Introduction
Fasciolosis is a parasitic infection of domestic ruminants, wildlife and humans caused by trematodes of the genus Fasciola (Admassu et al., Reference Admassu, Shite and Kinfe2015; Ibrahim, Reference Ibrahim2017). Fasciola hepatica (Linnaeus, 1758) and F. gigantica (Cobbold, 1856), commonly known as ‘liver flukes’, are the two main species responsible for most infections (Jaja et al., Reference Jaja, Mushonga, Green and Muchenje2012). Due to its ability to infect a variety of animal species (Jaja et al., Reference Jaja, Mushonga, Green and Muchenje2012), F. hepatica has a wider distribution, occurring mainly in temperate areas and highlands of tropical and subtropical regions (Abebe et al., Reference Abebe, Abdunna, Berhane, Megersa and Regassa2010; Admassu et al., Reference Admassu, Shite and Kinfe2015), whilst F. gigantica is more restricted to the tropical regions of Africa and Asia (Abebe et al., Reference Abebe, Abdunna, Berhane, Megersa and Regassa2010). However, both species overlap in many African and Asian countries where the ecological requirements of the flukes and their intermediate hosts (IHs) converge (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005).
The distribution of F. hepatica and F. gigantica is dependent on the availability of the IHs (Prasad et al., Reference Prasad, Tandon, Biswal, Goswami and Chatterjee2008). Lymnaeidae snail species act as the IHs of Fasciola spp. (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005) and play a prominent role in the epidemiology and distribution of fasciolosis (Mochankana & Robertson, Reference Mochankana and Robertson2016). The two species vary greatly in their ecological and IH preference requirements (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009a), and the distribution of the fasciolids is consequently in accordance with the ecological requirements of their respective snail IHs (Quayle et al., Reference Quayle, Appleton and Dickens2010).
Fasciola hepatica utilizes the European snail species Galba truncatula (Muller, 1774) as its IH in some parts of Africa (Brown, Reference Brown1994; De Kock et al., Reference De Kock, Wolmarans and Bornman2003) and other species from the genus Lymnaea/Galba or Fossaria as IHs (Mas-Coma, Reference Mas-Coma2005). In sub-Saharan Africa, the geographical distribution of both F. hepatica and G. truncatula may be divided into three categories, as suggested by Mas-Coma et al. (Reference Mas-Coma, Valero and Bargues2009b); (i) the north-western African region; (ii) the large western and central African region; and (iii) the Eastern Africa region from the northern Mediterranean shore to Southern Africa. The distribution of the latter includes large areas in South Africa and Lesotho, with large areas in Egypt in the northern regions, and another large but isolated area in Ethiopia, Kenya and Tanzania in the eastern region (Brown, Reference Brown1994). Fasciola gigantica, on the other hand, is found in the tropical and subtropical regions of Africa, Asia and the Far East (Mochankana & Robertson, Reference Mochankana and Robertson2018), and, according to Mas-Coma et al. (Reference Mas-Coma, Bargues and Valero2005), its limited geographical distribution is mainly linked to the weaker dispersion capacity of its intermediate snail hosts. This species is reported to utilize a greater range of IHs of the genus Radix species (Mas-Coma, Reference Mas-Coma2005). These are species that belong to Hubendick's (Reference Hubendick1951) superspecies of Radix auricularia (Linnaeus, 1758), which includes R. natalensis (Krauss, Reference Moema, King and Baker1848) in Africa and R. rubiginosa (Michelin, 1831) in Asia (Fretter & Peake, Reference Fretter and Peake1987; Brown, Reference Brown1994). According to Jaja et al. (Reference Jaja, Mushonga, Green and Muchenje2012), the extended geographical distribution of G. truncatula and R. natalensis is largely promoted by environmental factors such as rainfall, solar radiation and global warming.
An overlap in the geographical distribution of F. gigantica and F. hepatica in Africa has been reported (Mas-Coma et al., Reference Mas-Coma, Valero and Bargues2009b). Overlaps are distinguished as either local, when they occur in areas where the climate throughout the year favours the co-existence of both Galba and Radix species in the same locality, which has been observed in the Nile Delta in Egypt, or zonal, when overlap is observed in highland areas where the cold–mild weather favours F. hepatica and G. truncatula and the lowlands offer the warm–hot climate for Radix spp. and the F. gigantica system, and this overlap has been described in East Africa. Against this background, the present review focused on assessing the geographical distribution of F. hepatica and F. gigantica and their Lymnaeidae intermediate snail hosts in East and Southern Africa based on peer-reviewed publications and reports from this region between 1988 and 2018.
