INTRODUCTION
The application of real-time PCR (Klein, Reference Klein2002) in molecular diagnostics has boosted the use of nucleic acid-based detection methods. There is a large variety in the chemistry used for real-time detection of DNA amplification although Taqman® or hydrolysis probes are most commonly used, with the paper by Espy et al. as a good introductory text to these technologies (Espy et al. Reference Espy, Uhl, Sloan, Buckwalter, Jones, Vetter, Yao, Wengenack, Cockerill and Smith2006). Typically, amplification of the specific target is measured real-time during the thermal cycling process making post-PCR processing unnecessary, i.e. gel electrophoresis of amplicons. This closed-tube format of real-time PCR significantly reduces the risk of contamination but also reduces labour time, which in a busy diagnostic laboratory allows for task reallocation(s). Moreover, using different fluorescent molecules makes it possible to combine the detection of different targets which have amplicon sizes of similar lengths and therefore similar amplification efficiency, into one multiplex assay, co-detecting the presence or absence of several pathogens. This can also reduce costs of reagent allowing more wide-scale screening within existing budgets. Additionally, real-time PCR gives a (semi)-quantitative indication of the DNA representing the parasite load in the sample. In parasitology, the importance of power of application of DNA-based methods became apparent in the 1990s with the redescription of the potential invasive Entamoeba histolytica and the non-pathogenic Entamoeba dispar (Diamond and Clark, Reference Diamond and Clark1993). This urged the use of alternative diagnostic methods as the two species are morphologically indistinguishable and microscopy alone could no longer provide the answer for appropriate diagnosis and decision for treatment. From that time, nucleic acid-based detection methods were slowly introduced into the clinical laboratory for differentiation and confirmation in cases where microscopy was lacking specificity, as in the differentiation of E. histolytica/E. dispar. Other applications included those cases in which microscopy was extremely time-consuming or difficult; for example, in the detection of microsporidia (Franzen and Müller, Reference Franzen and Müller1999). These assays proved to be extremely sensitive and specific as compared with microscopy and when real-time PCR was introduced the idea of using multiplex real-time PCR for the simultaneous detection of various pathogens as first-line diagnosis gradually began to take shape (Verweij et al. Reference Verweij, Blange, Templeton, Schinkel, Brienen, van Rooyen, van Lieshout and Polderman2004). Since that time numerous multiplex real-time PCRs have been described and used in different settings (Table 1). Currently, laboratories that are starting to use molecular diagnostics for the diagnosis of parasitic infections may be overwhelmed at first by the large variety of assays and targets to choose from. This can lead to confusion as to how to incorporate molecular diagnostics within the algorithm of applying diagnostic techniques into their daily routine and weekly timetable. It is my intention that this paper will provide some guidance on answering these questions and overcoming hurdles to the introduction and adoption of molecular diagnostic methods in the clinical laboratory.
Table 1. Examples of real-time PCRs for the detection of intestinal parasitic infections

IS MOLECULAR DETECTION GOOD ENOUGH?
Over the last decade, the application of (multiplex) real-time PCR for the detection of intestinal parasitic infections has been validated against microscopy in a variety of different settings (ten Hove et al. Reference ten Hove, Schuurman, Kooistra, Moller, van Lieshout and Verweij2007, Reference ten Hove, van Esbroeck, Vervoort, van den Ende, van Lieshout and Verweij2009; Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009; Calderaro et al. Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzi2010a, Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzib; de Boer et al. Reference de Boer, Ott, Kesztyüs and Kooistra-Smid2010; Stark et al. Reference Stark, Al-Qassab, Barratt, Stanley, Roberts, Marriott, Harkness and Ellis2011; Basuni et al. Reference Basuni, Mohamed, Ahmad, Zakaria and Noordin2012; McAuliffe et al. Reference McAuliffe, Anderson, Stevens, Adams, Coleman, Mahagamasekera, Young, Henderson, Hofmann, Jennings and Murdoch2013; Mejia et al. Reference Mejia, Vicuna, Broncano, Sandoval, Vaca, Chico, Cooper and Nutman2013; Nazeer et al. Reference Nazeer, El, von Thien, El-Sibaei, Abdel-Hamid, Tawfik and Tannich2013). These studies have clearly shown that molecular diagnostics outperforms microscopy in the detection rate for the targets chosen. In a Dutch laboratory receiving stool samples from patients presenting with gastrointestinal complaints to the general practitioner the detection rate for Giardia lamblia increased from 5·7% to 9·3% comparing microscopy and real-time