Introduction
Humans have dramatically modified the world by altering biogeochemical cycles and eroding biodiversity (Crutzen Reference Crutzen2002; Terborgh and Estes Reference Terborgh and Estes2010). Large species are particularly vulnerable to human impacts, and many of them declined in population size, or went extinct, before we had the ability to study and understand their ecological significance (Jackson et al. Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001; Martin Reference Martin2005; Terborgh and Estes Reference Terborgh and Estes2010). Nevertheless, biologists still attempt to interpret the biology of extant species as if they had evolved in the current anthropogenic setting (Steadman Reference Steadman2006). In this context, a historical reference point is essential not only for understanding the evolutionary processes that operated in pristine environments, but also for providing goals for restoration and management (Jackson et al. Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001; Terborgh and Estes Reference Terborgh and Estes2010; Braje and Rick Reference Braje and Rick2011). Although much of the anthropogenic loss of biodiversity has been caused by the expansion of western societies, aboriginal exploitation also resulted in significant levels of extinction and habitat modification (Jackson et al. Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001; Martin Reference Martin2005; Terborgh and Estes Reference Terborgh and Estes2010). Thus, we must study deep time, using the stable isotope ratios in bone collagen to reconstruct paleodiets and trophic levels to understand the natural trophic ecology of modern species in their original environments (Ambrose and DeNiro Reference Ambrose and DeNiro1989; Schwarcz Reference Schwarcz2000).
Human impacts are not limited to terrestrial ecosystems; the world oceans also have been severely affected (Halpern et al. Reference Halpern, Walbridge, Selkoe, Kappel, Micheli, D’Agrosa, Bruno, Casey, Ebert, Fox, Fujita, Heinemann, Lenihan, Madin, Perry, Selig, Spalding, Steneck and Watson2008). Eared seals, or Otariids, may exert strong top-down effects on ecosystem structure where abundant enough (Yodzis Reference Yodzis1998; Koen-Alonso and Yodzis Reference Koen-Alonso and Yodzis2005) and are still major components of coastal ecosystems in the temperate regions of the Southern Hemisphere (Gentry Reference Gentry2009). However, most species of eared seals, commercially exploited for their pelts and fat, were hunted to the brink of extinction throughout the nineteenth and twentieth centuries, and currently some species remain well below their original numbers (Kovacs et al. Reference Kovacs, Aguilar, Aurioles, Burkanov, Campagna, Gales, Gelatt, Goldsworthy, Goodman, Hofmeyr, Härkönen, Lowry, Lydersen, Schipper, Sipilä, Southwell, Stuart, Thompson and Trillmich2012). Nevertheless, in some regions the human exploitation of eared seals predates the arrival of western sealers by several millennia, thus raising many questions about the actual impact of aboriginal exploitation (Porcasi et al. Reference Porcasi, Jones and Raab2000; Jones et al. Reference Jones, Hildebrant, Kennett and Porcasi2004; Newsome et al. Reference Newsome, Etnier, Kurle, Waldbauer, Chamberlain and Koch2007; Tivoli and Zangrando Reference Tivoli and Zangrando2011).
The South American sea lion Otaria flavescens is widely distributed along some 10,000 km of the coast of South America (Cappozo and Perrin Reference Cappozzo and Perrin2009). According to the zooarchaeological record, pinnipeds and other marine species were widely exploited by the hunter-gatherers inhabiting the Beagle Channel and northern Patagonia during the late Holocene, although the effect of aboriginal hunting on their populations remains uncertain (Schiavini Reference Schiavini1993; Orquera and Piana Reference Orquera and Piana1999; Gómez Otero Reference Gómez Otero2006; Favier Dubois et al. Reference Favier Dubois, Borella and Tykot2009; Favier Dubois and Kokot Reference Favier Dubois and Kokot2011; Orquera et al. Reference Orquera, Legoupil and Piana2011; Tivoli and Zangrando Reference Tivoli and Zangrando2011; Borella and Cruz Reference Borella and Cruz2012; Favier Dubois and Scartascini Reference Favier Dubois and Scartascini2012). Modern exploitation through its entire range began in the eighteenth century and lasted until the first half of the twentieth century, by which time the species had been severely depleted (Cappozo and Perrin Reference Cappozzo and Perrin2009). In Uruguay, the population is still decreasing, even though sealing ceased in 1995 (Páez Reference Páez2006). The population breeding in Argentina experienced a 90% decline from the 1920s to the 1960s and currently is approximately one-third of the original size (Crespo and Pedraza Reference Crespo and Pedraza1991; Dans et al. Reference Dans, Crespo, Pedraza and Koen-Alonso2004; Schiavini et al. Reference Schiavini, Crespo and Szapkievich2004). Simultaneously, numbers of sea lions declined in the Falkland (Malvinas) Islands, where the small remaining population is less than 1.5% of the original size (Thompson et al. Reference Thompson, Strange, Riddy and Duck2005).