Methods
Search strategy
A systematic search of literature between 1988 and 2018 (30 years) was done on the Google Scholar database, using the search terms: Fasciola spp., Lymnaeidae, Lymnaea, Pseudosuccinea, Galba, Radix, Southern Africa and East Africa, and Boolean operators (AND, OR) were used. The literature search was limited to articles published in English. Additionally, reference lists of the selected articles were screened as potential leads for inclusion. Full-text articles were retrieved and managed in EndNote reference manager version X8 (Clarivate Analytics, Philadelphia, PA, USA).
Inclusion and exclusion criteria
Both published and unpublished reports reporting on the distribution of Lymnaeidae and Fasciola species in East and Southern Africa were included in the review. Articles were identified by reading through the titles and abstracts. The literature search on the distribution of Fasciola species and their invertebrate hosts in East and Southern Africa included studies focusing on either the geographical distribution or prevalence and identification of F. hepatica and F. gigantica in naturally infected IHs in East and Southern African countries. All studies focusing on the economic loss and treatment were excluded. A systematic search on the distribution of Fasciola species and their IHs included field studies focusing on the association and distribution of both the IHs and Fasciola spp. and those that focused solely on the distribution or occurrence of the lymnaeid IHs and experimental studies conducted with Fasciola species and Lymnaeidae specimens from East and Southern African countries. The review excluded review papers and studies that did not identify the lymnaeid species up to species level.
Results
The systematic literature search yielded a total of 640 hits, which included abstracts, reports, books and duplicate articles (fig. 1). Twelve additional records obtained through the reference lists of the screened articles, and two unpublished articles, were also included in the list. Fifteen articles were excluded due to duplication, and a total of 639 articles were screened. Four hundred and eighty-seven abstracts and full-texts were excluded because they did not explicitly report on the distribution, occurrence or identification of Fasciola and Lymnaeidae species in East and Southern Africa, and four books were excluded. Ninety-one articles were excluded because the studies were either conducted on the same study area and animal species, or they did not identify Lymnaeidae species up to species level. Fifty-seven articles met the criteria and were considered and discussed in this review in relation to the identity and distribution of Fasciola and Lymnaeidae species in East and Southern African countries.

Fig. 1. PRISMA diagram.
Distribution and prevalence of Fasciola species and their vertebrate host(s)
The distribution of Fasciola species and their vertebrate hosts in East and Southern Africa is shown in tables 1 and 2. Results show that F. gigantica is the predominant species distributed in East and Southern Africa, occurring as the only species in 11 reviewed countries with the exception of South Africa and Zimbabwe in Southern Africa and Ethiopia and Tanzania in East Africa, where both F. gigantica and F. hepaica have been reported. Results show that both Fasciola species utilize a range of vertebrate animal species ranging from domestic to wild ruminants as definitive hosts. Although Fasciola infection was common in domestic ruminants (cattle, goats and sheep) in East and Southern African countries (table 1), infections in wildlife have only been reported in Southern African countries during the period under review (table 2). Fasciola gigantica infection was reported in nine species of wildlife, while F. hepatica was only reported in three (table 2). Fasciola tragelaphi infection was reported in cattle in Zimbabwe. Two cases of Fasciola spp. infection in humans have been reported in South Africa using Fasciola immune fluorescent antibody test (IFAT) assay.
Table 1. Checklist of Fasciola species and their domestic ruminant hosts reported in East and Southern Africa (1988–2018).

IFAT, immune fluorescent antibody test.
Table 2. Checklist of Fasciola species and their wildlife hosts reported in East and Southern Africa (1988–2018).

