Introduction
Necrotising otitis externa is a progressive infection of the external auditory canal which extends to affect the temporal bone and adjacent structures. This invasive infection originates between the cartilaginous and bony portions of the external auditory canal. The most common causative organism is Pseudomonas aeruginosa. The infection begins as an otitis externa that progresses into osteomyelitis of the temporal bone. Once infection and subsequent granulation occurs over the osseous canal, the VIIth cranial nerve can become compromised, resulting in a facial nerve palsy. If infection progresses to the base of the skull, IXth, Xth, XIth and XIIth cranial nerves can be affected, resulting in dysphagia, hoarseness, weakness of the shoulder and difficulty in swallowing. Therefore, if inadequately treated, this disease is associated with significant morbidity and mortality. If at-risk patients are identified early and treated adequately then improved outcomes can be achieved.
There is currently no formal published guideline on the treatment of necrotising otitis externa. This is likely to be a result of the heterogeneous nature of its presentation and progression, as well as contention amongst health professionals regarding its diagnosis. The authors’ aim was to incorporate salient points from the evidence base, as well as our practice, in the diagnosis and management of necrotising otitis externa. We have also advocated a multi-specialty approach, including input from ENT surgeons, infectious disease specialists, microbiologists, diabetes specialists (when applicable) and neuroradiologists, with the intention of producing a guideline that is consulted by all the specialties involved in treating this condition.
Necrotising otitis externa is certainly an increasing health burden, with incidence throughout the UK rising. Data from NHS National Services Scotland confirm an increase in cases from 0.2 cases per 100 000 in 2008 to 1.19 cases per 100 000 in 2018 (Information Services Division (‘ISD’) Scotland, direct communication). This is based on patients who are discharged from hospital with a diagnosis of necrotising otitis externa, and may underestimate the true incidence.
Most departments advocate prolonged courses of intravenous antibiotics, which may necessitate a lengthy hospital stay. There is a growing body of evidence outlining the demographic features of patients suffering from necrotising otitis externa, which includes a preponderance towards elderly, diabetic males.Reference Sylvester, Sanghvi, Patel, Eloy and Ying1 With an ageing population, it is therefore not surprising that the incidence is on the rise. A combination of increased age and diabetes predispose patients to microvascular abnormalities around the ear canal. Furthermore, pseudomonal infection in this environment can result in vasculitis with thrombosis, and coagulation necrosis of surrounding tissue.Reference Nadol2 The cerumen of diabetic patients is speculated to have a higher pH, making it more susceptible to bacterial growth.
Materials and methods
A retrospective review of necrotising otitis externa cases within NHS Lothian was undertaken, from April 2013 to January 2018. Clinical coding data based on the International Classification of Diseases (‘ICD’) code at discharge or death were used to extract cases for review. Here, we focused on presenting signs and symptoms, patient demographics, type and timing of investigations, organism identified, management instigated, and patient outcome in order to identify trends.
We also undertook a review of the current literature, using a PubMed search, to substantiate findings from our own data and generate an evidence-based guideline. The search terms used were ‘malignant otitis externa’, ‘necrotising otitis externa’ and ‘skull base osteomyelitis’.
Following implementation of our guideline, data were then collected prospectively on all patients presenting with clinical signs of necrotising otitis externa who subsequently underwent investigation, allowing assessment of its impact on the management and outcomes of this patient cohort.
Results
Twenty patients were identified in our search. On review of presenting signs and symptoms, 100 per cent of patients with radiologically proven necrotising otitis externa (20 out of 20) suffered from otalgia and 95 per cent (19 out of 20) suffered purulent otorrhoea. Eighty-five per cent (17 out of 20) demonstrated polypoidal granulation tissue within the external auditory canal. Twenty-five per cent (5 out of 20) suffered from cranial nerve palsies, most commonly the facial nerve, with two-thirds of these established on presentation (Table 1).
Table 1. Signs and symptoms*

Total n = 20.
* In patients with radiologically proven necrotising otitis externa from our retrospective case series.
Analysis of patient demographics established that 65 per cent of patients (13 out of 20) were diabetic. Of the non-diabetic group (n = 7), 71 per cent (5 out of 7) had undergone previous ear surgery, and 14 per cent (1 out of 7) suffered from systemic immunosuppression (Table 2). Eighty-five per cent (17 out of 20) were over the age of 65 years.
Table 2. Demographic data*

Total n = 20.
