Introduction
The use of pyrethroid insecticides to control the bollworm, Helicoverpa armigera (Hübner), in cotton-producing areas has, more or less, rapidly led to widespread resistance: successively Australia (Gunning et al., Reference Gunning, Easton, Greenup and Edge1984; Forrester et al., Reference Forrester, Cahill, Bird and Layland1993), China (Shen et al., Reference Shen, Tan, Zhou, Jin and Tan1992), India (McCaffery et al., Reference McCaffery, King, Walker and El-Nayir1989; Armes et al., Reference Armes, Jadhav, Bond and King1992; Kranthi et al., Reference Kranthi, Jadhav, Wanjari, Kranthi and Russel2001), Thailand (Ahmad & McCaffery, Reference Ahmad and McCaffery1988), Turkey (Ernst & Dittrich, Reference Ernst and Dittrich1992) and Pakistan (Ahmad et al., Reference Ahmad, Arif and Ahmad1995, Reference Ahmad, Arif and Attique1997) have been concerned. In Africa, pyrethroid resistance was diagnosed in the 1996–97 season in the southern (Van Jaarsveld et al., Reference Van Jaarsveld, Basson and Marais1997) and western regions of the continent (Vassal et al., Reference Vassal, Vaissayre and Martin1997; Martin et al., Reference Martin, Ochou Ochou, Hala N'Klo, Vassal and Vaissayre2000) where populations developed metabolic resistance to pyrethroids via overproduction of cytochrome P450-dependent monoxygenases (or MFOs) (Martin et al., Reference Martin, Chandre, Ochou, Vaissayre and Fournier2002). In Cameroon, where pyrethroid insecticides had been widely used on cotton for approximately 20 years because of their efficacy in controlling a wide range of cotton pests at low cost, a monitoring network was set up in 1999 for the early detection of bollworm resistance to pyrethroids (Brévault et al., Reference Brévault, Asfom, Beyo, Nibouche and Vaissayre2002). Several control failures in farmers' fields in the 2004 cotton-growing season were undoubtedly due to pyrethroid resistance (Brévault & Achaleke, Reference Brévault and Achaleke2005).
Because of its high polyphagy and its capability of dispersing over great distances, H armigera has the potential to rapidly colonize diverse cropping systems and to develop insecticide resistance (Fitt, Reference Fitt1989). In the cotton growing area of Central Africa, including on northern Cameroon and Chad, H. armigera is a major pest of cotton and vegetable crops, particularly tomato, and affects production by destroying flowering and fruit-bearing organs. Due to the annual sequence of dry and rainy seasons, the agricultural landscape, thus, consists of a shifting mosaic of habitats. At the beginning of the rainy season (June to mid-July), a common wild plant that grows in degraded soils and along roadsides, Cleome viscosa L. (Capparidaceae), provides a nursery for the first generation of H. armigera (fig. 1). From mid-July to the end of October, the bollworm simultaneously colonizes rainfed crops, such as silking maize (1 generation) and cotton (up to three generations), as a function of the pattern of planting dates. Although millet, groundnut, cowpea and sorghum are known as host plants in several different cropping systems in the world, local varieties cultivated in Central Africa are not significantly infested. At the end of the rainy season (late October), populations can persist on wild plants, such as Hyptis suaveolens (L.) Poit. (Lamiaceae) (Gadpayle et al., Reference Gadpayle, Rajgure, Vennila, Bambawale, Deole, Karanjkar, Panchbhai and Biradar2004). During the dry season (late October through March), local populations can persist as diapausing individuals, by dispersing to local small irrigated sites under tomato and maize crops or by migrating over long range distances to find suitable habitats (Nibouche, Reference Nibouche1994). H. armigera, thus, exploits a sequence of crops that offer a substantial but time-limited resource (maize and cotton) and uncultivated or scarce hosts (Cleome, Hyptis and tomato) that enable the bollworm to bridge the critical period (Nibouche et al., Reference Nibouche, Guérard, Martin and Vaissayre2007). This succession of host plants is likely to affect temporal and spatial population dynamics (Kennedy & Storer, Reference Kennedy and Storer2000) and, consequently, the gene flow that determines the pattern of insecticide resistance. As a rainfed crop, cotton occupies a considerable part of the Central African agricultural landscape. Each growing season, the widespread application of cypermethrin in the cotton-growing area exerts continuous selection pressure. Similarly, the indiscriminate use of insecticides on commercial vegetable crops, such as tomatoes, which are mostly cultivated in small irrigated plots during the dry season, significantly contributes to the selection of resistant genes. On the other hand, large areas of untreated maize crops and wild hosts may provide relatively insecticide-free refuges (Roush, Reference Roush1989).