Geographical distribution of lymnaeids in East and Southern Africa
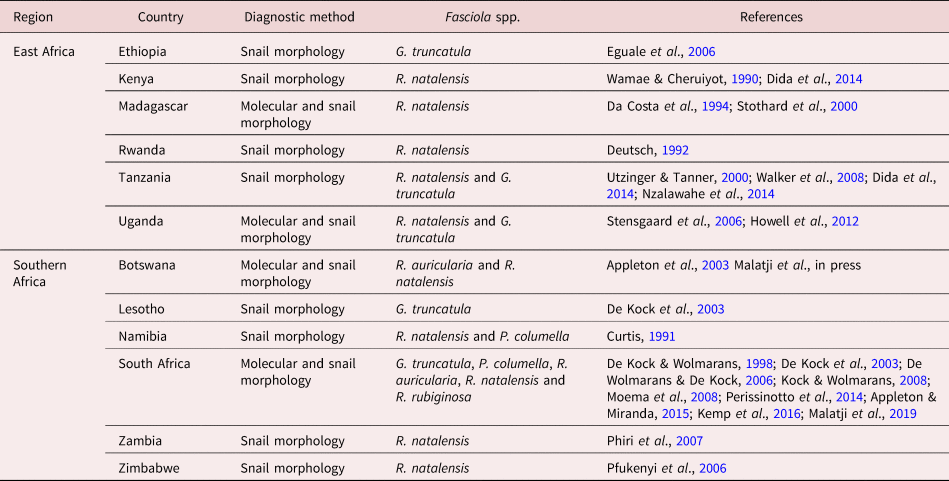
Radix natalensis, G. truncatula, Pseudosuccinea columella, R. auricularia and R. rubiginosa have been documented in East and Southern Africa (table 3). All five species were reported in Southern Africa, and only two (i.e. R. natalensis and G. truncatula) are documented in East African countries. Radix natalensis was found to occur in ten of the 12 East and Southern African countries and is presumed to act as the main IH of F. gigantica. This species was found to be the only lymnaeid species found in Rwanda, Kenya and Madagascar (East Africa) and Zimbabwe and Zambia (Southern Africa), where only F. gigantica was recorded, with the exception of Zimbabwe where a case of F. hepatica was also recorded (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015). All five lymnaeid species have been identified using both molecular and morphological techniques in South Africa. An overlap in the distribution and co-habitation among these species was observed and reported in Mpumalanga and KwaZulu-Natal provinces of South Africa. Galba truncatula was documented in Ethiopia, Tanzania, Uganda, South Africa and Lesotho – countries where F. hepatica has been documented, with the exception of Uganda. In Tanzania, R. natalensis and G. truncatula have been reported to occur in locations with different altitudes, with the former mainly found in lower altitudes and the latter in higher altitudes (Walker et al., Reference Walker, Makundi, Namuba, Kassuku, Keyyu, Hoey, Prödohl, Stothard and Trudgett2008). Similar observations were made in Uganda (Howell et al., Reference Howell, Mugisha and Davies2012) and Ethiopia (Kedir et al., Reference Kedir, Deressa and Tigre2012; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015). However, unlike in Tanzania and Uganda, an overlap in the distribution of both Fasciola species was observed in Ethiopia, which occur at the intermediate of the lower and the higher altitude (Kedir et al., Reference Kedir, Deressa and Tigre2012). In Namibia, R. natalensis and P. columella have been documented, with the former occurring abundantly in the northern rivers, where the latter has not yet been found, and these species also overlap in the Orange River (Curtis, Reference Curtis1991). In Botswana, R. natalensis and R. auricularia have been identified, and these species act as the IHs of F. gigantica, which has been documented in the country.
Table 3. Checklist of Lymnaeidae species reported in East and Southern Africa (1988–2018).
Prevalence of Fasciola spp. in East and Southern Africa
Results from the reviewed articles showed that the overall prevalence of fasciolosis in cattle was the same with both faecal egg count and fluke count at post-mortem, and prevalence as determined by faecal egg count was higher in goats and sheep compared to the prevalence using the fluke count at post-mortem (table 4). Based on the faecal egg count technique, the overall prevalence of fasciolosis was significantly higher in cattle (14.5%) (P = 0.002) compared to that of sheep (10.9%) and goats (9.4%) (table 4). The same trend was observed with the fluke count technique, where more flukes were found in cattle (14.5%), followed by sheep (3.7%) and, lastly, goats (2.5%) (table 4). In cattle, the prevalence ranged from 0.74% to 58% and from 0.09% to 75.8% based on the egg count and fluke count technique, respectively (table 4). In goats, the prevalence ranged from 2.4% to 26%, and in sheep from 3.6% to 26.3% based on the fluke count technique (table 4). The prevalence of fasciolosis in wildlife was 52.5% (21 of n = 40) for Kafue lechwe in Zambia (Munyeme et al., Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010), 12.5% (one of n = 8) for Kudu in South Africa (Van Wyk & Boomker, Reference Van Wyk and Boomker2015) and 0.52% (one of n = 191) for Impala in Swaziland (Gallivan et al., Reference Gallivan, Karker, Culverwell and Girdwood1996).
Table 4. Prevalence of fasciolosis in domestic ruminants in East and Southern Africa based on faecal egg and fluke counts (1988–2018).

CI, confidence interval.