* For patients with radiologically proven necrotising otitis externa from our retrospective case series.
Regarding investigation findings, P aeruginosa was cultured in 60 per cent (12 out of 20) and Staphylococcus aureus was cultured in 25 per cent (5 out of 20). In 83 per cent of cases where P aeruginosa was cultured, the organism was susceptible to ciprofloxacin. Aspergillus flavus was isolated from one patient (5 per cent) (Table 3); however, this was not initially targeted in treatment. C-reactive protein (CRP) was reviewed, and this ranged from 9 to 137 mg/l at diagnosis, with no demonstrable link to patient outcome. Computed tomography (CT) scanning was used for diagnosis in 100 per cent of cases and magnetic resonance imaging (MRI) was used as an adjunct in 35 per cent (7 out of 20).
Table 3. Organisms cultured*

Total n = 20.
* In patients with radiologically proven necrotising otitis externa from our retrospective case series.
In treating the condition, systemic ciprofloxacin was the most widely used antibiotic, prescribed in 60 per cent of cases (12 out of 20). Fifteen per cent of patients (3 out of 20) alternatively received meropenem, 5 per cent (1 out of 20) received piperacillin/tazobactam, and 10 per cent (2 out of 20) received piperacillin/tazobactam followed by meropenem.
Twenty-five per cent of patients (5 out of 20) in our retrospective audit demonstrated a deterioration in their condition under treatment from our service, which is a relatively high proportion. On review, the reasons for the observed deterioration included two delayed diagnoses and under-treatment from the ENT department, one inadequate course length and one possible missed fungal infection; three patients deteriorated on a course of oral ciprofloxacin without having received intravenous antibiotics. Only 1 case out of 20 relapsed following the completion of treatment during the study period.
Discussion
The first step in our guideline (Figure 1) is identifying patients for whom a diagnosis of necrotising otitis externa is suspected; although this may result in increased sensitivity of pick up, it ensures that cases are not missed. Lambor et al. stated that any diabetic patient presenting with otalgia and otorrhoea should be deemed to have necrotising otitis externa until proven otherwise.Reference Lambor, Das, Goel, Tiwari, Lambor and Fegade3 This concept has been adapted to define our patients with suspected necrotising otitis externa, along with findings from our retrospective case series and large-scale patient demographic studies in the literature.Reference Sylvester, Sanghvi, Patel, Eloy and Ying1,Reference Guerrero-Espejo, Valenciano-Moreno, Ramírez-Llorens