South American sea lions are no longer hunted in the region, but the intense exploitation of fishes, squids, and crustaceans experienced during the last 40 years has deeply modified the structure of the ecosystems and caused a dramatic decrease in average fish size (Koen-Alonso and Yodzis Reference Koen-Alonso and Yodzis2005; Dato et al. Reference Dato, Bambill, Cañete, Villarino and Aubone2006). South American sea lions are generalist predators (Thompson et al. Reference Thompson, Duck, McConnell and Garrett1998; Koen Alonso et al. Reference Koen Alonso, Crespo, Pedraza, Garcia and Coscarella2000; Campagna et al. Reference Campagna, Werner, Karesh, Marin, Koontz, Cook and Koontz2001; Riet-Sapriza et al. Reference Riet-Sapriza, Costa, Franco-Trecu, Marín, Chocca, González, Beathyate, Chilvers and Hückstädt2012) and during the twentieth century they have shifted their diet to adapt to the new environmental conditions created by industrial fishing (Suárez et al. Reference Suárez, Sanfelice, Cassini and Cappozzo2005; Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009; Romero et al. Reference Romero, Dans, Gonzalez, Svendsen, Garcia and Crespo2011), but at the cost of consuming less profitable prey and reducing body size (Drago et al. Reference Drago, Cardona, Crespo, Grandi and Aguilar2010). Nevertheless, intraspecific competition, and not competition with fisheries, has been proposed to be the major determinant of diet composition in South American sea lions (Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009, Reference Drago, Cardona, Crespo, Grandi and Aguilar2010), as they consume primarily large prey that feed near the bottom (demersal hereafter) when and where the population is small (Koen Alonso et al. Reference Koen Alonso, Crespo, Pedraza, Garcia and Coscarella2000; Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009; Riet-Sapriza et al. Reference Riet-Sapriza, Costa, Franco-Trecu, Marín, Chocca, González, Beathyate, Chilvers and Hückstädt2012) and shift to smaller benthic and pelagic prey when and where the population increases (Suárez et al. Reference Suárez, Sanfelice, Cassini and Cappozzo2005; Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009; Romero et al. Reference Romero, Dans, Gonzalez, Svendsen, Garcia and Crespo2011). If this hypothesis is true, the current trophic niche of South American sea lions would be different from that exploited in the past, when the population was much larger (Rodriguez and Bastida Reference Rodriguez and Bastida1998; Dans et al. Reference Dans, Crespo, Pedraza and Koen-Alonso2004; Schiavini et al. Reference Schiavini, Crespo and Szapkievich2004).
The presence of bones of the South American sea lion in the zooarchaeological record, together with extensive scientific collections of modern skeletal material, offers a unique opportunity to assess the magnitude of change in the trophic ecology of a marine top predator in response to human exploitation and compare the effect of aboriginal and modern exploitation. To do so, here we analyze the stable isotope ratios of carbon and nitrogen in the bone of ancient and modern South American sea lions from Argentina to assess the dietary changes of the species through the second half of the Holocene and determine the trophic niche of the species in ancient ecosystems. The stable isotope ratios of modern and ancient organisms cannot be compared directly, because temporal variations in the isotopic baseline may exist (Casey and Post Reference Casey and Post2011). Nonetheless, the proteins that make up the organic matrix of mollusc shells can become encased within mineral crystals and preserved, hence offering a material suitable to reconstructing the changes in the isotopic baseline (Crenshaw Reference Crenshaw1980; Bailey et al. Reference Bailey, Barrett, Craig and Milner2008; Casey and Post Reference Casey and Post2011). Accordingly, we also analyzed the stable isotope ratios in the shells of modern and ancient limpets and mussels to reconstruct, and compensate for, changes through time in the stable isotope baseline.