Factors associated with the distribution and prevalence of Fasciola spp. in East and Southern Africa
Diagnostic techniques
Faecal egg count and fluke count techniques showed no difference in the overall mean prevalence of fasciolosis (14.5%) in cattle. However, the overall mean prevalence in goats and sheep was higher with the faecal egg count technique (9.4% and 10.9%, respectively) when compared with the fluke count technique (2.5% and 3.7%, respectively).
Season
Phiri et al. (Reference Phiri, Phiri, Siziya, Sikasunge, Chembensofu and Monrad2005a) observed that the prevalence of fasciolosis was higher during the rainy and the cold–dry seasons in Zambia, using both the faecal egg and fluke count techniques. The hot–dry season exhibited low fluke abundance (13.8%), with a gradual increase in fluke prevalence (21.7%) during the rainy and the cold–dry seasons (24.8%). A similar trend was observed with the faecal egg count technique; 34.5% during the hot–dry season, increasing to 39.1% during the rainy season. Both flukes and faecal egg counts peaked during the post-rainy season, with a prevalence of 41.3% and 45.0%, respectively. Fasciola gigantica infection was also reported to be higher in the wet highlands as compared to the dry lowlands of Zimbabwe (Pfukenyi & Mukaratirwa, Reference Pfukenyi and Mukaratirwa2004). The authors recorded a highest prevalence of 54.3% in February and December (rainy months), and lowest (17.8%) in September (dry month). The wet season had a significantly higher prevalence (45.6%) (P < 0.001) as compared to the dry season (27.8%). In Tanzania, Nzalawahe & Komba (Reference Nzalawahe and Komba2013) showed a significant seasonal variation in the occurrence of fasciolosis, with the highest (77.5% and 28%) recorded during the dry season and the lowest (74% and 26.5%) during the wet season in cattle and goats, respectively. There were also variations observed in the cumulative monthly prevalence, with the highest prevalence in cattle obtained in October (89.4%) and lowest (61.7%) in June, whilst in goats the highest prevalence of 33.9% was obtained in September and the lowest (22.4%) in October (Nzalawahe & Komba, Reference Nzalawahe and Komba2013).
Age
Both young and adult animals seem to be equally susceptible to Fasciola infection. Studies in Ethiopia showed a significantly higher prevalence of Fasciola infection in young animals (Kedir et al., Reference Kedir, Deressa and Tigre2012; Regassa et al., Reference Regassa, Woldemariam, Damissie, Moje, Ayana and Abunna2012; Aregay et al., Reference Aregay, Beleke, Ferede and Hailemelekot2013; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015; Betebo, Reference Betebo2017), while other studies showed that adults had a significantly higher prevalence of Fasciola infection (Phiri et al., Reference Phiri, Phiri, Siziya, Sikasunge, Chembensofu and Monrad2005a, Reference Phiri, Phiri, Sikasunge and Monradb; Pfukenyi et al., Reference Pfukenyi, Mukaratirwa, Willingham and Monrad2006; Mebrahtu & Beka, Reference Mebrahtu and Beka2013; Moje et al., Reference Moje, Mathewos, Desissa and Regassa2015; Amsalu, Reference Amsalu2017; Eshetu et al., Reference Eshetu, Thomas, Awukew, Goa and Butako2017; Mochankana & Robertson, Reference Mochankana and Robertson2018).
Sex
Both males and females seem to be equally likely to have fasciolosis. Studies showed that Fasciola infection due to either F. gigantica or F. hepatica was more prevalent in males (Kithuka et al., Reference Kithuka, Maingi, Njeru and Ombui2002; Njeruh et al., Reference Njeruh, Kithuka, Maingi and Ombui2004; Kedir et al., Reference Kedir, Deressa and Tigre2012; Munyeme et al., Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010; Betebo, Reference Betebo2017); however, the difference was not significant, with the exception to that reported by Kedir et al. (Reference Kedir, Deressa and Tigre2012) in small ruminants. Other studies showed that fasciolosis occur mostly in females (Phiri et al., Reference Phiri, Phiri, Siziya, Sikasunge, Chembensofu and Monrad2005a, Reference Phiri, Phiri, Sikasunge and Monradb; Aregay et al., Reference Aregay, Beleke, Ferede and Hailemelekot2013; Mebrahtu & Beka, Reference Mebrahtu and Beka2013; Amsalu, Reference Amsalu2017; Eshetu et al., Reference Eshetu, Thomas, Awukew, Goa and Butako2017; Mochankana & Robertson, Reference Mochankana and Robertson2018). However, the difference in the prevalence between sex was not significant with exception to that reported by Mochankana & Robertson (Reference Mochankana and Robertson2018).