and Pérez-Monteagudo4 This is the evidence used to constitute our diagnostic criteria. The diagnosis should also be considered in patients with a cranial nerve palsy, most commonly the VIIth cranial nerve, and signs of ear infection; however, this will often be a late presenting sign.Reference Soundry, Joshua, Sulkes and Nageris5 We also felt it prudent to include a failure to respond to one week of conservative otitis externa treatment as a ‘red flag’ sign to consider necrotising otitis externa.
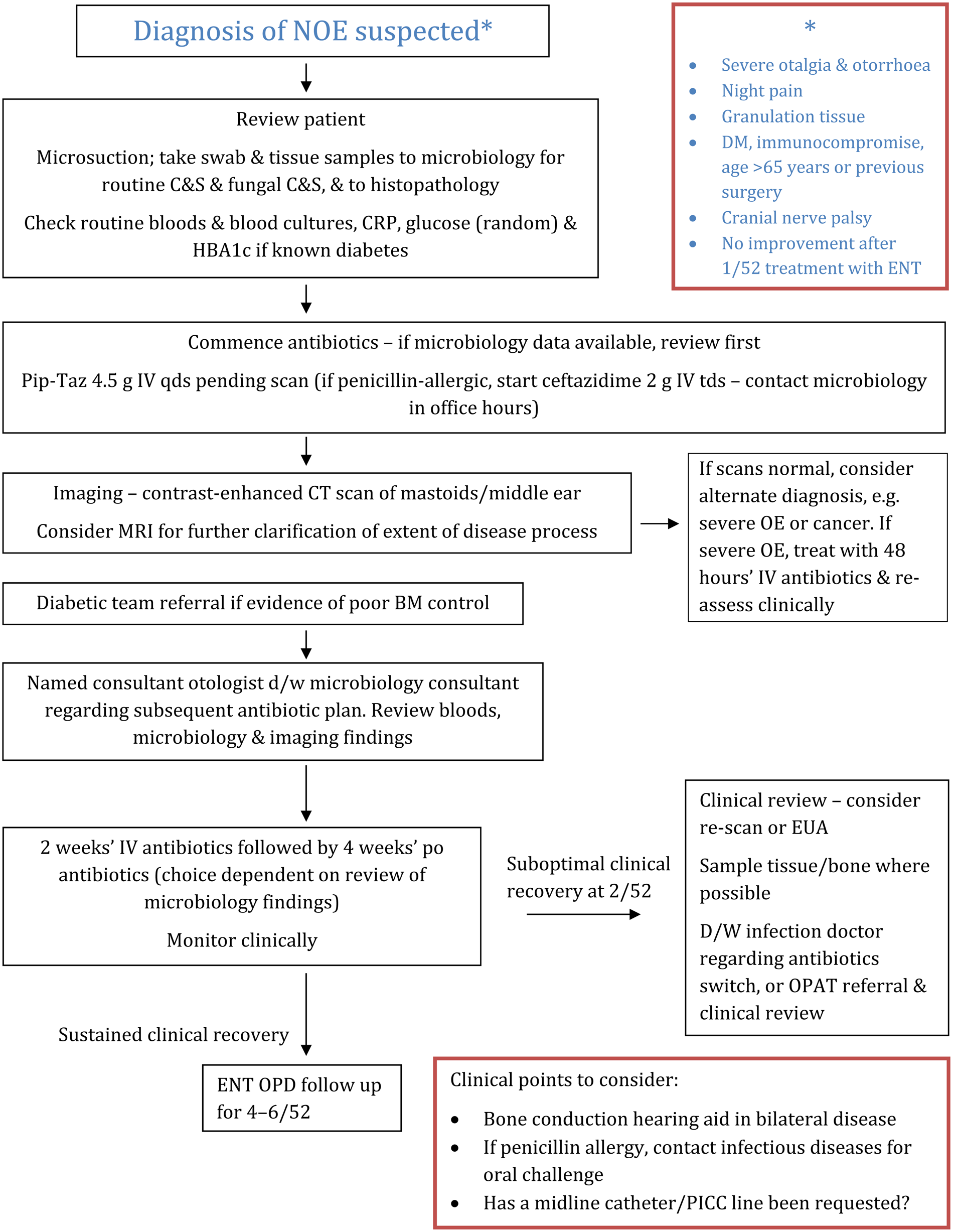
Fig. 1. NHS Lothian guideline for the diagnosis and management of necrotising otitis externa (NOE). DM = diabetes mellitus; C&S = culture and sensitivity testing; CRP = C-reactive protein; HBA1c = haemoglobin A1c; Pip-Taz = piperacillin/tazobactam; IV = intravenous; qds = four times a day; tds = three times a day; CT = computed tomography; MRI = magnetic resonance imaging; OE = otitis externa; BM = blood glucose measurement; d/w = discussion with; po = orally; EUA = examination under anaesthesia; OPAT = out-patient parenteral antibiotic therapy; OPD = out-patient department; PICC = peripherally inserted central catheter
On clinical review of patients with suspected necrotising otitis externa, microsuction should initially be undertaken for adequate assessment of the ear canal. At this stage, swab samples of discharge should be sent for culture and sensitivity testing – both routine and fungal – to determine the organisms involved and to guide antibiotic choice. Tissue samples of any granulation should be obtained, and sent for both culture and sensitivity testing and histopathology. This is important in the potential identification of organisms that may be missed from superficial swab cultures. Furthermore, alternative diagnoses such as squamous cell carcinoma of the ear canal can, rarely, present similarly to necrotising otitis externa and must be excluded.Reference Zainuddin and Abdullah6 There is evidence that CRP is correlated with disease status (although our local audit findings do not support this) and therefore should be checked on admission.Reference Hopkins, Harris and Cuddihy7
It is quoted that patients with good diabetic control do not progress to the more severe form of necrotising otitis externa with involvement of surrounding anatomical structures.Reference Lee, Lee, Seon, Jung, Lee and Choi8 Therefore, in patients with diabetes, particular attention should be placed on glycaemic control, with measurement of haemoglobin A1c and serial monitoring of blood glucose. Referral to the diabetic specialist team for review of diabetic control thus forms part of the management of necrotising otitis externa.