Material and Methods
Study Site and Sample Collection
We measured the stable isotope ratios of carbon and nitrogen of both modern and archaeological bone collagen samples of South American sea lions from two areas in Argentina (Fig. 1), northern-central Patagonia (from 39°S to 46°S) and southern Patagonia (from 46°S to 55°S). Modern samples of turbinate bones from South American sea lions were collected from specimens at the scientific collections at Centro Nacional Patagónico (Puerto Madryn, Argentina) and Museo Acatushún (Ushuaia, Argentina); the corresponding stable isotope ratios of carbon and nitrogen had been published previously elsewhere (Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009). Zooarchaeological bone samples from different skeletal elements were recovered from different layers of shell middens in northern-central Patagonia and southern Patagonia by researchers from Centro Nacional Patagónico, Centro Austral de Investigaciones Científicas, and Instituto Multidisciplinario de Historia y Ciencias Humanas (Table 1).

Figure 1 Location of archaeological sites from which sea lions and shells were sampled for stable isotope analysis. Sample sizes are listed in parentheses. The filled circles show archaeological sites for sea lions and the triangles denote sites for shells: 1=Los Abanicos 1; 2=Las Ollas Conchero 1; 3=Ecocentro Fogón 3; 4=Playa Las Lisas 2; 5=Cracker 6; Túnel VII; 7=Shamakush X; 8=Imiwaia I.
Table 1 Ratios of stable isotopes of carbon and nitrogen in the bone tissue of South American sea lions from the archaeological sites of northern-central Patagonia (Río Negro and Chubut) and southern Patagonia (Santa Cruz and Tierra del Fuego).

* Paleontological site
** Unspecified
1The regional marine reservoir effect of 266±51 years was included in the calibration of the samples (Favier Dubois Reference Favier Dubois2009).
2The regional marine reservoir effect of 516±85 years was included in the calibration of the samples (Cordero et al. Reference Cordero, Panarello, Lanzelotti and Favier Dubois2003).
The samples were dated in different laboratories and using different methods, in particular samples from northern-central Patagonia, where all dated samples were marine shells instead of charcoal. We calibrated radiocarbon ages using the package Clam 2.2 (Blaauw Reference Blaauw2010) and the new curve for Southern Hemisphere ShCal13 (Hogg et al. Reference Hogg, Hua, Blackwell, Niu, Buck, Guilderson, Heaton, Palmer, Reimer, Reimer, Turney and Zimmerman2013). Reservoir effects data for the northern Patagonia region have emerged only recently, and they suggest variable differences between marine and terrestrial ages (Cordero et al. Reference Cordero, Panarello, Lanzelotti and Favier Dubois2003; Favier Dubois Reference Favier Dubois2009).
From December 2009 to February 2010 we collected the shells of modern molluscs from the two study regions (Supplementary Table). We have also analyzed zooarchaeological shell samples recovered from different layers of shell middens in northern-central Patagonia and the Beagle Channel, Tierra del Fuego (Fig. 1). Clementz and Koch (Reference Clementz and Koch2001) pointed out that five samples are enough to provide robust estimates of mean and standard deviation for stable isotope ratios in tissues that integrate dietary information over long periods of time, and hence sample size was set at five for each species, locality, and zooarchaeological stratum where available (Supplementary Table). The limpet Nacella magellanica was sampled everywhere, but the rubbed mussel (Aulacomya atra atra) was sampled in northern-central Patagonia and the blue mussel (Mytilus edulis) in southern Patagonia, according to availability in regional shell middens.