Body condition score
Animals with a poor (low) body condition score seemed to be more susceptible to infection and had a higher fasciolosis prevalence than those with moderate and higher body condition scores. For instance, studies showed a prevalence ranging from 26.92% to 60.48% in cattle with poor body condition scores, compared to 20.83%–36.1% in those with moderate and 11.0%–22.45% in those with good body condition (Mebrahtu & Beka, Reference Mebrahtu and Beka2013; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015; Moje et al., Reference Moje, Mathewos, Desissa and Regassa2015; Betebo, Reference Betebo2017). Amsalu (Reference Amsalu2017) and Kedir et al. (Reference Kedir, Deressa and Tigre2012) also showed a similar trend in sheep and goats; a prevalence of 13.5%, 8.2% and 7.9% in sheep, and 44.6%, 22.7% and 15.4% in goats with poor, moderate and good body condition scores, respectively. Some studies showed a significant association between the body condition scores and Fasciola infection prevalence (P < 0.05) (Mebrahtu & Beka, Reference Mebrahtu and Beka2013; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015; Moje et al., Reference Moje, Mathewos, Desissa and Regassa2015; Betebo, Reference Betebo2017; Eshetu et al., Reference Eshetu, Thomas, Awukew, Goa and Butako2017), whilst others found no statistical association (Mebrahtu and Beka, Reference Mebrahtu and Beka2013). However, Aregay et al. (Reference Aregay, Beleke, Ferede and Hailemelekot2013) and Munyeme et al. (Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010) showed that cattle and Kafue lechwe with good condition scores were largely infected (39.30% and 75%) with fasciolosis compared to those with poor condition scores (39.30% and 12.5%), but the association was not statistically significant (Munyeme et al., Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010; Aregay et al., Reference Aregay, Beleke, Ferede and Hailemelekot2013).
Discussion
Fasciola hepatica and F. gigantica have been proven to utilize diverse mammalian species as their vertebrate hosts, ranging from domestic ruminants to wildlife (Jaja et al., Reference Jaja, Mushonga, Green and Muchenje2012) in East and Southern Africa. However, Fasciola infection due to one or both Fasciola species seems to be common in domestic ruminants throughout the reviewed countries, but rare in wildlife and humans, probably due to limited studies in wildlife in the two regions.
Results showed that both Fasciola species are present in Southern African wildlife. Fasciola hepatica was documented in kudu in South Africa (Van Wyk & Boomker, Reference Van Wyk and Boomker2015), sable antelope and common duiker in Zimbabwe (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015). Fasciola gigantica was reported in ten wildlife species in Zimbabwe (Jooste, Reference Jooste1989; Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015), in an impala from a game reserve previously used as a cattle ranch in Swaziland (Gallivan et al., Reference Gallivan, Karker, Culverwell and Girdwood1996) and Kafue lechwe in the Kafue basin in Zambia (Munyeme et al., Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010). According to Boomker (Reference Boomker2007), fasciolosis is rare in free-ranging antelopes, and the presence of F. hepatica infection in kudu might have been accidental as they are generally browsers and are less likely to get exposed to aquatic vegetation. Fasciola hepatica infection recorded in kudu may be attributed to the water source (dam), which provided a favourable environment for freshwater snails that serve as IHs for the liver fluke, and was regularly accessed by cattle (Van Wyk & Boomker, Reference Van Wyk and Boomker2015). According to Muma et al. (Reference Muma, Samui, Oloya, Munyeme and Skjerve2007) and Munyeme et al. (Reference Munyeme, Muma, Skjerve, Nambota, Phiri, Samui, Dorny and Tryland2008), contact between wildlife and cattle via sharing grazing land or drinking water most likely facilitates bi-modal transmission of the parasite.
The reviewed studies also showed that the highest prevalence of fasciolosis in domestic ruminants in East Africa was reported in Ethiopia in cattle (Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015), sheep and goats (Kedir et al., Reference Kedir, Deressa and Tigre2012), and in Tanzanian cattle and sheep in communal grazing areas where irrigation was practiced (Nzalawahe & Komba, Reference Nzalawahe and Komba2013). In Southern Africa, the highest prevalence of fasciolosis was reported in domestic ruminants from the Kafue basin in Zambia (Yabe et al., Reference Yabe, Phiri, Phiri, Chembensofu, Dorny and Vercruysse2008). In wildlife, the prevalence of fasciolosis was highest in the Kafue lechwe from the Kafue wetlands in Zambia (Munyeme et al., Reference Munyeme, Mingang'andu, Muma, Nambota, Biffa and Siamudaala2010), followed by kudu in South Africa (Van Wyk & Boomker, Reference Van Wyk and Boomker2015) and impala from a game reserve previously used as a cattle ranch in Swaziland (Gallivan et al., Reference Gallivan, Karker, Culverwell and Girdwood1996).