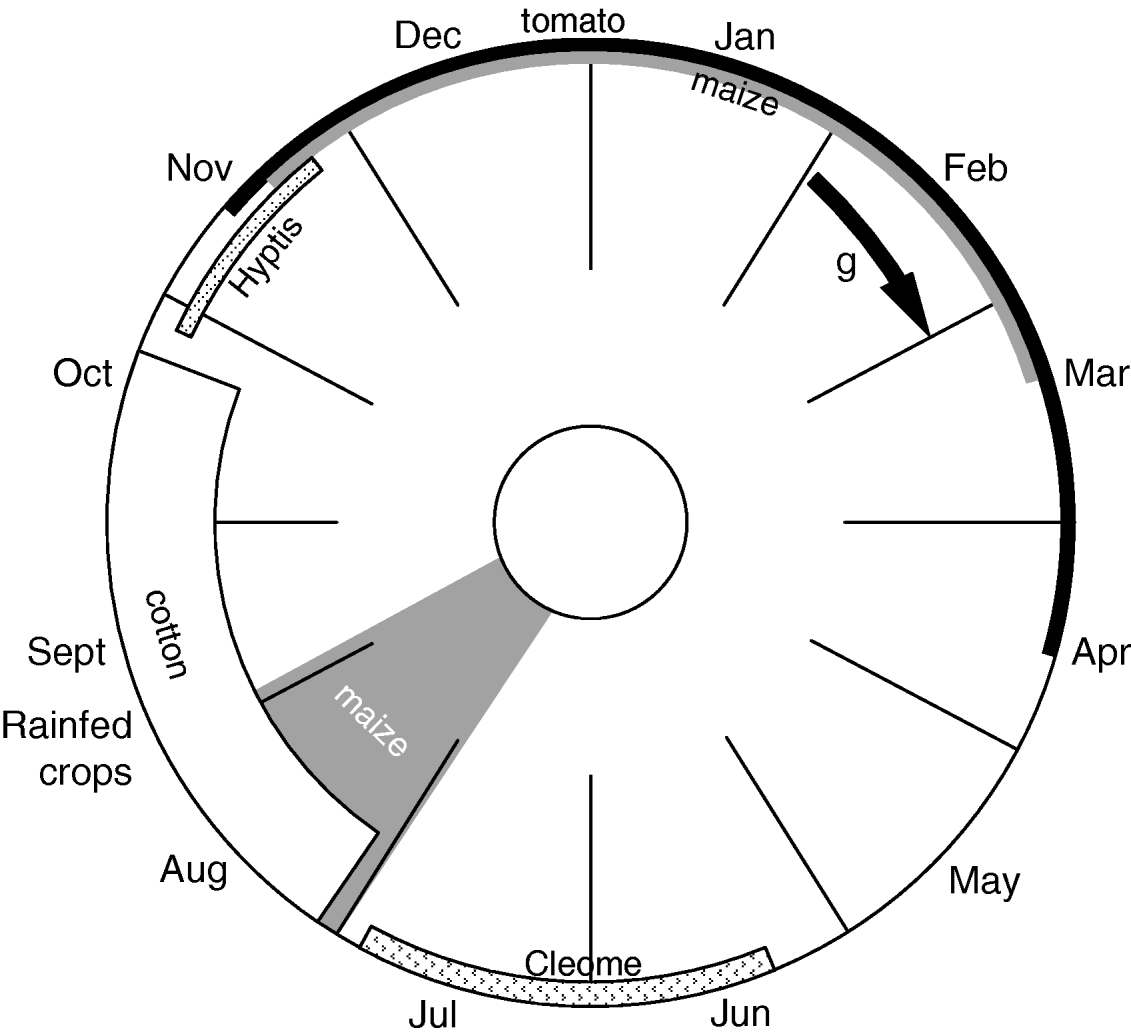
Fig. 1. Typical host plant succession of Helicoverpa armigera in the cotton-growing area of Central Africa. Band thickness is approximately proportionate to host abundance. g, generation time.
While the relationship between heavy insecticide use and insecticide resistance in cotton pests have been well documented in several cotton-growing areas worldwide, overall pyrethroid resistance has not been established at the regional scale of Central Africa. In addition, few detailed studies have dealt with the impact of the life system of polyphagous insects (particularly the sequence of host plants) on the evolution of insecticide resistance in annual cropping systems. This paper reports the 2002 to 2006 dynamics of pyrethroid resistance in field-collected H. armigera larvae from different sampling sites in the Central African cotton-growing area and through a sequence of crop and wild hosts at a local scale in northern Cameroon. Understanding the population dynamics of the polyphagous bollworm in a shifting agricultural landscape, both in time and space, is a key step in developing sustainable strategies for local and regional insecticide resistance management (IRM).
Materials and methods
Vial tests
To assess the spatial and temporal patterns of resistance in the cotton-growing area, H. armigera larvae were collected in cotton fields from four sampling areas in Cameroon (fig. 2) at the beginning (mid-August) and at the end (early October) of the infestation period from 2002 to 2006. Four additional sampling areas in Chad and Nigeria were monitored to validate our results at a broader regional scale. Local resistance was monitored from July 2003 to February 2007 by collecting larvae on successive major host plants (fig. 1) in an area located 25 km from Garoua (9°23 N, 13°45 E). Resistance to pyrethroid insecticides was assessed by vial tests (McCutchen et al., Reference McCutchen, Plapp, Nemec and Campanhola1989) adapted to bollworm larvae (Vaissayre et al., Reference Vaissayre, Vassal, Irving and Staetz2002). This method is based on the use of glass vials whose internal wall has been coated with an insecticide. For this experiment, cypermethrin was chosen as the active ingredient as the most commonly used pyrethroid in Central Africa. We used technical grade cypermethrin (94%, Arysta Life Science). Two treatments were tested in the laboratory in 40 ml vials: 10 untreated vials, used as control, and 30 vials, each treated with 30 μg of cypermethrin, a diagnostic dose reported to kill all the individuals of a susceptible strain (BK77) and 60–80% of a resistant population collected in Benin in 1997 (Vaissayre et al., Reference Vaissayre, Vassal, Irving and Staetz2002). Fourth instar larvae of between 10 and 15 mm long were collected from host plants not less than five days after the last insecticide application (if any). They were then placed individually in vials without food and maintained at ambient temperature. The number of dead larvae was recorded 24 hours later. For each sampling area, a minimum of two replications were carried out in different villages, except when there were not enough larvae. At least two replications were performed for each survey, depending on the number of larvae collected.