PCR, respectively (ten Hove et al. Reference ten Hove, Schuurman, Kooistra, Moller, van Lieshout and Verweij2007). Specific staining for Cryptosporidium was performed on request only and therefore not performed during the study period, whereas it was detected in 5% of the cases in the total study population and in more than 20% of the children under the age of 5. In the same laboratory, an increased detection rate of G. lamblia infections was found after the introduction of a routinely used molecular screening approach in combination with bacterial gastrointestinal pathogens (de Boer et al. Reference de Boer, Ott, Kesztyüs and Kooistra-Smid2010). In another study in the Netherlands, comparing microscopy performed on two sodium-acid-formalin (SAF) preserved samples and one non-preserved stool sample, G. lamblia was detected in 7·3% of cases as compared with 11·1% using real-time PCR (Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009). In this study, Dientamoeba fragilis real-time PCR was included as well, showing almost double the number of detected cases as compared with microscopy performed on the two SAF preserved samples, 30·7 and 17·4% respectively. Similar findings on the performance of D. fragilis real-time PCR as compared with microscopy and culture have been reported by a laboratory in Italy (Calderaro et al. Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzi2010a, Reference Calderaro, Gorrini, Montecchini, Peruzzi, Piccolo, Rossi, Gargiulo, Manca, Dettori and Chezzib). Increased detection rates using multiplex real-time PCR for the detection of G. lamblia, Cryptosporidium and Strongyloides stercoralis and excellent specificity differentiating E. histolytica from E. dispar were also shown in travellers when comparing the results of expert microscopy and molecular diagnostics (ten Hove et al. Reference ten Hove, van Esbroeck, Vervoort, van den Ende, van Lieshout and Verweij2009). The higher sensitivity of S. stercoralis PCR in this study could be explained by failure of Baermann and culture methods through the death of S. stercoralis larvae during transport. In rural non-industrialized settings, differences between detection rates obtained by microscopy compared with PCR can be more extreme; in Ecuador, Mejia et al. found 5·8% G. lamblia with microscopy compared with 31·5% with real-time PCR (Mejia et al. Reference Mejia, Vicuna, Broncano, Sandoval, Vaca, Chico, Cooper and Nutman2013). Molecular diagnostics also has found its way into veterinary medicine; a neat example of crossover into zoonotic disease was illustrated in the application of molecular techniques to confirm intestinal schistosomiasis in chimpanzees on Ngamba Island, Uganda (Standley et al. Reference Standley, Mugisha, Verweij, Adriko, Arinaitwe, Rowell, Atuhaire, Betson, Hobbs, van Tulleken, Kane, van Lieshout, Ajarova, Kabatereine and Stothard2011).
IS MOLECULAR DETECTION TOO GOOD TO BE TRUE?
The higher detection rates found using molecular diagnostics as compared with conventional methods are sometimes looked upon with some scepticism; are these additional positives in fact false positives? Reasons for the development of new diagnostic methods will usually be that the old methods are unsatisfactory regarding sensitivity, specificity, ease of use and so forth. Using well-defined controls in the initial validation will probably not lead to many problems regarding specificity and sensitivity when new diagnostic methods are compared with the old (gold standard) methods but this highlights the need for reference biological databanks to compare against as new assays come online. Thereafter, however, when newly developed more sensitive tools are compared with the old (gold standard) method in the target population it is often difficult to define true positive and true negative samples resulting in a low specificity for the new test. To overcome these problems usually new gold standards combining different tests or statistical methods to estimate the performance of new diagnostics without a gold standard are being used.
With the wealth of information of genomic data, in the development of nucleic acid-based diagnostic tests, sensitivity and especially specificity are assured in the design and in silico testing of the primers and probes used. Therefore, knowledge of inter-specific and intra-specific genetic diversity among the organisms targeted, and concerns regarding the accuracy and limitation of the data available are indispensable in the development of molecular diagnostics (Stensvold et al. Reference Stensvold, Lebbad and Verweij2011a). Moreover, unknown intra-specific variation should also be considered when samples are analysed from ‘new’ geographical areas. Additionally the specificity is tested against a challenging panel of well-defined controls. As mentioned, defining true positive and true negative test results when new (molecular) diagnostic methods are applied within a target population is often difficult and results of the technical validation have to be known to the reader.
