke, and progressive or degenerative neurological disease. Complications of profound oropharyngeal dysphagia include malnutrition, dehydration, aspiration pneumonia, empyema, depression, social isolation and death.Reference Garcia-Peris, Paron, Velasco, de la Cuerda, Camblor and Breton 4 The overall impact of dysphagia on the healthcare system is costly. Recent reports estimate that dysphagia is associated with nearly double the length of average hospital stays and is responsible for a total cost of greater than $500 billion annually in the USA.Reference Altman, Yu and Schaefer 5
Dysphagia is most successfully treated using a multidisciplinary team approach, and management begins with conservative therapies. However, surgery may be necessary when conservative treatment fails or in cases of life-threatening aspiration. The development of novel surgical treatments is limited by the absence of a validated research model. This study seeks to establish the cadaveric head and neck of a Dorper cross ewe as a suitable model for profound oropharyngeal dysphagia.
The ‘gold standard’ treatment for patients with life-threatening swallowing dysfunction and aspiration pneumonia is surgical separation of the respiratory and deglutitive tracts.Reference Takano, Suga, Sakamoto, Sato, Samejima and Ando 6 – Reference Eisele 8 Two primary operations can achieve this goal: total laryngectomy and laryngotracheal separation.Reference Snyderman and Johnson 7 , Reference Cannon and McLean 9 The advantage of laryngotracheal separation over total laryngectomy is that the procedure is theoretically reversible, making it preferable in persons with transient profound oropharyngeal dysphagia.
The comparative benefits of these procedures in terms of swallowing outcomes have not been systematically evaluated. While both are effective in eliminating aspiration, we hypothesise that the larynx itself is a significant barrier to bolus transit through the pharynx and that total laryngectomy may result in superior swallowing outcomes. This investigation aimed to evaluate the validity of an ovine model of profound oropharyngeal dysphagia and compare swallowing outcomes after total laryngectomy with those after laryngotracheal separation in a validated animal model (PC Belafsky, unpublished data).
Materials and methods
One fresh human cadaver and two fresh cadaveric Dorper cross ewes were utilised in this study. This sheep model was selected based on previous work establishing the animal as a valid surrogate model of the human larynx and upper oesophagus.Reference Zrunek, Happak, Hermann and Streinzer 10 , Reference Cates, Plowman, Mehdizadeh, Yen, Domer and Gilden 11
The human cadaveric head and neck were secured in the lateral fluoroscopic orientation using a large surgical basin (Medline Industries, Mundelein, Illinois, USA), which also allowed collection of the barium runoff (Figure 1a). The head and neck of a Dorper cross ewe was secured using a Rochard-OR-Table Fixation system (Medicon, Tuttlingen, Germany) placed at the edge of the table and a C-clamp attached to the vertical portion. Two Allis clamps were used to grasp the forehead of the ewe. A piece of twine was placed through both handles of the clamps to stabilise the ewe's head and neck in a 90-degree upright position on the fixation system, and the head and neck was placed in the lateral fluoroscopic orientation (Figure 1b).
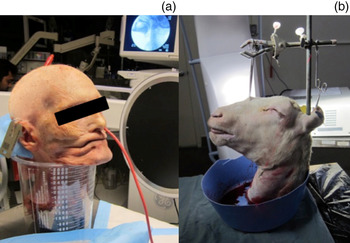
Fig. 1 (a) Cadaver secured in lateral fluoroscopic orientation. (b) Modifications used to secure ovine specimen.
An 18 Fr Red Rubber Catheter (Bard Medical Division, Covington, Georgia, USA) was placed transnasally, with the tip positioned within 1 cm of the epiglottis. Using the rubber catheter, 20 cc of liquid contrast agent (Liquid E-Z-Paque barium sulphate suspension (60 per cent weight/volume, 41 per cent weight/weight); E-Z-EM, Westbury, New York, USA) was delivered to the oropharynx of each specimen, and five successive trials were performed.
All radiographic images were captured using a properly collimated fluoroscopy unit with a 12-inch input phosphor diameter (OEC Medical Systems, Salt Lake City, Utah, USA). The videofluoroscopic recordings were then evaluated and scored using the Penetration Aspiration Scale (Table I)Reference Rosenbek, Robbins, Roecker, Coyle and Wood 12 and the National Institutes of Health Swallow Safety Scale (Table II).Reference Belafsky and Kuhn 13 All videofluoroscopic evaluations were performed by a clinician blind to the study condition and trial. The Penetration Aspiration Scale was modified for use in a cadaver study by eliminating volitional responses (Table I).