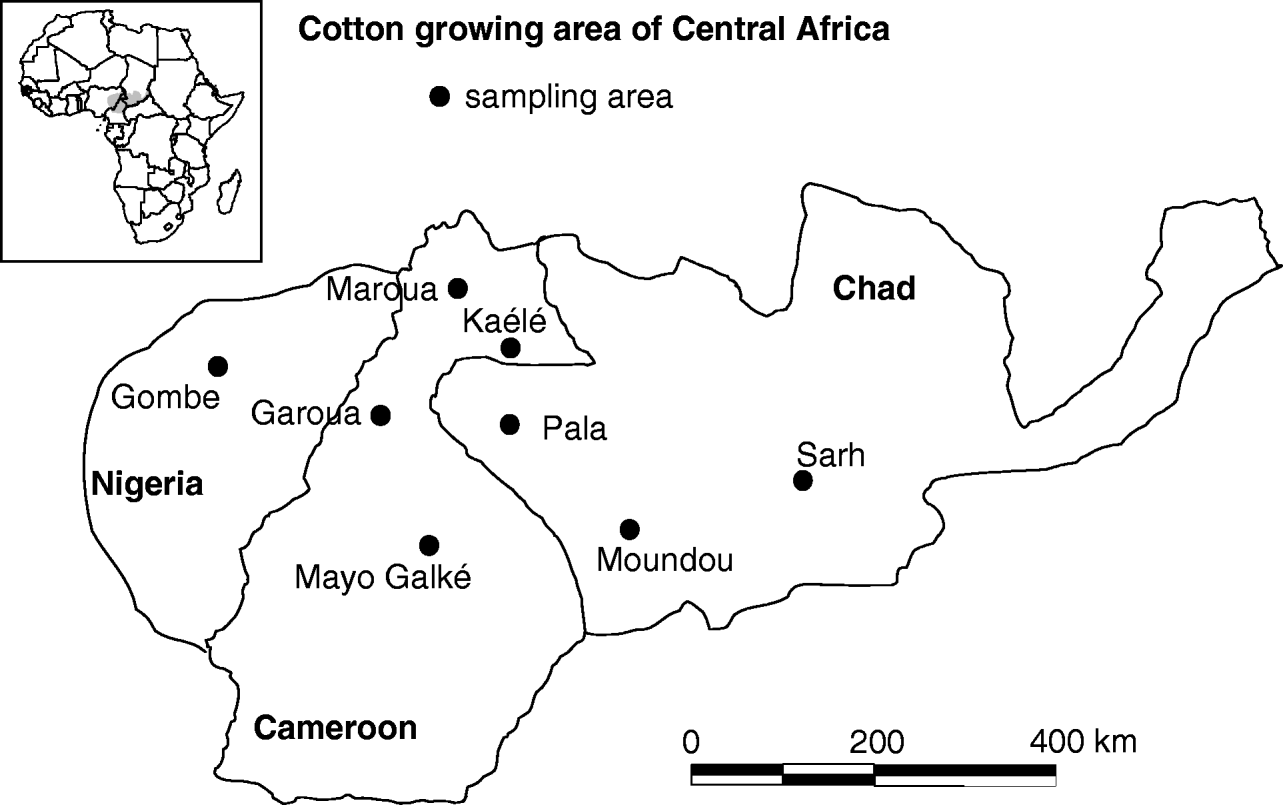
Fig. 2. Location of sampling areas on the survey of cypermethrin resistance of Helicoverpa armigera in the cotton-growing region of Central Africa, including on Nigeria, Cameroon and Chad.
Topical bioassays
Each insect colony was established with a minimum of 300 larvae collected from cotton or tomato crops around Gaschiga and Djalingo, two neighbouring localities in the vicinity of Garoua (fig. 2). First generation larvae of each colony were used for toxicological tests. The insecticide solution used for the topical application was prepared from the above-mentioned technical grade insecticide diluted with acetone. Fourth instar larvae were allocated into weight classes from 16 to 40 mg, and then individually held in six cell-boxes containing artificial diet. A quantity of insecticide solution (0.6–1.0 μl), depending on larva weight class expressed in micrograms per gram of larva, was applied on the thorax with an Arnold micro-applicator (Burkard, UK). A minimum of five doses were applied to build a regression line representative of the relation between dosage and mortality. For each test, a control population was treated with pure acetone. Treated larvae were kept at a constant temperature of 25±2°C and a L14:D10 photoperiod cycle. Mortality was observed 48 hours later. Larvae were considered dead if they gave no coordinated response to stimulation by touch with a blunt needle.
Data analysis
Vial test results were expressed as the percentage survival, corrected by the mortality observed in the control using Abbott's (Reference Abbott1925) formula. Square root transformed data were statistically compared by ANOVA using SAS GLM (SAS Institute, 1989) followed by Duncan's multiple range test. For topical application bioassays, the analysis of data was performed with the WINDL software (Cirad, France), according to Finney's log-probit method (Finney, Reference Finney1971). The lethal concentration (LC50) was expressed as micrograms of active ingredient per gram of larva. For each strain, the resistance factor (RF) was calculated by dividing the LC50 of the tested strain by that of the susceptible reference strain BK77 (Martin et al., Reference Martin, Ochou Ochou, Hala N'Klo, Vassal and Vaissayre2000).