Moreover, in many studies, additional data on the specificity are somewhat hidden. For example, in most studies, microscopy of only one sample is compared with PCR performed on one sample. However, the gold standard for microscopy is repeated (three times) examination of consecutive stool samples which may already explain the differential sensitivities. Unfortunately, such data are only documented rarely and in some studies given as examples only (Verweij et al. Reference Verweij, Schinkel, Laeijendecker, van Rooyen, van Lieshout and Polderman2003c, Reference Verweij, Blange, Templeton, Schinkel, Brienen, van Rooyen, van Lieshout and Polderman2004; Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009). In general, for most intestinal parasitic infections PCR performed on one sample appears to be at least as sensitive as microscopy performed on three samples (van Mens et al. Reference van Mens, Aryeetey, Yazdanbakhsh, van Lieshout, Boakye and Verweij2013). Or maybe better, PCR on one sample provides a true positive or negative test result with the same confidence interval as obtained by microscopy of three samples. This implies that when PCR is used in some cases pathogens remain undetected in a first sample and will only be detected in a second or even a third sample.
The lower DNA load, reflected as higher Ct-values, found in microscopy negative samples compared with the higher DNA load found in microscopy-positive samples shows that the higher detection rates found by PCR are due to a higher sensitivity rather than lower specificity of molecular diagnostics (ten Hove et al. Reference ten Hove, Schuurman, Kooistra, Moller, van Lieshout and Verweij2007; Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009; de Boer et al. Reference de Boer, Ott, Kesztyüs and Kooistra-Smid2010). Another piece of presumptive evidence can be found in the fact that using the same PCR in a different population gives high detection rates in one study group and is reaching towards almost negative in another group. For example, high detection rates of Cryptosporidium were found in a study performed in late summer and as expected low detection rates were found in a study performed in winter using real-time PCR (ten Hove et al. Reference ten Hove, Schuurman, Kooistra, Moller, van Lieshout and Verweij2007; Bruijnesteijn van Coppenraet et al. Reference Bruijnesteijn van Coppenraet, Wallinga, Ruijs, Bruins and Verweij2009). Similar results can even be found within one study if detection rates are analysed in different age groups, showing for example a similar age distribution of G. lamblia infections with higher numbers detected with PCR as compared with microscopy but leaving the general picture intact, showing high prevalence in children and (grand) parents age groups and low prevalence in young adults (ten Hove et al. Reference ten Hove, Schuurman, Kooistra, Moller, van Lieshout and Verweij2007).
Although the microscopic detection of, for example, one Giardia cyst in a third sample is taken for granted as a fact beyond doubt there is some debate on the relevance of a positive PCR result found with a high (>35) Ct-value (i.e. a low DNA load) and it is sometimes suggested to regard such samples as being negative. Technically, this is incorrect; a Ct-value measured as a consequence of an exponential curve is the result of amplification of the target DNA. True, at the edge of the detection limit of any assay, including microscopy, non-reproducible results will be obtained and as discussed above even with PCR it remains possible that a pathogen stays undetected in a first sample and will only be detected in a consecutive sample. Therefore one could consider requesting an additional sample in cases in which a low load of pathogenic DNA is detected and there is doubt on its clinical significance. The question remains, however, would a patient or medical practitioner be willing to wait with treatment against E. histolytica even if detected at very low DNA load and be willing to take the risk of a liver abscess when regarding a high Ct-value for E. histolytica as being negative?
WILL MOLECULAR DETECTION BE CONFOUNDED BY ‘dead’ dna?
Since DNA-based methods for the detection of intestinal parasitic infections are now being introduced in daily clinical practice one of the key questions is, how long can DNA be detected in stool after successful treatment? It is a fact that DNA can still be detected after the organism has died; however, bearing in mind that the transit time of the intestinal contents and regeneration time of the intestinal epithelium is within days it is expected that parasitic remains are rapidly cleared from the gut. Although cases have been described mentioning that PCR turns negative after successful treatment, to date there are very few larger follow-up studies using PCR to monitor the effect of treatment. Enterocytozoon bieneusi DNA became undetectable in stool in two patients at day 7 and day 4 after treatment with fumagillin, respectively (Lanternier et al. Reference Lanternier, Boutboul, Menotti, Chandesris, Sarfati, Mamzer Bruneel, Calmus, Mechaï, Viard, Lecuit, Bougnoux and Lortholary2009). In a cohort of 125 children in Ecuador, Ascaris lumbricoides DNA could not be detected 21 days after albendazole/ivermectin treatment in those children that were PCR positive before treatment (Mejia et al. Reference Mejia, Vicuna, Broncano, Sandoval, Vaca, Chico, Cooper and Nutman2013). In the same study, approximately 35% of the subjects remained positive for G. lamblia after albendazole/ivermectin therapy, which might be due to the non-optimal treatment regime and the high degree of infection in this region. In a recent study in the Netherlands, G. lamblia DNA could not be detected one week after metronidazole treatment in children and adults that were diagnosed with giardiasis using real-time PCR (Bijllaard et al. Reference van den Bijllaardt, Overdevest, Buiting and Verweij2014). Rapid disappearance of DNA is expected in other gut infections that are limited to the lumen as well. It is postulated that, for example, remaining Schistosoma eggs in the gut wall could cause ‘leakage’ of parasitic DNA for longer periods.