The overall mean prevalence of fasciolosis was highest in cattle, followed by sheep and then goats. According to Kedir et al. (Reference Kedir, Deressa and Tigre2012), the interspecies variation in the prevalence is probably due to the difference in the feeding behaviour of the animal species, as well as their general immunological response to parasitic infection. Cattle and sheep are generally grazers, while sheep nibble nearer to the ground and goats browse; hence, the feeding behaviour of cattle and sheep increases the chances of exposure to infective stages of parasites compared to goats (Kedir et al., Reference Kedir, Deressa and Tigre2012; Legesse et al., Reference Legesse, Asfaw, Tolossa and Beyene2014), and contact of goats with infective stages may be caused by the scarcity of browse feed or due to a decrease in vegetation cover, compelling goats to graze around water points and irrigation lands (Legesse et al., Reference Legesse, Asfaw, Tolossa and Beyene2014). Furthermore, Fasciola infection in goats (Nzalawahe & Komba, Reference Nzalawahe and Komba2013) and sheep (Wamae & Cheruiyot, Reference Van Wyk and Boomker1990; Mungube et al., Reference Mungube, Bauni, Tenhagen, Wamae, Ngini and Mugambi2006) has been reported to cause an acute form of fasciolosis, characterized by high mortality rate (Mungube et al., Reference Mungube, Bauni, Tenhagen, Wamae, Ngini and Mugambi2006; Nzalawahe & Komba, Reference Nzalawahe and Komba2013). In cattle, fasciolosis generally manifests as a chronic disease due to immunity acquired following the first infection (Mungube et al., Reference Mungube, Bauni, Tenhagen, Wamae, Ngini and Mugambi2006), which reduces the migration of the immature flukes to the liver (Mungube et al., Reference Mungube, Bauni, Tenhagen, Wamae, Ngini and Mugambi2006; Nzalawahe & Komba, Reference Nzalawahe and Komba2013). According to Nzalawahe & Komba (Reference Nzalawahe and Komba2013), this phenomenon has not been described in sheep and goats. However, Kedir et al. (Reference Kedir, Deressa and Tigre2012) reported that unlike goats, sheep acquire resistance to fasciolosis.
Our results also showed that the overall prevalence of fasciolosis in cattle was similar based on faecal egg count and fluke count methods, but higher in sheep and goats based on the faecal egg count as compared to the fluke count method. However, most studies recorded a higher prevalence with the fluke count method. The egg count method has a low sensitivity due to the fact that Fasciola eggs only appear in faeces about 8–15 weeks post-infection (Sanchez-Andrade et al., Reference Sanchez-Andrade, Paz-Silva, Suaurez, Panadero, Pedreira, Lopez, Diez-Banos and Morrondo2002). Pfukenyi & Mukaratirwa (Reference Pfukenyi and Mukaratirwa2004) reported higher prevalence of fasciolosis based on the fluke count method, as post-mortem studies include immature flukes, which cannot be detected by coprological examinations. Although the faecal egg count technique is commonly used in the diagnosis of fasciolosis in animals and humans, species identification is often based on assumptions since Fasciola eggs cannot be used to distinguish species.
Risk factors that may influence the transmission of Fasciola spp. may include age, sex and body condition of the host. However, according to Rangel-Ruiz et al. (Reference Rangel-Ruiz, Marquez-Izquierdo and Bravo-Nogueira1999), determining the major risk factors influencing the transmission of fasciolosis is complicated, mainly because adult Fasciola may persist inside the definitive host for more than one year, consistently producing eggs.