Results
Resistance among cotton fields
From 2002 onwards, the annual survey of cotton crops showed a significant increase in the resistance frequency of H. armigera cotton populations to cypermethrin (F=100.5, P<0.001), culminating at the end of the 2005 growing season (fig. 3). Pyrethroid resistance increased systematically within cotton-growing seasons (F=45.1, P<0.001), except in 2006. It was also observed that the mean survival rate at the beginning of one cotton season (August, year n) was very similar to that obtained at the end of the previous cotton season (October, year n–1). On the other hand, the resistance frequency of cypermethrin was seen to vary between sampling sites (table 1). While no difference was observed between populations in August 2002, the first sign of pyrethroid resistance was detected in October of the same year in the central part of the cotton-growing region (Garoua, Cameroon). In the following growing season (2003), only bollworm populations collected from the southern part, including Mayo Galké (Cameroon) and Moundou (Chad), could be considered as not resistant (% survival<20). Although overall resistance continuously increased until October 2004, the resistance frequency of bollworm populations from Mayo Galké was still significantly lower than that of areas sampled in the central (Garoua and Pala) and northern area (Maroua) of the cotton-growing region. Surveys from October 2005 showed that Mayo Galké still displayed the lowest resistance, but significant differences were only observed between Mayo Galké and Moundou. In October 2006, pyrethroid resistance grew significantly worse in the central part of the cotton growing area, particularly Pala and Kaélé. At the beginning of cotton infestation by H. armigera (August), significant spatial differences were only detected in 2005 and 2006, with the lowest resistance frequencies observed in samples from Mayo Galké.
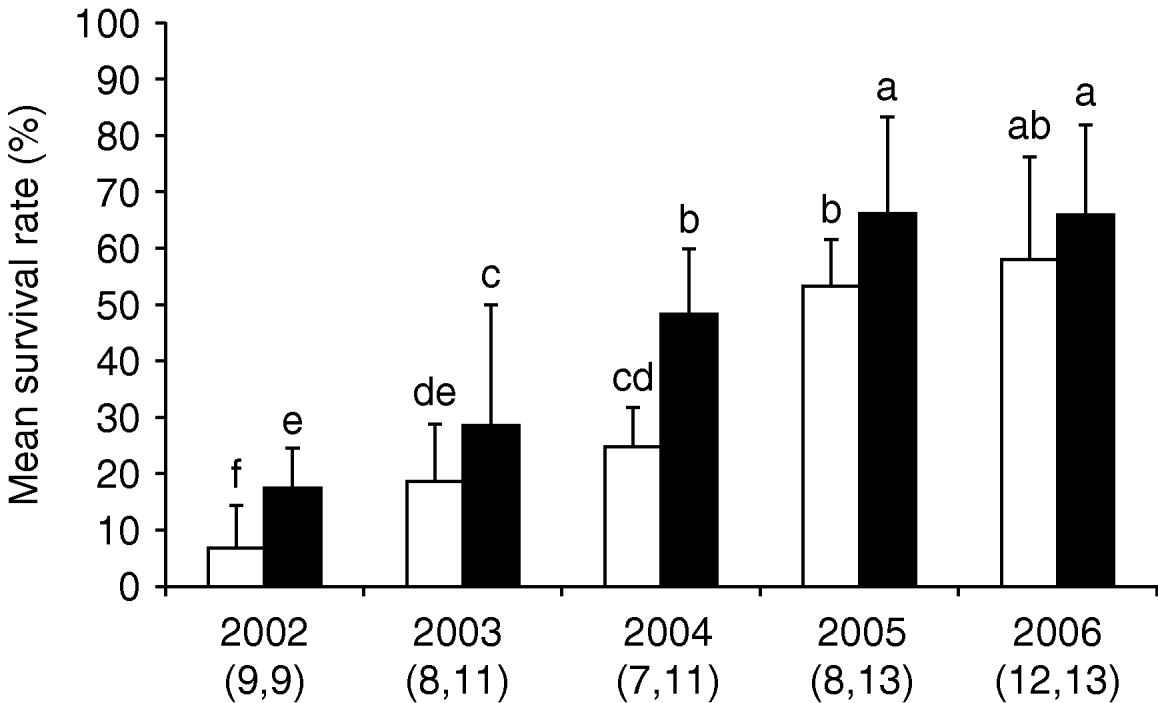
Fig. 3. Mean survival rate (%) of Helicoverpa armigera field-collected larvae exposed to 30 μg cypermethrin at the beginning (August) and at the end (October) of infestation of cotton crops. Bars indicate maximum value and the numbers in parentheses refer to the total numbers of vial tests conducted in August and October, respectively. Bars followed by different letters are significantly different (ANOVA, P<0.05). Numbers in parentheses indicate the number of vial tests.
Table 1. Mean survival rate (%) of Helicoverpa armigera field-collected larvae exposed to 30 μg cypermethrin at the beginning (August) and at the end (October) of infestation of cotton crops in the cotton-growing area of Central Africa.