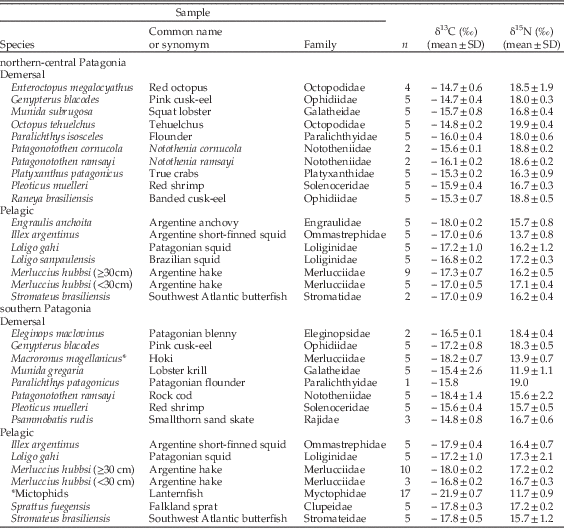
Bones of some fish species are abundant in the zooarchaeological record of both northern-central and southern Patagonia (Favier Dubois et al. Reference Favier Dubois, Borella and Tykot2009: Favier Dubois and Kokot Reference Favier Dubois and Kokot2011; Favier Dubois and Scartascini 2011; Tivoli and Zangrando Reference Tivoli and Zangrando2011), but the remains of cephalopods, shrimp and squat lobsters are missing. These taxa are important prey for modern South American sea lions (Thompson et al. Reference Thompson, Duck, McConnell and Garrett1998; Koen Alonso et al. Reference Koen Alonso, Crespo, Pedraza, Garcia and Coscarella2000; Suárez et al. Reference Suárez, Sanfelice, Cassini and Cappozzo2005; Romero et al. Reference Romero, Dans, Gonzalez, Svendsen, Garcia and Crespo2011) and hence necessary for comparisons between the stable isotope ratios of ancient South American sea lions and those of potential prey from the same period and region. For this reason, we analyzed muscle samples from the prey species currently consumed by South American sea lions (Table 2) and inferred the likely stable isotope ratios expected for ancient prey after correcting for the changes in the isotopic baseline revealed by the analysis of mollusc shells. Furthermore, we computed a diet-to-bone discrimination factor by combining published information about diet-to-vibrissa fractionation in marine carnivores (Hobson et al. Reference Hobson, Schell, Renouf and Noseworthy1996; Newsome et al. Reference Newsome, Bentall, Tinker, Oftedal, Ralls, Estes and Fogel2010) and the stable isotope ratios of paired samples of vibrissa and bone from eight adult South American sea lions dead-stranded in northern Patagonia between Reference Dato, Bambill, Cañete, Villarino and Aubone2006 and 2011 (see below for details about the calculations). This discrimination factor is necessary for comparing the stable isotope rations in the tissue of the predator with those in the tissue of its prey.
Table 2 Ratios of stable isotopes of carbon and nitrogen (mean±standard deviation) in the muscle of modern potential prey of the South American sea lion off northern-central Patagonia and southern Patagonia.
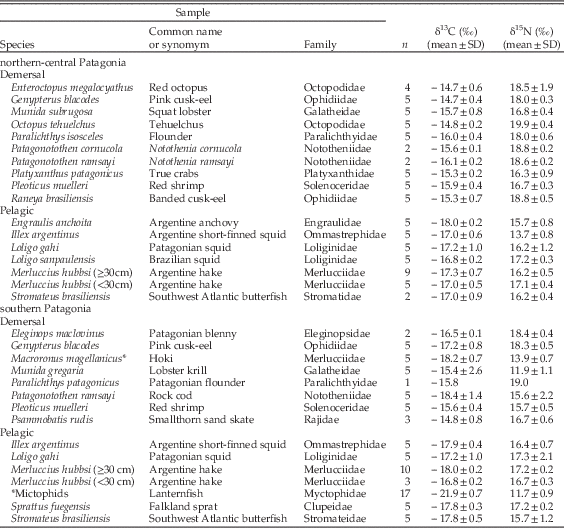
* Reference: Ciancio et al. Reference Ciancio, Pascual, Botto, Frere and Iribarne2008
Bone and shell samples were stored dry at room temperature. Samples from potential prey were stored at −20°C prior to analysis.
Stable Isotope Analysis
Bones were cleaned of sediment and dried in a stove at 50°C. Shell samples were polished with sandpaper and with a diamond wheel drill to remove impurities and subsequently rinsed with distilled water and dried in a stove at 50°C. White muscle from fish and mantle from squids were thawed and dried in a stove at 50°C. Once dry, all samples were ground to a fine powder with a mortar and pestle. Because shells and bone contain high concentrations of inorganic carbon, which may bias δ13C values (Lorrain Reference Lorrain2003), they were divided in two aliquots. One of them was decarbonized by soaking during in 0.5 N (bone) or 1 N (shell) hydrochloric acid (HCl) until no more CO2 was released (Newsome et al. Reference Newsome, Koch, Etnier and Aurioles-Gamboa2006). The HCl treatment adversely affects δ15N values (Bunn et al. Reference Bunn, Loneragan and Kempster1995), so the other aliquot was not treated with HCl and was used for δ15N determination. Lipids were extracted from bone samples with a chloroform/ methanol (2:1) solution (Bligh and Dyer Reference Bligh and Dyer1959).