Table I Modification of penetration aspiration scale for use in cadavers

Table II National institutes of health swallow safety scale

The numbers at the bottom of each Score column represent the maximum possible score. A score of 0 indicates normal swallowing, with a maximum score of 8. UOS = upper oesophageal sphincter
Intra- and inter-species reproducibility was evaluated. To determine criterion-based validity, five 20 ml barium trials were administered pre- and post-laryngohyoid suspension in one cadaveric Dorper cross ewe head and neck. A Wilcoxon signed-rank test was used to compare post-laryngohyoid suspension with baseline Penetration Aspiration Scale and National Institutes of Health Swallow Safety Scale scores.
In the second animal, five trials were conducted under each of the following conditions: prior to intervention (baseline), following laryngotracheal separation and following narrow-field laryngectomy. During each trial, 60 ml of barium sulphate was delivered into the oropharynx and recorded as described above. With the head and neck positioned as stated above, a large surgical basin (Medline Industries) was placed under the neck to collect barium from the oesophagus. Laryngotracheal separation was performed first, followed by narrow-field laryngectomy.
The primary outcome measures were: the modified Penetration Aspiration Scale, the National Institutes of Health Swallow Safety Scale, and the volume (in millilitres) of aerodigestive tract residue (i.e. the quantity of barium that did not enter the oesophagus). Statistical analyses were performed with SPSS 17 for Macintosh (IBM, Armonk, New York, USA). Swallowing outcomes were evaluated with repeated measures analysis of variance and confirmed with the non-parametric Wilcoxon signed-rank test. A probability of type I error (α) of 0.05 was used to ascertain statistical significance.
Our techniques for performing the above procedures are described below.
Laryngohyoid suspension
Surgery was carried out via a midline cervical incision, with the dissection of strap muscles to expose the laryngeal framework and the hyoid bone. The thyroid cartilage was brought up to the hyoid and this was secured in place using size 2-0 PDS suture (Ethicon, Somerville, New Jersey, USA). The entire framework was then brought up, and the hyoid was secured to the mandible using size 2-0 PDS suture. The wound was then closed in layers using size 4-0 nylon suture (Ethicon).
Laryngotracheal separation
A small horizontal incision was made, inferior to the cricoid cartilage, followed by elevation of subplatysmal flaps. The strap muscles were separated in the midline to expose the cricoid cartilage and trachea. An incision was made between the second and third tracheal rings. Mucosa was elevated from the inside of the trachea up to the level of the cricoid ring and then secured using size 2-0 chromic suture (Ethicon). The distal trachea was sutured to the skin using size 2-0 chromic suture in standard half-mattress stitches.
Narrow-field laryngectomy
In standard narrow-field laryngectomy, a vertical incision is made from the tracheostoma to the hyoid bone. In this model, the horizontal incision from the laryngotracheal separation was used. The strap muscles were separated in the midline. The stoma had previously been created during laryngotracheal separation. The post-cricoid mucosa was elevated from the cricoid. The pharynx was entered at the level of the arytenoid cartilages. This was followed by a limited infrahyoid pharyngotomy with preservation of as much mucosa as possible. The hyoid bone was left in vivo, and the larynx and cricoid were removed in their entirety. Closure of the mucosa was performed with a running vertical stitch using size 3-0 vicryl suture (Ethicon).
Results
The mean (± standard deviation) Penetration Aspiration Scale and National Institutes of Health Swallow Safety Scale scores for both sheep and the human cadaver at baseline prior to intervention were 8 ± 0 and 6 ± 0 respectively (Table III), indicating profound swallowing dysfunction. Both intra- and inter-species reproducibility was perfect (100 per cent).
Table III PAS and NIH-SSS scores in ovine and human cadavers

Data are presented as means (± standard deviations). PAS = Penetration Aspiration Scale; NIH-SSS = National Institutes of Health Swallow Safety Scale
Swallow safety improved significantly following laryngohyoid suspension (Figure 2). The mean Penetration Aspiration Scale and Swallow Safety Scale scores in the ovine cadaver improved from 8 ± 0 and 6 ± 0 pre-laryngohyoid suspension to 1 ± 0 and 2 ± 0 post-laryngohyoid suspension (p < 0.01), indicating excellent criterion-based validity (Table IV).
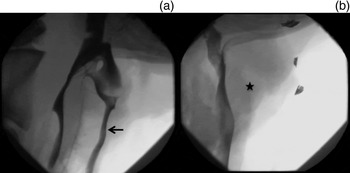
Fig. 2 (a) Videofluoroscopy showing gross aspiration (arrow) in the ovine model of profound oropharyngeal dysphagia pre-laryngohyoid suspension. (b) No aspiration (star) seen in the ovine model post-laryngohyoid suspension.