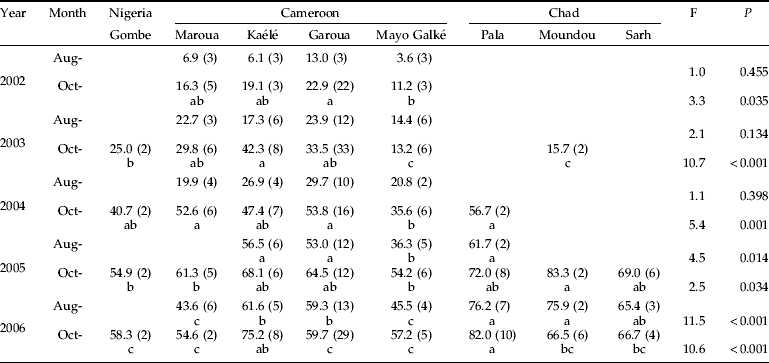
Values in the same row followed by different letters are significantly different (ANOVA, P<0.05). Numbers in parentheses indicate the number of vial tests.
Resistance among host plants
To measure seasonal resistance dynamics, local scale monitoring was conducted of larvae collected from a sequence of major host plants within 25 km around Garoua. In the 2003/04 season, larvae sampled from Cleome and maize at the beginning of the rainy season (July and August), presented a low resistance frequency (fig. 4). However, a significant increase of resistance to cypermethrin was recorded through the sequence of host plants (F=14.1, P<0.001), including cotton, Hyptis and tomato. Interestingly, populations sampled from unsprayed Hyptis (October 2003) or irrigated maize (December 2003 and February 2004) showed comparable resistance frequency to larvae from sprayed plants of cotton (F=0.01, P=0.909) or tomato (F=2.7, P=0.181), respectively. Between March and May 2004, the scarcity of host plants and larvae restricted vial tests. This dry transition period was associated with a marked decrease in resistance frequency in populations colonizing Cleome and maize at the beginning of the following rainy season in June 2004 (F=30.6, P<0.001). In the 2004/05 season, a significant increase in resistance was also recorded through the cotton-tomato sequence (F=19.1, P<0.001), although a significant decrease in resistance frequency was observed on irrigated tomato crops from December 2004 to February 2005 (F=11.0, P=0.045). On the other hand, the similarity in the frequency of resistant larvae from synchronously sprayed and unsprayed host plants was confirmed in the case of the cotton-maize in August 2004 (F=0.4, P=0.536), the cotton-Hyptis in October 2004 (F=1.9, P=0.194) and the tomato-irrigated maize in February 2005 (F=2.3, P=0.226) but not the tomato-maize in December 2004 (F=8.5, P=0.043). The 2005/06 and 2006/07 seasons recorded a new pattern. At the beginning of the rainy season, first populations sampled on Cleome presented a high resistance frequency. For the first time, no further increase in cypermethrin resistance was observed in larvae sampled on cotton (2004/05, F=3.0, P=0.103; and 2005/06, F=0.02, P=0.890), whereas a significant increase in resistance was observed in larvae sampled on tomato in December (2004/05, F=19.6, P<0.001; and 2005/06, F=8.2, P=0.017). Resistance frequency in tomato populations significantly decreased from December to February (F=6.9, P=0.022; F=5.4, P=0.036) in the 2004/05 and 2005/06 seasons, but not in the 2006/07 season (F=0.24, P=0.636). Based on this four-year study, we observed that the overall resistance frequency of local H. armigera populations significantly increased (F=27.3, P<0.001), depending on the season (F=49.7, P<0.001, 2003/04<2004/5<2005/06=2006/07) and on the host plant (F=27.4, P<0.001, Cleome=maize<cotton<Hyptis<tomato), and that there was a significant interaction between the two parameters (F=4.2, P<0.001). Unexpectedly, cotton significantly selected H. armigera larvae for cypermethrin resistance during the first two seasons only. Tomato systematically selected resistance until December, but resistance frequency did not increase and sometimes even decreased in February in the three last seasons. Finally, except in 2004/05, the resistance frequency decreased during the dry transition period from March to June.