• Necrotising otitis externa presents with severe otalgia, otorrhoea and granulation tissue in the external auditory canal; cranial nerve palsies indicate advanced disease
• Risk factors include old age, poor diabetic control and immunocompromise
• There is variation in necrotising otitis externa management between centres, due to its heterogeneous nature and lack of robust evidence for treatment
• This paper presents the first published health board based guideline for necrotising otitis externa management
• The protocol integrates local data, an up-to-date evidence base and a multi-specialty approach
• Necrotising otitis externa represents a spectrum of disease; avoiding under-treatment may prevent disease progression and relapse
Regarding antimicrobial therapy, if there is a high level of clinical suspicion of necrotising otitis externa, empirical intravenous treatment should be commenced after microbiological sampling. Locally, piperacillin/tazobactam is the first-line therapy because of its efficacy against the most prevalent organisms in necrotising otitis externa, in particular P aeruginosa. Ceftazidime may be suitable for patients with a history of non-urticarial rash following penicillin use, although the risk of diarrhoea due to Clostridium difficile is greater. A true penicillin allergy requires elucidation through careful history taking; if necessary, patients can be referred for further investigations to our penicillin allergy clinic (the authors acknowledge this is not always available), or review on the ward by an infection specialist. All antibiotics should be reviewed following the analysis of culture and sensitivity findings.
From our retrospective data, a factor identified as having potential to improve patient outcomes was the presence of a named consultant otologist responsible for decision-making for individual patients. This enables a clear management plan to be followed and encourages accountability for a patient's care. Furthermore, such individuals are best placed to ensure the correct condition is being pursued. Under inexperienced care, the diagnosis can easily be confused with other conditions such as squamous cell carcinoma of the ear canal or external ear canal cholesteatoma.
The next area to consider is what happens when the patient has clinically suspected necrotising otitis externa but lacks radiological features of disease. As discussed, alternative diagnoses such as malignancy or cholesteatoma should be considered and ruled out. Otherwise, we would classify this under the ‘severe’ otitis externa category. These patients should be treated actively in the first instance, with reviews at 48-hour intervals to determine the response to treatment, as early necrotising otitis externa can be missed on imaging.Reference Sudhoff, Rajagopal, Mani, Moumoulidis, Axon and Moffat9 Experience has shown that if risk factors for necrotising otitis externa are present and the patient is under-treated, there is a potential for the condition to develop into osteomyelitis of the temporal bone.Reference Rubin Grandis, Branstetter and Yu10
Those with diagnosed necrotising otitis externa undergo a two-week induction course of intravenous antibiotics, in line with current practice of treating osteomyelitis at other sites, although this concept has recently been challenged.Reference Li, Rombach, Zambellas, Walker, McNally and Atkins11 A comprehensive re-assessment is required at this stage, as suboptimal clinical recovery should prompt consideration of further tissue sampling, further scanning, and discussion with the infectious disease team or microbiologist regarding the appropriateness of the chosen antibiotics. At this time, the surgeons may want to consider whether local debridement or incision and drainage might be a useful adjunct to treatment.Reference Carfrae12 Indeed, a body of evidence suggests that samples from this deep debridement may harvest fungal organisms that have not been identified on previous cultures.Reference Gruber, Sela, Doweck, Roitman, Uri and Srouji13,Reference Mion, Bovo, Marchese-Ragona and Martini14
Should a clinical improvement be observed, with no adverse clinical features present, and P aeruginosa susceptible to ciprofloxacin has been isolated, then an oral switch may be considered at this stage. We would advocate the prescription of a subsequent 4-week course of ciprofloxacin (750 mg) every 12 hours, unless renal impairment is present. Ciprofloxacin has excellent bone penetration; furthermore, this therapy allows the patient to be treated at home. Data from our audit investigating pseudomonal isolates support ciprofloxacin as a valid option in the majority of patients. The caveats are that this could change with ongoing antibiotic pressure and sub-therapeutic doses being administered topically. Patients also require counselling on the risk of tendon inflammation or rupture, neurological complications, risk of ruptured aneurysm,Reference Pasternak, Inghammar and Svanstrom15 and the increased risk of C difficile infection.16
Patients should be monitored clinically until recovery is ensured, with regular microsuction and examination of the external auditory canal. Subsequent to this, we advocate that patients attend follow up with their named otologist at four to six weeks to ensure clinical recovery is sustained. However, regarding relapse, local data appear to be reassuring, with only 1 out of 20 cases relapsing following completion of treatment.