Table IV PAS and NIH-SSS scores pre- and post-laryngohyoid suspension in an ovine cadaver

Data are presented as means (± standard deviations). PAS = Penetration Aspiration Scale; NIH-SSS = National Institutes of Health Swallow Safety Scale
Prior to performing laryngotracheal separation and narrow-field laryngectomy, baseline modified Penetration Aspiration Scale and Swallow Safety Scale scores were re-assessed and were 8 ± 0 and 6 ± 0 respectively, confirming profound swallowing dysfunction. After laryngotracheal separation, these scores were 8 ± 0 and 6 ± 0 respectively (p > 0.05). As the airway had been completely separated from the digestive tract, these measures could not be assessed after narrow-field laryngectomy.
Aerodigestive tract residue was 18.6 ± 2.4 ml at baseline, 15.4 ± 3.8 ml following laryngotracheal separation and 3.0 ± 0.7 ml after narrow-field laryngectomy (Table V and Figure 3). There was no difference in aerodigestive tract residue between baseline and post-laryngotracheal separation trials (p > 0.05). Airway residue was significantly reduced after narrow-field laryngectomy, indicating improvement of bolus transit into the oesophagus (p < 0.001).
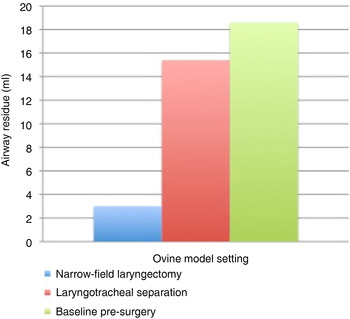
Fig. 3 Comparison of airway residue (60 ml bolus trials).
Table V Barium swallow results in an ovine model of profound dysphagia

NIH-SSS = National Institutes of Health Swallow Safety Scale; PAS = Penetration Aspiration Scale; N/A = not applicable
Although not quantified, the upper oesophageal sphincter showed improved opening following narrow-field laryngectomy (Figure 4), while there was no obvious change in upper oesophageal sphincter opening between baseline (Figure 5) and post-laryngotracheal separation (Figure 6).
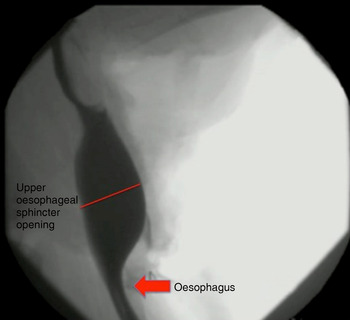
Fig. 4 Fluoroscopic image of ovine model following narrow-field laryngectomy.
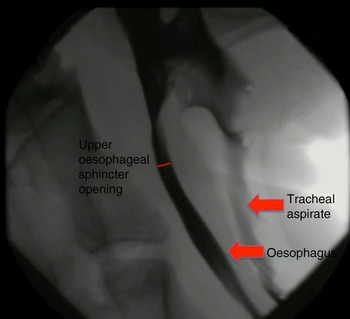
Fig. 5 Fluoroscopic image of ovine model at baseline.
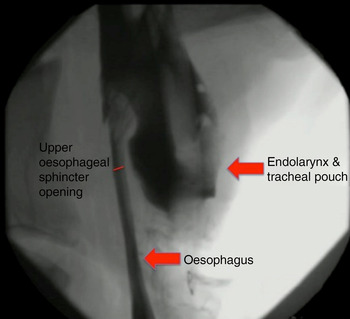
Fig. 6 Fluoroscopic image of ovine model following laryngotracheal separation.
Discussion
Profound swallowing dysfunction is a devastating condition associated with significant morbidity and mortality. Fortunately, conservative measures are sufficient to improve symptoms and prevent illness for most affected individuals. For others, vocal fold medialisation, cricopharyngeus muscle myotomy, laryngohyoid suspension, tubed epiglottoplasty and glottic closure may help to decrease aspiration risk, and may even be curative in less severe cases. The development of further innovative treatment strategies for swallowing dysfunction is limited by the lack of a reliable profound oropharyngeal dysphagia model. The cadaveric state represents a worst-case swallowing scenario, with complete absence of lingual and pharyngeal contraction, laryngohyoid elevation and upper oesophageal opening.
The development of novel surgical techniques and devices that improve swallowing safety in this model may lead to advancements which improve swallowing in patients with profound oropharyngeal dysphagia. Some devices we have tested using the human cadaveric model of oropharyngeal dysphagia include the Swallow Expansion Device, innovative pharyngoesophageal dilation techniques and a swallow propulsion system.Reference Belafsky 14 – Reference Belafsky, Plowman, Mehdizadeh, Cates, Domer and Yen 16 A validated cadaveric animal model for dysphagia has theoretical advantages over a human cadaver.