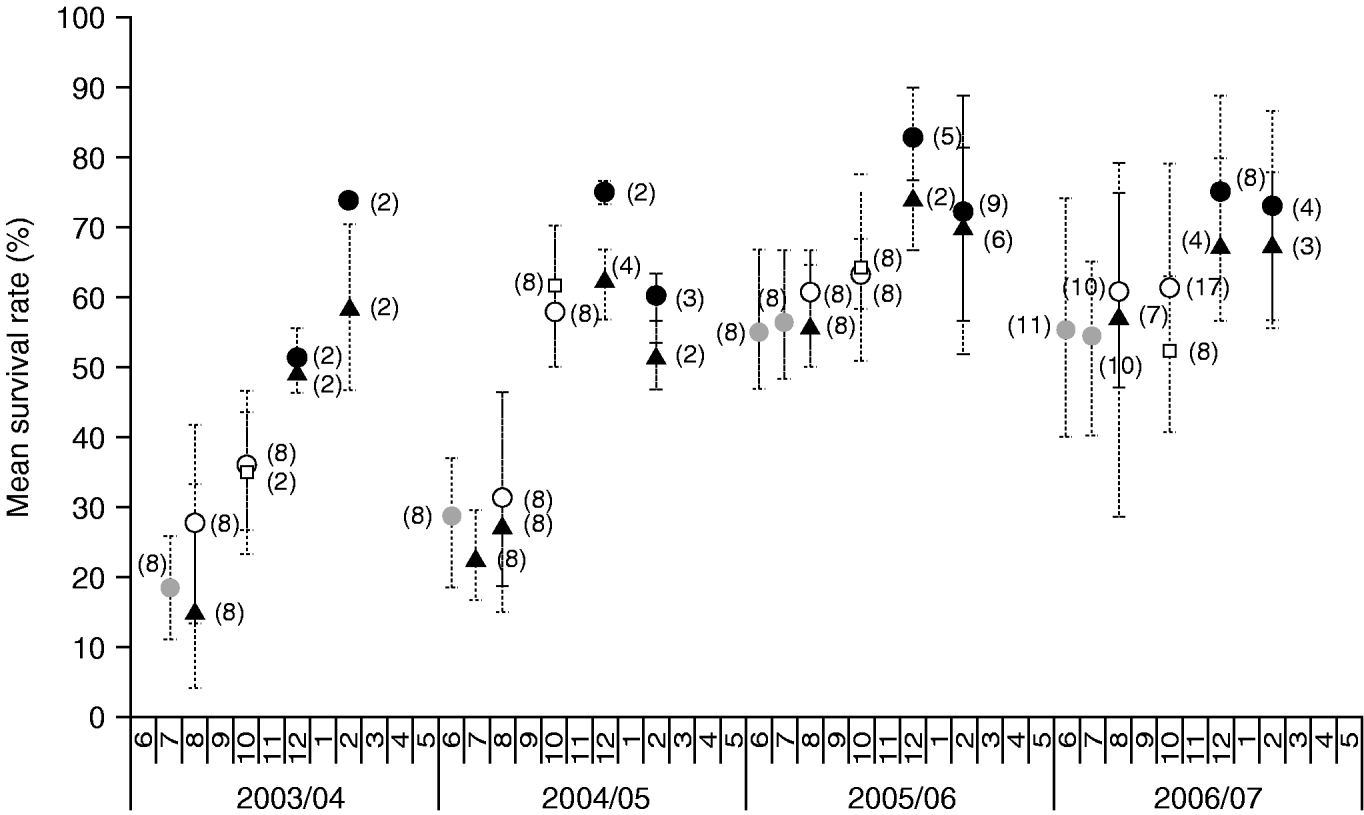
Fig. 4. Mean survival rate (%) of Helicoverpa armigera field-collected larvae exposed to 30 μg cypermethrin through a sequence of crop and wild host plants (within the range of 25 km of Garoua, Cameroon). Bars indicate maximum/minimum values and numbers in parentheses indicate the number of vial tests (![]() , Cleome; ▲, maize; ○, cotton; □, Hyptis; •, tomato).
, Cleome; ▲, maize; ○, cotton; □, Hyptis; •, tomato).
To complete the field survey, larval resistance was assessed in the laboratory by topical application on reared F1 colonies originating from cotton or tomato crops in two villages situated within a range of 25 km from Garoua. LC50 measurements confirmed the results of vial tests with a significant increase of resistance in wild populations of H. armigera across years and with a marked increase in resistance in populations sampled on tomato (table 2).
Table 2. Response of Helicoverpa armigera populations to topically applied cypermethrin.
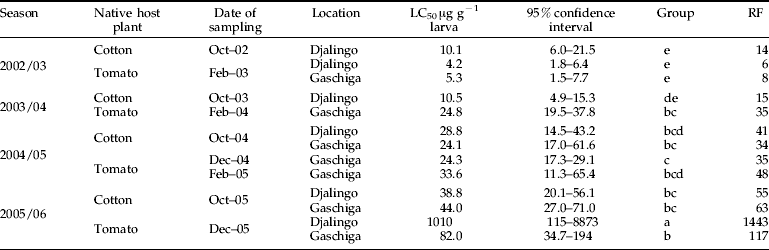
LC50: lethal concentration expressed in μg g−1 larva. RF (resistance factor)=LC50 of the tested strain/LC50 of the reference strain BK77. Groups were built based on overlapping confidence intervals.
Discussion
Resistance among cotton fields
The 2002 field survey of pyrethroid resistance in the cotton bollworm, H. armigera, revealed pyrethroid resistance in some local populations of the bollworm, H. armigera, in Cameroon. By 2004, resistance frequency of field-sampled larvae had gradually increased to reach a worrying situation. Laboratory bioassays on field-sampled populations showed that most control failures reported by farmers in 2004 were definitely due to pyrethroid resistance. Subsequent monitoring results indicated that pyrethroid resistance in H. armigera concerned the entire cotton-growing area of Central Africa. The cotton pest management programme in northern Cameroon was designed to provide small-scale cotton growers with a simple and cheap calendar-based spraying programme. Approximately six insecticide treatments are applied. The spraying programme begins from about day 45 after seedlings emerge (usually at squaring), with two consecutive endosulfan applications at biweekly interval, followed, irrespective of the pest status or level of infestation, by applications of a pyrethroid (usually cypermethrin) associated with an organophosphate (usually profenofos) (Vaissayre et al., Reference Vaissayre, Sement and Trijau1984; Ochou et al., Reference Ochou, Martin and Hala1998). In Chad, pyrethroids-profenofos mix is used throughout the cotton-growing season, while control methods in Nigeria are unknown. In South India, although recommended insecticides were becoming inefficient, the use of alternative chemistry that would have dramatically increased the cost of pest control was rejected in small-scale farming systems, where farmers preferred to increase the number of sprays. To control the high pressure from resistant H. armigera larvae, farmers had applied over 30 sprays as opposed to the recommended 6–10 sprays (Ramasubramanian, Reference Ramasubramanian2004). In Australia, conventional cotton production relies on chemical pesticides for pest control with 8–15 sprays applied to conventional crops (Fitt, Reference Fitt2000).