The vibrissae were washed in methanol in an ultrasonic bath for 20 min in order to remove residual deposits or any lipid contamination from the vibrissae’s surface as a result of handling, and then were dried again for 48 hr at 50°C. Vibrissae were cut into 3-mm-long consecutive sections starting from the proximal end. This is because each section integrates diet during one month (Hirons et al. Reference Hirons, Schell and St. Aubin2001)
Approximately 0.8 mg of bone, 0.3 mg of vibrissae, 0.4–9.9 mg of shell, and 0.3 mg of white muscle from fish and mantle from cephalopods were weighed into tin cups (3.3×5 mm), combusted at 900°C, and analyzed in a continuous-flow isotope ratio mass spectrometer (Flash 1112 IRMS Delta C Series EA; Thermo Finnigan, Bremen, Germany). Atropine was used as a system check for elemental analyses. Samples were processed at Centres Cientifics i Tecnològics de la Universitat de Barcelona. The samples from modern South American sea lions had already been analyzed in the same laboratory and the results had been reported by Drago et al. (Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009).
Stable isotopes abundances, expressed in delta (δ) notation, in which the relative variations of stable isotope ratios are expressed in parts permil (‰) deviations from predefined international standards, were calculated as
where X is 13C or 15N, and R sample and R standard are the 13C/12C and 15N/14N ratios in the sample and standard, respectively. The standards used were Vienna Pee Dee Belemnite (VPDB) calcium carbonate for 13C and atmospheric nitrogen (air) for 15N.
Stable Isotope Discrimination Factors
Animals are related isotopically to their environment by means of an isotopic diet-tissue discrimination factor (Hobson Reference Hobson1999). These factors vary significantly, within and between species, with diet, physiology, and tissue (Gannes et al. Reference Gannes, Obrien and Martínez del Rio1997; Olive et al. Reference Olive, Pinnegar, Polunin, Richards and Welch2003; Koch Reference Koch2007). Discrimination factors from diet to enamel and bone have been assessed in ungulates (Passey and Cerling, Reference Passey and Cerling2002; Nardoto et al. Reference Nardoto, Godoy, Ferraz, Ometto and Martinelli2006), but they are unlikely to be useful because enamel and bone may differ in fractionation factors (Riofrío-Lazo and Aurioles-Gamboa Reference Riofrío-Lazo and Aurioles Gamboa2013) and nutrient routing is different between omnivores and carnivores (Martínez del Rio et al. Reference Martínez del Rio, Wolf, Carleton and Gannes2009). For this reason, we have computed a diet-to-bone fractionation factor using published information about diet-to-vibrissa fractionation in marine carnivores (Hobson et al. Reference Hobson, Schell, Renouf and Noseworthy1996; Newsome et al. Reference Newsome, Bentall, Tinker, Oftedal, Ralls, Estes and Fogel2010) and comparing the stable isotope ratios of vibrissa and bone of South American sea lions (eq. 2), as bone is expected to integrate diet over several years (Newsome et al. Reference Newsome, Koch, Etnier and Aurioles-Gamboa2006) and the same is true for long otariid vibrissa, with each few millimeters corresponding to several weeks (Cherel et al. Reference Cherel, Kernaleguen, Richard and Guinet2009):
Data Analysis
The δ13Cshell and δ15Nshell values of limpets and mussels allowed tracking changes in the stable isotope baseline through time. The δ13Cshell and δ15Nshell values of modern and ancient individuals of each species from the same region were compared using the nonparametric Kruskal-Wallis test for multiple comparisons, because the assumptions of normality (using Lilleford test) and homoscedasticity (using Leven test) were seldom met (Zar Reference Zar1984).
Stable isotope ratios in archaeological and modern bone samples were compared only after correcting for changes in the isotopic baseline (Casey and Post Reference Casey and Post2011). When statistically significant differences were found between modern and ancient stable isotope ratios, a correction factor was computed as the difference between the average stable isotope ratio of modern and ancient shells from each locality and age. Secondly, the difference was added to the stable isotope ratio of ancient bones from the same locality and age, to allow comparison with modern samples. For instance, if the δ15N value of modern shells was 2‰ above that of ancient ones, the δ15N value of ancient bones had to be increased 2‰ to be compared with that of modern bones. When bones came from a stratum without associated mollusc shells, bone stable isotope ratios were corrected using the time-weighted average of the correction factors computed for nearest strata below and above. Ideally, a bottom grazer (limpet) and a suspension feeder (ribbed mussel and blue mussel) were combined from each locality, but this was not always possible. A detailed description of those calculations and the resulting correction factors are shown in Table 3.
Table 3 Baseline correction factor for shells and sea lions to each radiocarbon year (ybp) where we obtained samples. Underlined numbers are the correction factors utilized for calculating the weighted values.