Advantages of an animal model of profound oropharyngeal dysphagia include: reduced cost, no transmission of human communicable disease, optimisation of resources and increased specimen availability. The use of animal cadavers does not require permission for use from a donated bodies programme, needs no approval from an institutional review board or animal care and rights committee, and does not interfere with animal rights initiatives. This affords efficient and expedited research without administrative delay. The data from this investigation suggest that the ovine model of profound oropharyngeal dysphagia is reliable and a valid surrogate when compared to the human cadaveric model. The elimination of gross aspiration following laryngohyoid suspension suggests that criterion-based validity in this model is also excellent.
After establishing the validity of the ovine model of profound oropharyngeal dysphagia, we sought to compare the results of laryngotracheal separation and total laryngectomy, the only two current options for treatment of intractable aspiration in profound oropharyngeal dysphagia. The allure of reversal has made laryngotracheal separation a favoured solution in the paediatric population. Laryngotracheal separation is successful in the resolution of aspiration and allows for decreased care requirements.Reference Cook 17 – Reference Gelfand, Duncan, Albright, Roy, Montagnino and Edmonds 19 However, these studies do highlight that the neurological deficits causing aspiration often do not resolve, which precludes the possibility of reversal. For many surgeons, total laryngectomy has been considered the gold standard for treatment of chronic aspiration.Reference Miller and Eliachar 20 For patients without cancer, narrow-field laryngectomy has been devised to allow for a safer closure, with lower rates of fistula formation. In narrow-field laryngectomy, the resection is limited to the epiglottis, thyroid cartilage, cricoid cartilage and arytenoid cartilages, while preserving the hyoid bone, piriform sinuses and posterior pharyngeal wall, thus preserving more mucosa for closure.
Though both laryngotracheal separation and narrow-field laryngectomy resolve aspiration, their effects on swallowing outcomes have not been previously reported. The present investigation suggests that swallowing outcomes are superior after narrow-field laryngectomy in comparison with laryngotracheal separation. In the ovine model, aspiration was eliminated in both laryngotracheal separation and narrow-field laryngectomy, as expected. However, modified Penetration Aspiration Scale and National Institutes of Health Swallow Safety Scale scores, and airway residue, were improved only after narrow-field laryngectomy (p < 0.001). Using standard 60 ml boluses of barium, narrow-field laryngectomy demonstrated near-complete passage of the bolus, with little residual barium. After laryngotracheal separation, however, over one-quarter of the barium remained in the airway, indicating diminished bolus transit into the oesophagus.
The larynx is a significant barrier to bolus transit. The elastic recoil of the larynx and cricoid cartilages against the spine contributes to the majority of upper oesophageal sphincter pressure. In persons with profound oropharyngeal dysphagia, the ability to elevate the larynx and cricoid off the spine is diminished, and barium flow into the oesophagus is limited. Our results confirm that removing the larynx and cricoid improves upper oesophageal sphincter opening and bolus transit into the oesophagus. This supports the hypothesis that swallowing outcomes are superior after narrow-field laryngectomy compared with laryngotracheal separation.
-
• Cadaveric head and neck of a Dorper cross ewe can be used as a surrogate model of profound oropharyngeal dysphagia in humans
-
• The cadaveric ovine model can be utilised in research to find novel treatment for profound oropharyngeal dysphagia in humans
-
• Laryngotracheal separation and laryngectomy effectively eliminate aspiration
-
• While irreversible, laryngectomy provides superior swallowing outcomes to laryngotracheal separation
The central limitation of this investigation lies within the cadaveric model of profound oropharyngeal dysphagia, which itself is only a surrogate for the living human condition. Additionally, barium was delivered to the pharynx with a red rubber catheter, which does not replicate the typical swallowing conditions of oropharyngeal dysphagia. Nonetheless, the data from this study support the utility of the model. The availability, low cost and reproducibility of the ovine model make it a viable option for future dysphagia research and innovation. Further, the findings from this investigation, performed under the worst-case (cadaveric) conditions, demonstrate that swallowing outcomes are superior after narrow-field laryngectomy in comparison with laryngotracheal separation.
Conclusion
The ovine model displayed perfect intra- and inter-species reliability in terms of Penetration Aspiration Scale and National Institutes of Health Swallow Safety Scale scores. Criterion-based validity was excellent. The data support the utility of the ovine cadaveric model as a surrogate for the human dysphagia model. In this animal model of profound oropharyngeal dysphagia, outcome measures of swallowing function were superior after narrow-field laryngectomy compared with laryngotracheal separation (p < 0.001). These findings lend support to the application of narrow-field laryngectomy over laryngotracheal separation in certain cases of profound oropharyngeal dysphagia with intractable aspiration.