Why did resistance increase so rapidly without any significant changes in the agricultural landscape or in insecticide use in previous years? It can be hypothesized that resistant genes were recently introduced by resistant immigrant moths from West Africa, where resistance to pyrethroids in H. armigera was confirmed earlier (Vassal et al., Reference Vassal, Vaissayre and Martin1997; Martin et al., Reference Martin, Ochou Ochou, Hala N'Klo, Vassal and Vaissayre2000). In the 1998–2002 period, vial tests with 30 μg cypermethrin performed on field-collected larvae at the end of the cotton season gave survival rates of 1–35% in Ivory Coast (Martin et al., Reference Martin, Ochou Ochou, Vaissayre and Fournier2003), whereas values above 20% were obtained in neighbouring countries such as Mali, Burkina Faso and Benin (Vaissayre et al., Reference Vaissayre, Vassal, Irving and Staetz2002). If we consider that control is very likely to fail as soon as survival rate exceeds 20%, then most sampling sites in Cameroon should have been considered as pyrethroid-resistant from the end of the 2003 growing season, with the exception of the southern cotton-growing area (Mayo Galké). Knowing that the use of pyrethroid was homogeneous throughout the cotton-growing area of Cameroon, this particular situation is probably explained by landscape components where predominant maize crops and natural ecosystems served as insecticide-free refuges, which would have diluted resistance frequency in the latter area. Resistance worsened in 2005, followed by a relative stabilization in October 2006. Generally, the resistance of larvae samples did not decrease between the end of one season (October n) and the subsequent early season (August n+1), despite a long dry season of six to seven months without cotton crops. This stability could be explained by different non-exclusive factors that remain to be explored: (i) persistence of resistance alleles in the absence of a significant biological fitness cost and reconstitution of populations at the onset of the rainy season from diapausing populations vs. migrating populations; (ii) maintenance of selection pressure by the use of pyrethroids on intermediate cultivated host plants, such as tomato crops; and (iii) disappearance of refuge patches with the continual reduction of natural ecosystems in the landscape (Madden et al., Reference Madden, Holt and Armes1995; De Souza et al., Reference De Souza, Holt and Colvin1995). In Australia, Daly & Fitt (Reference Daly and Fitt1990) considered that fitness cost was irrelevant to explain the rapid off-season drop in resistance frequencies. In India, De Souza et al. (Reference De Souza, Holt and Colvin1995) noted that diapause-inducing conditions occurred before spraying against H. armigera had been completed and, in many areas, coincided with a peak density. These authors suggested that this phenomenon may have led to a lower resistance frequency in the proportion of the diapausing population, i.e. a refuge in time as opposed to a refuge in space. Their model showed that a significant drop in resistance could be achieved with only 1–2% of the H. armigera population entering diapause.
Resistance among host plants
Field monitoring at a local scale across the sequence of host plants showed that resistance frequency can be used as a marker to assess the gene flow of H. armigera between host plants. The similarity between the resistance frequency of larvae collected at the same time from treated and untreated hosts, such as cotton-maize (August), cotton-Hyptis (October) and tomato-maize (December and February), as well as the annual trend, confirmed the hypothesis of the absence of reproductive isolation between populations sampled on different host plants (Achaleke et al., Reference Achaleke, Brévault, Blondin and Vassal2005). Furthermore, Martin et al. (Reference Martin, Chandre, Ochou, Vaissayre and Fournier2002) showed, in Ivory Coast, that the same resistance mechanism (MFO-mediated) was involved in strains from different host plants, particularly cotton and tomato.
Results from the 2003 and 2004 surveys of host plants confirmed the key role of cotton in the seasonal increase in resistance. It can be assumed that dispersal of H. armigera occurs only at particular times during or after the cropping season, when resources are no longer available, which eventually modifies resistance patterns across the cotton-growing area. Han et al. (Reference Han, Wang, Zhang, Li and Li1999), in China, and Madden et al. (Reference Madden, Holt and Armes1995), in India, reported a seasonal pattern with a maximum at the end of the cropping season. Similarly, in West Africa, the resistance level to pyrethroids was generally highest in populations collected at the end of cotton treatments and decreased during the dry season (Martin et al., Reference Martin, Ochou Ochou, Vaissayre and Fournier2003). However, in 2005 and 2006, our results showed that local resistance frequency did not increase within the cotton season, probably due to the temporary exclusion of cypermethrin. In Cameroon, this strategy for the management of pest resistance have been implemented since the 2005 cotton season in some cotton areas where resistance had caused severe pest outbreaks. In 2006, cypermethrin was temporarily replaced by endosulfan or indoxacarb in the whole cotton area from September 9 to 22, the period which coincides with the onset of a predictable peak in H. armigera infestation of cotton. On the other hand, due to unfavourable conditions, the sowing of maize was abnormally delayed and extended in both years; so that, susceptible stages of cotton and maize were very synchronous throughout September (J. Achaleke, unpublished data). If the usual planting dates had been respected, cotton would have been the only available host in September. We hypothesize that maize might have served as a consistent refuge acting as a reservoir of non-resistant moths (De Souza et al., Reference De Souza, Holt and Colvin1995; Han et al., Reference Han, Wang, Zhang, Li and Li1999; Wu et al., Reference Wu, Feng and Guo2004).