AS MICROSCOPY COVERS ALL, WHAT WILL BE MISSED?
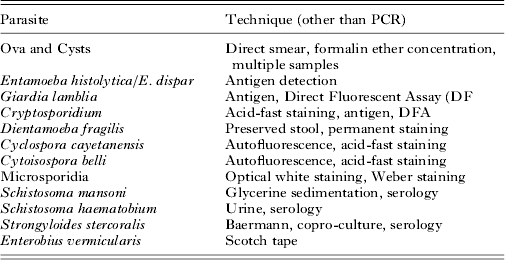
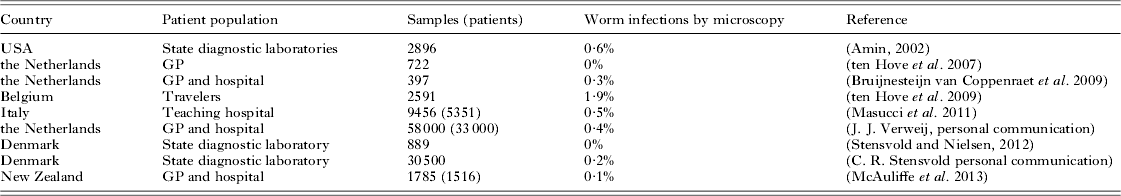
One of the most discussed tensions between microscopy and molecular diagnostics is the overall capacity of microscopy to detect ‘all’ parasites and the high target specificity of PCR, i.e. you'll find just the thing you are looking for and nothing more. The latter I cannot deny, however, in microscopy a whole range of species-specific additional staining, culture, and concentration techniques are used to achieve ample sensitivity and specificity (Table 2). In daily practice, these techniques will not be performed on all samples that are submitted for parasitology but choices will be made with an algorithm based on travel history (including adoption), eosinophilia, increased IgE level, urticaria, immune status, and so forth. While it is true that in rare cases microscopy can provide an accidental discovery of an unexpected parasite, it is also true that, whether we like it or not, microscopy expertise is waning. Moreover, in most clinical settings in industrialized countries the number of cases with intestinal parasites other than G. lamblia and Cryptosporidium will usually be very limited (Table 3). Therefore, the same algorithm used to decide whether or not to perform additional conventional techniques besides the general ova and cysts microscopy can be used in the decision to apply additional conventional or DNA-based techniques specific for the diagnostic question in a particular patient or patient group in addition to a multiplex PCR for the most common intestinal parasites.
Table 2. Examples of different species-specific additional techniques

Table 3. Helminth infections in a non-endemic setting

FUTURE DIRECTIONS
One of the most appealing benefits of molecular diagnostics is the possibility of automation providing a short sample-to-answer time with limited hands-on time. Furthermore, bacterial, viral and parasitic targets can be detected using one overall technique combined in setting-specific panels. Such an automated overall screening approach will result in a considerable cost reduction as compared with conventional methods (Fig. 1). Alternative semi- and fully automated high-throughput platforms allowing multiplexing of up to tens of different targets have been developed and will find their way into commercially available assays that are FDA and/or CE approved (Claas et al. Reference Claas, Burnham, Mazzulli, Templeton and Topin2013; Goldfarb et al. Reference Goldfarb, Dixon, Moldovan, Barrowman, Mattison, Zentner, Baikie, Bidawid, Chan and Slinger2013; Liu et al. Reference Liu, Gratz, Amour, Kibiki, Becker, Janaki, Verweij, Taniuchi, Sobuz, Haque, Haverstick and Houpt2013; Mengelle et al. Reference Mengelle, Mansuy, Prere, Grouteau, Claudet, Kamar, Huynh, Plat, Benard, Marty, Valentin, Berry and Izopet2013; Wessels et al. Reference Wessels, Rusman, van Bussel and Claas2013). Platforms that combine nucleic-acid isolation, amplification and detection of gastrointestinal pathogens e.g. Filmarray (Biofire diagnostics, USA) and BDMax (Becton, Dickinson and Company, USA) are in development phase. Although such assays might be appealing for smaller laboratories that are handling small numbers of samples, the cost effectiveness of these low throughput approaches compared with existing high throughput formats needs to determined.

Fig. 1. Flowchart of automated nucleic acid isolation, PCR set-up, and PCR integrated through middle-ware with the laboratory information management system (LIMS). Published with permission by the American Society for Microbiology. Image created by Patrick Lane. Copyright 2014.