Clearly, avoiding the progression of disease to severe necrotising otitis externa is essential. We classify severe necrotising otitis externa here as osteomyelitis of the petrous portion of the temporal bone, involving surrounding structures, and resulting in cranial nerve palsies, which, once present, are usually irreversible and life-altering. Indeed, any patient suffering from necrotising otitis externa with cranial nerve involvement would be subject to at least six weeks of intravenous antipseudomonal antibiotics. Furthermore, patients who have received two weeks of intravenous antibiotics without observation of sustained clinical recovery, or worse, an observation of disease progression, would also be considered for a further four weeks of intravenous therapy.
Current intravenous antipseudomonal antibiotics require frequent dosing, precluding the use of many out-patient parenteral antibiotic therapy services. Consequently, many patients are confined to hospital wards for this protracted period, which is detrimental to patient psychological health, as well as being a burden on a service where in-patient bed availability is increasingly under pressure. Our out-patient parenteral antibiotic therapy team has developed a service that facilitates the training of family or caregivers in administering intravenous antibiotics at home. This is offered in selected cases, whilst ensuring the patients receive regular review of their clinical status, particularly with regard to the appearances of the external auditory canal.
Necrotising otitis externa caused by invasive fungal infection, usually aspergillus species, is rare but must not be overlooked. These infections often develop from the mastoid or middle ear, and can be much more invasive. Consequently, identifying the pathogen early is essential. The ‘gold standard’ examination involves testing for the presence of hyphae in tissue samples. Recognition that a single isolate of mould or yeast from a swab must not be considered an indication for prolonged systemic antifungal treatment is essential. If invasive infection with aspergillus species is confirmed, then prolonged treatment (more than 12 weeks) with voriconazole is advised.Reference Mouas, Lutsar, Dupont, Fain, Herbrecht and Lescure17–Reference Patterson, Thompson, Denning, Fishman, Hadley and Herbrecht19 This should be overseen by the local consulting infection team, with levels assessment after 7 days or following any dose adjustment. Yeasts isolated from superficial swabs are not generally treated with antifungals; however, such a finding should prompt consideration of deep tissue sampling to determine any requirement for systemic antifungal therapy.
Evidence on topical antibiotics remains ambiguous and therefore our current guideline does not require specific topical treatment to be prescribed. Many ENT specialists advocate the use of topical treatment, and further work investigating the efficacy of this therapy is needed.
A recent systematic review was conducted by Phillips and Jones, which investigated the evidence for using hyperbaric oxygen therapy as an adjunct in necrotising otitis externa, and it was found to be equivocal.Reference Phillips and Jones20 Therefore, this has not been suggested as a therapeutic option in our guideline.
There is a paucity of evidence on surgical debridement, with much discrepancy between studies and with no common factors linking debridement to a positive outcome.Reference Carfrae12,Reference Singh and Bhardwaj21 We therefore concluded that this should be considered on a case by case basis by the patient's named otologist. It is reasonable to suggest that local soft tissue debridement or incision and drainage of abscesses would be a useful therapeutic adjunct, particularly in patients whose progress appears to have stalled.
Imaging modality remains a point of contention. The bulk of the evidence suggests that contrast-enhanced CT of the petrous portion of the temporal bone should be used as a gold standard for the detection of temporal bone osteomyelitis,Reference Hopkins, Harris and Cuddihy7,Reference Chawdhary, Pankhania, Douglas and Bottrill22 given its sensitivity, ease of access and cost-effectiveness. As a result, this is our first-line radiological investigation. However, there is evidence to suggest that CT may miss early stages of disease; therefore, we have reserved MRI for further clarification of disease extent in cases where clinical and radiological findings are incongruous.Reference Sudhoff, Rajagopal, Mani, Moumoulidis, Axon and Moffat9 From our own data, we have observed a case where the findings of both CT and MRI were negative, yet necrotic bone was excised on debridement. This episode precipitated a large-scale review of imaging modality and reporting. Good results are achieved with a dedicated ENT radiologist who has the opportunity to meet regularly with the ENT surgical department and who is given full clinical details (facilitated by radiology referrals being made by more senior members of the team).