In our survey, a significant increase in resistance frequency was recorded in tomato fields in irrigated sites. In 2005, strong resistance to cypermethrin was observed in a population collected on Gaschiga (RF>1000). Vegetable crops that are mostly located in the vicinity of towns offer conditions that are favourable for the rapid evolution of resistance. Indeed, irrigated sites form very small ‘oases’ surrounded by desert landscapes that probably prevent gene flow from outside while insecticides that are generally intended for cotton are indiscriminately applied on tomato crops. However, these crops offer a weak carrying capacity for adult production due to high temperatures, high insecticide use and limited acreage. The decreased resistance observed in February 2005 and 2006 may have resulted from the emergence of late season diapausing moths from cotton fields.
The resistance frequency of the populations colonizing Cleome at the beginning of the rainy season was quite similar to that of cotton populations in the previous year. This observation indicates that populations colonizing Cleome may be recruited via diapausing individuals that survive the hot and dry season and emerge with early rains. However, Nibouche (Reference Nibouche1994) showed that diapause occurring in H. armigera in West Africa was of limited duration and did not allow the pest to survive the whole dry season. In fact, the pest possibly survives the dry season by migrating. Rainfed crop areas are re-colonized at the beginning of every rainy season (May) by moths produced by populations that colonize vegetable crops located at varying distances from the northern region during the off-season. The reverse migration (from rainfed crops grown in the north to vegetable crops southwards) occurs at the beginning of the dry season (November). To conclude, the increment acreage of cotton crops linked to the disappearance of insecticide-free refuges in the agricultural landscape may have contributed to a certain extent to strengthening the selection of resistant alleles. Even if some irrigated sites, where tomato crops are grown, strongly concentrate resistance, densities are probably too low to contribute significantly to populations colonizing Cleome at the beginning of the rainy season. Long range migration from more humid areas, associated with air movements (Inter Tropical Front), may be more consistent, as shown by the relative spatial homogeneity of resistance frequency in populations sampled on cotton at the beginning of infestation (August).
Temporal positioning of host crops and pest management practices in annual cropping systems result in a shifting mosaic of habitats that influence resistance dynamics at local and regional scales, both within and across seasons (Kennedy & Storer, Reference Kennedy and Storer2000; Sequeira & Playford, Reference Sequeira and Playford2001). This information can help improve system-based resistance management by preserving the efficacy of both synthetic and natural insecticides, including Bt toxins expressed by transgenic crops (Gustafson et al., Reference Gustafson, Head and Caprio2006). The results of the study clearly showed that the window strategy that prescribes endosulfan (mid-July to mid-August) at the beginning of the cotton spraying programme did not overcome resistance in Cameroon. According to our results, strategies for managing resistance should exclude the use of pyrethroids on one generation if cotton is the only available host plant in the agricultural landscape (window from early September to early October), or on the last generation (mid-September to mid-October) when populations experience bottlenecks in food resources associated with southward emigration. Furthermore, alternative insecticide with no cross resistance (spinosad, indoxacarb, etc.) should be more widely used to control H. armigera in high value cash crops, such as tomato. In Australia, the threat of pyrethroid resistance in H. armigera is countered by a maximum of two consecutive pyrethroids sprays to coincide with peak bollworm damage (Forrester et al., Reference Forrester, Cahill, Bird and Layland1993). In the absence of reproductive isolation in populations of different hosts, diversified cropping systems should be encouraged with the planting of alternative host plants (e.g. maize, sorghum, cowpea, sunflower, etc.) to provide a greater mosaic of habitats, which, in return, would increase insecticide-free refuges or trap crops (Sequeira, Reference Sequeira2001) with enhanced biological control (Fitt, Reference Fitt2000; Grundy et al., Reference Grundy, Sequeira and Short2004). On the other hand, uncultivated hosts, such as Cleome and Hyptis, which serve as nurseries at the beginning and at the end of the cropping season, should be systematically destroyed prior to the flowering stage.
Priority actions should focus on the rational and concerted use of pesticides in cotton areas at the regional scale, with cooperation between the agrochemical industry, research and extension services in communicating with and explaining strategies to farmers (Denholm & Rowland, Reference Denholm and Rowland1992; Martin et al., Reference Martin, Ochou, Djihinto, Traore, Togola, Vassal, Vaissayre and Fournier2005). Resistance in H. armigera populations should be monitored at the onset of the rainy season on Cleome to evaluate the initial resistance frequency and, subsequently, across the season to evaluate programmes. On the whole, reduced reliance on insecticides should be based on sampling systems and thresholds, to better target sprays (Silvie et al., Reference Silvie, Deguine, Nibouche, Michel and Vaissayre2001), as well as on the use of more selective insecticides and the integration of a range of non-chemical tactics (Fitt, Reference Fitt2000; Vaissayre et al., Reference Vaissayre, Ochou, Hema and Togola2006).
More fundamental research is needed to understand the genetic basis of resistance mechanisms, including fitness cost, and the role of ecological factors that favour the increase in frequency and the further spread of resistance alleles in H. armigera populations. In particular, detailed knowledge is required on the origin of populations that first colonize Cleome at the beginning of the rainy season and, subsequently, colonize cotton (diapause vs. immigration) and, more generally, on the role of selection, drift and migration in the genetic structuring of H. armigera populations at a regional scale. Such results could help identify practical ways of reducing insecticide exposure and increase the use of host plants as potential refuges in IRM programmes for sustainable cotton production.
Acknowledgements
We express our sincere gratitude to the Cameroon cotton development corporation SODECOTON for providing the financial and technical assistance that permitted the gathering of field data on the resistance status of H. armigera. We also acknowledge PRASAC-ARDESAC Project who enabled completion of the regional aspect of this work. We are grateful to the two anonymous reviewers for comments and helpful suggestions.