1,2 weighted value, calculated by: 1(0.90 *−3.4 + 0.10 *−3.6); 2 (0.9 *1.5 + 0.10 *3.1)
Once we had corrected for isotope baseline shifts, we compared stable isotope ratios in bone samples with those of modern potential prey, after applying the diet-to-bone discrimination factors for South American sea lions (Δδ13C=3.5±0.8‰; Δδ15N=4.4±0.8‰) obtained in this study. Mann-Whitney U-tests were used for testing differences in the δ13C and δ15N signatures between demersal and pelagic modern prey.
Data are presented as mean±standard deviation (SD) and significance was assumed at the 0.05 level. All statistical analyses were carried out with PASW Statistics (Version 17.0 for Windows, SPSS).
Results
Although the δ13Cshell values of the mussels and limpets from northern-central Patagonia did not vary throughout the late Holocene (Fig. 2; Kruskal-Wallis test; A. atra atra: χ2=9.418, df=4, p=0.052; N. magellanica: χ2=5.352, df=4, p=0.253), those of the mollusc species collected in southern Patagonia exhibited a remarkable variability and changes run in parallel in both species (Fig. 2; Kruskal-Wallis test; M. edulis: χ2=15.714, df=4, p=0.003; N. magellanica: χ2=11.765, df=4, p=0.008). Likewise, differences through time in the δ15Nshell values of mussels and limpets were statistically significant both in northern-central Patagonia (Kruskal-Wallis test; A. atra atra: χ2=19.549, df=4, p<0.001; N. magellanica: χ2=17.000, df=4, p=0.002) and in southern Patagonia (Kruskal-Wallis test; M. edulis: χ2=15.684, df=4, p=0.001; N. magellanica: χ2=14.392, df=4, p=0.002), and the two species from the same area exhibited the same pattern of temporal variation (Fig. 2) although each region evolved independently. These results revealed major changes in the stable isotope baseline, and hence correction factors were computed for each region and period to allow comparison of the stable isotope ratios in the bone of ancient and modern South American sea lions (Table 3).

Figure 2 Temporal trends throughout the late Holocene of the δ13Cshell and δ15Nshell values is molluscs from northern-central Patagonia and southern Patagonia. Differences through time were statistically significant; except for the δ13C shell values of Aulacomya atra atra and Nacella magellanica from northern Patagonia (see the text for details about the statistical analyses).
Vibrissae were depleted both in 13C and 15N relative to bone (mean δ13C: vibrissae=−13.1±0.8; bone=−12.3±0.8; mean δ15N: vibrissae=21.2±0.9; bone=22.5±1.5; p=0.01), which resulted in an average vibrissa-to-bone discrimination factor of 0.8±0.8‰ for δ13C and 1.2±1.0‰ for δ15N. Combining this with the diet-to-vibrissa discrimination factor (δ13C=2.7±0.7‰; δ15N=3.2±0.5‰) reported by Hobson et al. (Reference Hobson, Schell, Renouf and Noseworthy1996) and Newsome et al. (Reference Newsome, Bentall, Tinker, Oftedal, Ralls, Estes and Fogel2010) resulted in a diet-to-bone discrimination factor of 3.5±0.8‰ for δ13C and 4.4± 0.8‰ for δ15N. Bone (δ13C=−11.9±0.4; δ15N=22.2±0.8) was enriched relative to dentine (δ13C =−12.0±0.5; δ15N=21.4±0.6), so the diet-to-dentine discrimination was of 3.5±0.8‰ for δ13C and 3.6±0.8‰ for δ15N.
The uncorrected δ13Cbone and δ15Nbone values of ancient and modern sea lions are shown in the Table 1. After correction for changes in the isotope baseline (Fig. 3), the δ13Cbone values of modern South American sea lions from northern-central Patagonia were higher than those of the South American sea lions that inhabited the region during the second half of the Holocene (δ13C Kruskal-Wallis test: χ2=7.094; df=1; p<0.05) and the same was true for the δ15N values (Kruskal- Wallis test: χ2=43.628; df=1; p<0.05). The δ13Cbone values of South American sea lions from southern Patagonia also varied through time (δ13C Kruskal-Wallis test: χ2=5.262; df=1; p<0.05), but there were no consistent differences between modern and ancient South American sea lions. However, the δ15Nbone values of modern South American sea lions from southern Patagonia were higher than those of ancient South American sea lions from that area (δ15N Kruskal-Wallis test: χ2=44.480; df=1; p<0.05).

Figure 3 Mean (±SD) values of δ13Cbone (A, C) and δ15Nbone (B, D) for South American sea lions collected in northern-central Patagonia and southern Patagonia through the middle and late Holocene of Argentina, after being corrected for changes in stable isotope baseline.