Although both Fasciola species have been observed to occur in both regions of study, F. gigantica was more common, and geographical distribution was found to be consistent with that of its IHs. Radix natalensis, the African native IH of F. gigantica, was observed to occur in ten of the 11 countries where lymnaeid species have been reported as either the only species or in conjunction with other species. In some cases, this species has been found with more than one type of cercariae, including Gymnocephalous cercariae, which represent either fasciolids or amphistomes; however, co-infection by two different cercariae has never been reported (Wamae & Cheruiyot, Reference Wamae and Cheruiyot1990; Phiri et al., Reference Phiri, Phirir, Chota and Monrad2007; Moema et al., Reference Moema, King and Baker2008; Walker et al., Reference Walker, Makundi, Namuba, Kassuku, Keyyu, Hoey, Prödohl, Stothard and Trudgett2008). Other lymnaeid species have been reported to transmit F. gigantica elsewhere in the world (Brown, Reference Brown1994; Grabner et al., Reference Grabner, Mohamed, Nachev, Meabed, Sabry and Sures2014; Appleton & Miranda, Reference Appleton and Miranda2015) and R. auricularia has been reported in South Africa and Botswana (Malatji et al., Reference Malatji, Lamb and Mukaratirwa2019), R. rubiginosa in South Africa (Appleton & Miranda, Reference Appleton and Miranda2015) and P. columella in South Africa (De Kock & Wolmarans, Reference De Kock and Wolmarans1998; Wolmarans & De Kock, Reference Wolmarans and De Kock2006; De Kock & Wolmarans, Reference De Kock and Wolmarans2008; Perissinotto et al., Reference Perissinotto, Miranda, Raw and Peer2014; Kemp et al., Reference Kemp, De Kock and Wolmarans2016) and Namibia (Curtis, Reference Curtis1991). In South Africa, P. columella has been found naturally infected with F. gigantica and Echinostoma spp. (Malatji & Mukaratirwa, Reference Malatji and Mukaratirwa2019) and found to co-exist with R. natalensis in KwaZulu-Natal and Mpumalanga provinces, where both F. gigantica and F. hepatica overlap (De Kock & Wolmarans, Reference De Kock and Wolmarans1998; Wolmarans & De Kock, Reference Wolmarans and De Kock2006; De Kock & Wolmarans, Reference De Kock and Wolmarans2008; Malatji et al., Reference Malatji, Lamb and Mukaratirwa2019). These findings have led to the assumption that this species could be playing a major role in the transmission of both Fasciola species in the two provinces of South Africa, whereas its role in the transmission of Fasciola species in Namibia is not known.
Fasciola hepatica infections have been reported in ruminants in Ethiopia (Abebe et al., Reference Abebe, Abdunna, Berhane, Megersa and Regassa2010; Kedir et al., Reference Kedir, Deressa and Tigre2012; Regassa et al., Reference Regassa, Woldemariam, Damissie, Moje, Ayana and Abunna2012; Aregay et al., Reference Aregay, Beleke, Ferede and Hailemelekot2013; Mebrahtu & Beka, Reference Mebrahtu and Beka2013; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015; Moje et al., Reference Moje, Mathewos, Desissa and Regassa2015; Amsalu, Reference Amsalu2017; Betebo, Reference Betebo2017; Eshetu et al., Reference Eshetu, Thomas, Awukew, Goa and Butako2017), South Africa and Zimbabwe (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015). In Tanzania, this species has been detected in G. truncatula (Walker et al., Reference Walker, Makundi, Namuba, Kassuku, Keyyu, Hoey, Prödohl, Stothard and Trudgett2008) and this lymnaeid is known as the main IH of F. hepatica worldwide (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2005). Galba truncutula has been documented in Ethiopia (Eguale et al., Reference Eguale, Tilahun and Medhin2006), South Africa (De Kock et al., Reference De Kock, Wolmarans and Bornman2003), Tanzania (Walker et al., Reference Walker, Makundi, Namuba, Kassuku, Keyyu, Hoey, Prödohl, Stothard and Trudgett2008) and Uganda (Howell et al., Reference Howell, Mugisha and Davies2012). According to Howell et al. (Reference Howell, Mugisha and Davies2012), the presence of this lymnaeid species within the Mount Elgon National park of Uganda, where the presence of F. hepatica has not been reported, raises concerns of transmission of F. hepatica should it be introduced in the area. Although F. hepatica has been reported in cattle and wildlife in Zimbabwe (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015), the IH of this species in Zimbabwe is yet to be determined. Other than F. hepatica and F. gigantica, F. tragelaphi has been reported in cattle in Zimbabwe (Mukaratirwa & Brand, Reference Mukaratirwa and Brand1999); however, the IH of this species is yet to be identified.