The use of broad-range 16S rDNA PCR has been established for identification of cultured bacterial isolates but is also increasingly used as a detection method for difficult or non-culturable bacteria in potential sterile clinical samples (Sontakke et al. Reference Sontakke, Cadenas, Maggi, Diniz and Breitschwerdt2009). An extended-range PCR including ITS1, ITS2 regions and 28S eukaryotic ribosomal subunit primers for the amplification of fungal and parasitic DNA followed by sequencing detected Taenia solium in brain biopsy tissue of two patients with solitary central nervous system lesions (Harrington et al. Reference Harrington, Creutzfeldt, Sengupta, Hoogestraat, Zunt and Cookson2009). Using the same approach, Cystoisopora belli was identified in ileal biopsy material from a patient with AIDS (Murphy et al. Reference Murphy, Hoogestraat, Sengupta, Prentice, Chakrapani and Cookson2011). This type of development makes it possible to take advantage of the high sensitivity of DNA amplification without knowing the specific target beforehand. Using traditional Sanger sequencing this approach could give confusing overlaid sequence profiles resultant in cases of mixed infections. However, the increasing user friendliness and accessibility of next-generation sequencing will also allow the use of such a broad-range approach in samples with multiple organisms.
Another interesting development is the application of blood-based real-time PCR for the diagnosis of (acute) schistosomiasis. In a European-wide multicentre study the sensitivity of PCR was 92% at initial presentation of patients with acute schistosomiasis, in contrast with serology and microscopy with a sensitivity of 70% and 24%, respectively (Wichmann et al. Reference Wichmann, Poppert, Von Thien, Clerinx, Dieckmann, Jensenius, Parola, Richter, Schunk, Stich, Zanger, Burchard and Tannich2013). Another successful example of a non-faecal PCR approach was shown in the diagnosis of human neurocysticercosis in which T. solium-specific PCR performed on DNA isolated from cerebrospinal fluid showed a sensitivity of 95·9% compared with a sensitivity of 53·7%, 81% and 90·1% using Antigen-ELISA, Antibody-immunoblot and Ab-ELISA respectively (Michelet et al. Reference Michelet, Fleury, Sciutto, Kendjo, Fragoso, Paris and Bouteille2011).
While this paper focuses more on the application of molecular diagnostics in the clinical laboratory in industrialized countries, it is worth mentioning that this kind of equipment is available in an increasing number of research facilities in low- and moderate-income countries. Multiplex DNA-based techniques can and are being used in epidemiological studies and for the monitoring of integrated control programmes (Basuni et al. Reference Basuni, Mohamed, Ahmad, Zakaria and Noordin2012; Arndt et al. Reference Arndt, John-Stewart, Richardson, Singa, van Lieshout, Verweij, Sangare, Mbogo, Naulikha and Walson2013; Wiria et al. Reference Wiria, Hamid, Wammes, Kaisar, May, Prasetyani, Wahyuni, Djuardi, Ariawan, Wibowo, Lell, Sauerwein, Brice, Sutanto, van Lieshout, de Craen, van Ree, Verweij, Tsonaka, Houwing-Duistermaat, Luty, Sartono, Supali and Yazdanbakhsh2013a, Reference Wiria, Wammes, Hamid, Dekkers, Prasetyani, May, Kaisar, Verweij, Tamsma, Partono, Sartono, Supali, Yazdanbakhsh and Smitb).
CONCLUSIONS
The combination of bacterial, viral and parasitic targets makes molecular diagnostics an achievable and cost-effective approach in a clinical diagnostic laboratory. DNA-based methods provide highly sensitive and specific detection of intestinal parasitic infections. The use of additional (molecular) diagnostics is driven through an algorithm based on individual or population patient characteristics. Quality assessment schemes are essential to assure a high diagnostic accuracy among the variety of protocols used in different laboratories. Such schemes for the molecular diagnosis of a range of diarrhoea-causing pathogens have been started (http://www.qcmd.org and http://www.skml.nl) and will be expanded in future. Alternative platforms with assays for simultaneous detection of multiple targets within one sample have been developed and are in the process of commercialization. The application of broad-range PCRs in combination with sequencing or next-generation sequencing offer an interesting non-target specific approach. Non-faecal PCRs, for example on cerebral spinal fluid and serum have shown high sensitivity and specificity for the diagnosis of cysticercosis and schistosomiasis respectively.
ACKNOWLEDGEMENTS
Thanks to the BSP for the invitation to write this paper and thanks to Professor Russell Stothard and Dr Leslie Chappell for editorial suggestions that greatly improved this manuscript.