Short-term outcomes
Following the development of our protocol, prospective data are being collected to observe both adherence to our guidelines and the monitoring of our patient outcomes, to facilitate its adaptation over time. This unprecedented approach in combining different medical and surgical specialties in the creation of a necrotising otitis externa guideline has helped accommodate the different areas of expertise, offering a more holistic view of the patients’ care. Furthermore, it enhances the working relationship between departments that should seek to optimise management. Our data show us that 100 per cent of cases on the ENT wards are managed with input from the infectious disease and microbiology teams. The cases are also discussed at weekly infection multidisciplinary team (MDT) meetings and neuroradiology MDT meetings, where outcomes are fed back to the patient's team. Following the patient's progress and outcomes, as well as regularly considering and re-considering our definition and the published best practice for this condition, is certainly a useful approach to enhance our understanding of the disease.
Since the implementation of our guideline, 14 cases subsequent and separate to the initial dataset, have been recorded through the department. With excellent adherence to our guidance, 2 out of 14 cranial nerve palsies have been observed (1 of which was already present from a previous episode), as well as 1 out of 14 deaths within three months of diagnosis (to an unrelated condition). Ten out of 14 patients had made a complete and sustained recovery at the four-week follow up following discharge, with the other patients dying, suffering persistent cranial nerve palsies or relapsing. Clearly these numbers are small and therefore it is difficult to draw any robust conclusions; however, it does appear that the early diagnosis and appropriate management of at-risk patients can result in positive outcomes.
Important messages drawn from the data included: all patients had a named consultant otologist in charge of their care, and patients with a haemoglobin A1c level of over 70 mmol/mol (indicating poor glycaemic control) on admission had prompt referrals to diabetic nurse specialist for optimisation of blood glucose control. Although all the patients presented with clinical necrotising otitis externa, only 8 out of 14 demonstrated bony erosion on imaging and thus met the radiological criteria for necrotising otitis externa. Nonetheless, all the patients received a minimum of six weeks of antibiotic therapy; one of these patients was switched onto oral ciprofloxacin after two weeks of intravenous treatment.
The question remains, are we now over-diagnosing and over-treating? Are some patients being subjected to longer courses of treatment who would otherwise have made a full recovery? This possibility exists; to refute it, more data are required to compare against our retrospective results and outcomes across other centres. Our rationale is that imaging may miss early osteitis or cartilaginous inflammation. This was demonstrated in one case of severe otitis externa with clinical features of necrotising otitis externa, which showed no radiological changes in keeping with necrotising otitis externa. Subsequent examination under anaesthesia and debridement revealed necrotic cartilage and bone under the squamous epithelium of the ear canal, thus confirming a diagnosis of necrotising otitis externa.
Conclusion and further work
Necrotising otitis externa is a condition that is increasing in incidence (Information Services Division Scotland, direct communication).Reference Chawdhary, Liow, Democratis and Whiteside23 Through local and global evidence, we are continuously enhancing our understanding of the patient demographics, disease course and management of this serious condition. Creation of this guideline has drawn upon a body of evidence with the intention of optimising our management of necrotising otitis externa, ensuring that we avoid inadequate treatment and thereby mitigate the risk of serious complications.
With contention in the literature regarding a true definition of necrotising otitis externa, as well as challenges in its diagnosis, the clinical condition should be reviewed in stages, and those at-risk should be treated. We recognise that there is a spectrum of disease, ranging from those patients who demonstrate risk factors or typical clinical signs on presentation, through to those with fulminant skull base osteomyelitis. We recommend that patients who meet the clinical criteria are treated sufficiently to avoid progression to a more severe form of the disease. Following identification of these patients in primary care, rapid access to ENT assessment clinics is important, in order to start the investigation and management pathway early.
As per our guideline, there is opportunity for treatment to be de-escalated during the course of management should there be a notable change in circumstances. For example, the scan demonstrates no evidence of bony involvement, and the patient's symptoms respond rapidly to intravenous therapy. It is accepted that we may be over-treating some patients; however, under-treatment resulting in serious complications must be avoided. A prospective review of our subsequent outcomes will be helpful in identifying these patients.
Going forward, further prospective data are being collected following the implementation of this guideline, with the aim of assessing patient outcomes. This will help to validate our guideline and allow further refinements, to optimise management of this challenging patient cohort.
The limitations of any clinical guidelines are the neglect of clinical experience and the heterogeneous manner in which conditions present and progress. Therefore, it has never been suggested that this flow chart should be strictly adhered to. It has been designed primarily to provide guidance and ensure that even experienced clinicians are not missing salient aspects of the patient's management.
Acknowledgement
The authors would like to thank Information Services Division Scotland for their help in providing up-to-date statistics on the incidence of necrotising otitis externa in Scotland.
Competing interests
None declared