The geographical distribution of F. hepatica and F. gigantica have been observed to be influenced by different altitudes, associated with the existence of the ecological conditions conducive for their IHs. Fasciola hepatica has been observed to be predominant in cold (temperate) regions, with an altitude of approximately 1800 m above sea level in Ethiopia (Kedir et al., Reference Kedir, Deressa and Tigre2012; Assefa et al., Reference Assefa, Assefa, Beyene and Desiss2015), 3000 m in Kiulo Plateau in Tanzania (Walker et al., Reference Walker, Makundi, Namuba, Kassuku, Keyyu, Hoey, Prödohl, Stothard and Trudgett2008) and an altitude above 3500 m in Mount Elgon of Uganda (Stensgaard et al., Reference Stensgaard, Jørgensen, Kabatereine, Rahbek and Kristenses2006). These altitudes are not conducive for the survival of R. natalensis, the IH of F. gigantica. Fasciola gigantica is commonly found in areas with a lower altitude. In Ethiopia, this species is found in areas with an altitude below 1200 m above sea level (Kedir et al., Reference Kedir, Deressa and Tigre2012). In Uganda, F. gigantica was found in cattle between altitudes of 1000 m and 1500 m, declining concurrently with the growing scarcity of the population of the IH, R. natalensis, ceasing at an altitude of 1800 m (Howell et al., Reference Howell, Mugisha and Davies2012). Both Fasciola species have been observed to overlap in South Africa (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015) and Ethiopia, where mixed infection of both species have been found in cattle (Abebe et al., Reference Abebe, Abdunna, Berhane, Megersa and Regassa2010; Kedir et al., Reference Kedir, Deressa and Tigre2012; Regassa et al., Reference Regassa, Woldemariam, Damissie, Moje, Ayana and Abunna2012; Aregay et al., Reference Aregay, Beleke, Ferede and Hailemelekot2013; Moje et al., Reference Moje, Mathewos, Desissa and Regassa2015; Amsalu, Reference Amsalu2017; Betebo, Reference Betebo2017; Eshetu et al., Reference Eshetu, Thomas, Awukew, Goa and Butako2017). In South Africa, both Fasciola species have been reported to overlap in Mpumalanga and KwaZulu-Natal (Mucheka et al., Reference Mucheka, Lamb, Pfukenyi and Mukaratirwa2015), where the invasive species P. columella exists (De Kock & Wolmarans, Reference De Kock and Wolmarans1998; Wolmarans & De Kock, Reference Wolmarans and De Kock2006; De Kock & Wolmarans, Reference De Kock and Wolmarans2008; Perissinotto et al., Reference Perissinotto, Miranda, Raw and Peer2014). According to Kedir et al. (Reference Kedir, Deressa and Tigre2012), the overlap and co-infection of the two Fasciola species is common in areas with an altitude range of 1200–1800 m (Kedir et al., Reference Kedir, Deressa and Tigre2012).
Conclusion
In conclusion, this review has revealed that both F. hepatica and F. gigantica are present in East and Southern Africa. Furthermore, both species mainly infect cattle, followed by small ruminants (sheep and goats) and, to a lesser extent, wildlife. Both Fasciola species utilize different lymnaeid species with different geographical distributions, influenced by their ecological requirements and the environmental conditions. Fasciola gigantica utilizes R. natalensis as the IH, and this species is mainly found in areas with lower altitudes, while F. hepatica uses G. truncatula as the IH and is found in areas with high altitudes and lower temperatures. In South Africa and Ethiopia, F. hepatica and its IH, G. truncatula, are present, whereas in Zimbabwe, only a single case of F. hepatica was reported and the IH of this species has not been reported. Galba truncatula has been reported in Mount Elgon of Uganda, where F. hepatica has apparently not been reported, and the presence of this species raises concerns of potential rapid transmission of fasciolosis due to F. hepatica should the species be introduced into the area. Fasciola gigantica transmitted by R. natalensis was observed to be more prevalent and widespread in all reviewed countries, save South Africa and Ethiopia, where F. hepatica was reported to be the more prevalent species. Apart from R. natalensis and G. truncatula being reported as the IH species of F. gigantica and F. hepatica, respectively, other species have also been identified in South Africa, Namibia and Botswana, although their role in the transmission of Fasciola species has not been proven. Radix auricularia has been identified in Botswana and South Africa, R. rubiginosa in South Africa and P. columella in South Africa and Namibia, where it has been observed to share habitats with R. natalensis. Their role in the transmission of Fasciola spp. in Eastern and Southern Africa is yet to be determined.
Author ORCIDs
M.P. Malatji, 0000-0002-6188-4204
Acknowledgement
The authors would like to acknowledge the University of KwaZulu-Natal library for assisting with the full-text re-prints of some of the articles.
Financial support
This study was supported by the National Research Foundation (NRF) of South Africa and incentive funds awarded to Professor Samson Mukaratirwa.
Conflicts of interest
None.