The stable isotope ratios of modern potential prey from northern-central Patagonia and southern Patagonia are shown in Table 2. Demersal fishes from northern-central Patagonia were more enriched than pelagic fishes from the same region in both δ13C and δ15N (δ13C; Mann-Whitney U=0.000, Z=−3.428, df=17, p<0.000; δ15N; Mann-Whitney U=6.000, Z=−2.841, df=17, p<0.003), and, accordingly, the simultaneous increase in the δ13Cbone and δ15Nbone values of the South American sea lions from that region throughout the Holocene indicates an increase in the consumption of demersal prey (Fig. 4). Conversely, most pelagic and demersal prey from southern Patagonia exhibit similar δ13C and δ15N values (δ13C; Mann-Whitney U=7.500, Z=−1.934, df=13, p>0.051; δ15N; Mann-Whitney U=21.000, Z=0.000, df=13, p=1.000), although myctophid fishes and the squat lobster Munida gregaria were more depleted in 15N than any other species. Accordingly, the low δ15Nbone values typical of ancient South American sea lions suggest a diet dominated by prey at a low trophic level like M. gregaria (Fig. 4), whereas modern South American sea lions consume a larger proportion of prey at a higher trophic level like the rock cod Patagonotothen ramsayi, the Argentine hake Merluccius hubbsi, and the Argentine shrimp Pleoticus muelleri. Interestingly, the two samples from the nineteenth century fell outside the mixing polygon, suggesting they had a totally different diet or came from an area with a different isotopic baseline.

Figure 4 Bi-plot of the isotopic signal of the South American sea lion from northern-central (A) and southern (B) Patagonia, after correcting for differences in isotope baseline and for the diet-to-bone fractionation. Circles denote sea lions from different periods and triangles denote main prey.
Discussion
The overall evidence reported here indicates that South American sea lions currently forage at a higher trophic level than they did during the late Holocene. Furthermore, those inhabiting northern-central Patagonia forage more benthically than they used to do in the past. Ignorance about the actual age and sex of the ancient South American sea lions recovered from the shell middens, the analysis of different skeletal elements, and the inference made about the stable isotope ratios of ancient prey species might bias the results (Balasse et al. Reference Balasse, Bocherens and Mariotti1999), but the difference between ancient and modern stable isotope ratios is so large that current South American sea lions certainly occupy a totally different trophic level, both in northern-central and southern Patagonia.
The data also reveal the twentieth century as the period when most of the change in the trophic level of South American sea lions happened, following the massive removal of individuals by hunting along the coast of Argentina (Dans et al. Reference Dans, Crespo, Pedraza and Koen-Alonso2004; Schiavini et al. Reference Schiavini, Crespo and Szapkievich2004; Grandi et al. Reference Grandi, Oliveira, Dans and Crespo2012). Major changes also occurred in southern Patagonia during the early nineteenth century, as all the samples fell outside the mixing polygon formed by the stable isotope ratios of modern prey, even after correction for a shift in the isotope baseline. Western sealing had actually decimated otariid populations in the southern Patagonia during the late eighteenth and the early nineteenth centuries and only isolated otariids were sporadically recorded in the region during the second half of the nineteenth century (Bridges Reference Bridges1949). Rookeries persisted on Staten Island (Argentina), on the Falkland Islands (Malvinas), and in northern Argentina (Bridges Reference Bridges1949; Rodriguez and Bastida Reference Rodriguez and Bastida1998; Dans et al. Reference Dans, Crespo, Pedraza and Koen-Alonso2004; Dickinson Reference Dickinson2007), and the high δ13C values of the sea lion samples recovered at the Beagle Channel from the early nineteenth century are best explained by dispersal from distant areas with a distinct isotope baseline rather than by a dietary shift of the local population.
The dramatic impact of western sealing on the diet and ecology of South American sea lions is in sharp contrast with the rather stable diet of South American sea lions during the period of aboriginal exploitation. Evidence is particularly compelling in southern Patagonia, where the zooarchaeological record spans several millennia and the stable isotope ratios in the bones of South American sea lions were rather stable. Variability in stable isotope ratios was larger in northern-central Patagonia, but considering the scarcity of samples older than 2000 14C ybp and the variability associated with the sampling of different skeletal elements, dietary shifts during the aboriginal period are uncertain.
Nevertheless, it should be noted that the diet of South American sea lions inhabiting truly pristine environments remains unknown. Most of the samples analyzed here came from archaeological sites and the oldest skeletal remains of South American sea lions from southern Patagonia are 1000 years younger than the oldest evidence of human exploitation of the marine resources in the area (Orquera and Piana Reference Orquera and Piana1988, Reference Orquera and Piana1999; Orquera et al. Reference Orquera, Legoupil and Piana2011; Tivoli and Zangrando Reference Tivoli and Zangrando2011). Likewise, the oldest skeletal remains of South American sea lions from northern-central Patagonia are 3000 years younger than the oldest archaeological evidence of the exploitation of fishes, marine birds, and crustaceans in the area (Favier Dubois et al. Reference Favier Dubois, Borella and Tykot2009; Favier Dubois and Kokot Reference Favier Dubois and Kokot2011; Favier Dubois and Scartascini Reference Favier Dubois and Scartascini2012; Gómez Otero et al. Reference Gómez Otero, Weiler, Banegas and Moreno2013). Whether such previous exploitation had modified the trophic niche of South American sea lions remains unknown and can be answered only if paleontological sites predating the arrival of humans were discovered.
In any case, the overall evidence indicates that South American sea lions currently forage at a higher trophic level than they did originally and that most of the change was related to human exploitation during the twentieth century. On the contrary, there is no evidence that aboriginal exploitation had a major effect on the trophic ecology of South American sea lions. Accordingly, the current ecology of South American sea lions is a poor guide to understanding the evolutionary forces that operated on the species throughout most of its history. For instance, there is no justification for claims that sexual differences in body mass evolved to reduce trophic overlap, as differences in the diets of male and females vanish as population size approaches carrying capacity (Drago et al. Reference Drago, Crespo, Aguilar, Cardona, García, Dans and Goodall2009, Reference Drago, Cardona, Crespo, Grandi and Aguilar2010). Likewise, the pelagic diet of South American sea lions prior to exploitation by western sealers (this study) suggests that the current resource partitioning between demersal South American sea lions and epipelagic South American fur seals (Franco-Trecu et al. Reference Franco-Trecu, Aurioles-Gamboa, Arim and Lima2012) is an artifact resulting from human exploitation, and the same might be true for other sympatric pairs where sea lion and fur seal species differentiate along a demersal-pelagic gradient (e.g., Antonelis et al. Reference Antonelis, Stewart and Perryman1990; Páez-Rosas et al. Reference Páez-Rosas, Aurioles-Gamboa, Alava and Palacios2012).
The results reported here have also implications for conservation. Preventing extinction due to human activity is the first step in wildlife conservation, but restoring the role of species in ecosystem dynamics has to be the long-term goal (Jackson and Hobbs Reference Jackson and Hobbs2009; Terborgh and Estes Reference Terborgh and Estes2010; Bullock et al. Reference Bullock, Aronson, Newton, Pywell and Rey-Benayas2011). Legal protection has certainly allowed the partial recovery of the population of South American sea lions in Argentina (Dans et al. Reference Dans, Crespo, Pedraza and Koen-Alonso2004; Schiavini et al. Reference Schiavini, Crespo and Szapkievich2004; Grandi et al. Reference Grandi, Oliveira, Dans and Crespo2012), but has not restored the ecological role of the species (this study). Fishing is currently the major ecological driver of coastal ecosystems in the southwestern Atlantic (Koen-Alonso and Yodzis, Reference Koen-Alonso and Yodzis2005) and has established a new carrying capacity for South American sea lions (Drago et al. Reference Drago, Cardona, Crespo, Grandi and Aguilar2010). Information about the deep-time ecology of South American sea lions may assist managers in assessing whether the original niche has been restored, independently of population size. Restoring the original trophic ecology of the species is important, because only in this way will the evolutionary forces than once operated on the species also be restored.
Acknowledgments
This research was funded by Fundación BBVA through the project “Efectos de la explotación humana sobre depredadores apicales y la estructura de la red trófica del Mar Argentino durante los últimos 6.000 años” (BIOCON 08 - 194/09 2009-2011) and Agencia Nacional de Promoción Científica y Tecnológica (PICT N° 2110), and the Mohamed bin Sayed Conservation Fund (0925516). L.Z. was supported by a Fellowship Comisión Nacional de Investigación Científica y Tecnológica (CONICYT-Chile) and F.S. was supported by a Fellowship from Ministerio de Ciencia e Innovación (Spain). Sample collection was carried out under permits of the Provinces of Rio Negro, Chubut, Santa Cruz, and Tierra del Fuego.
Supplementary material
Supplemental materials deposited at Dryad: doi:10.1017/pab.2015.9