Intensive care unit (ICU) patients are at high risk for developing healthcare-associated infections, and ventilator-associated pneumonia (VAP) is the most common of these infections. 1 VAP is defined as pneumonia occurring 48 h following endotracheal intubation with the ventilator being in place the date of event or the day before. 2 The incidence of VAP was reported to be as high as 42%,Reference Ego, Preiser and Vincent 3 – Reference Elliott, Elliott and Burrell 5 but estimates vary substantially depending on different diagnostic scoring systems.Reference Ego, Preiser and Vincent 3 , Reference Wallace, Alexander, Spencer, Naisbitt, Moore and McGrath 6 Also, VAP is associated with substantial morbidity, an increase in mortality, and excess costs.Reference Safdar, Dezfulian, Collard and Saint 7 , Reference Branch-Elliman, Wright and Howell 8
Multiple international guidelines regarding the prevention of VAP are available. 9 – 13 Most hospitals implement VAP prevention elements as part of a prevention bundle, but the components of such bundles may vary from hospital to hospital.Reference Klompas 14 Unfortunately, sufficient evidence about the efficacy of single bundle components in preventing VAP is lacking.Reference Klompas 14 Some prevention measures, such as oral care with chlorhexidine, have recently come under suspicion as being potentially harmful.Reference Klompas, Li, Kleinman, Szumita and Massaro 15 , Reference Price, MacLennan, Glen and Su 16 Nevertheless, the potential to decrease VAP rates using VAP prevention bundles has been demonstrated by many authors,Reference Okgun Alcan, Demir Korkmaz and Uyar 17 – Reference Khan, Al-Dorzi and Al-Attas 22 and the preventable proportion of VAP was estimated to be 52%–55%.Reference Umscheid, Mitchell, Doshi, Agarwal, Williams and Brennan 23 , Reference Lambert, Silversmit and Savey 24
Effective implementation is as important as choosing the right bundle components.Reference Klompas 14 Adherence to and knowledge about VAP prevention measures were shown to be poor in several studies.Reference Daniel, Booth, Ellis, Maher and Longmate 19 , Reference Aloush 25 , Reference Yeganeh, Yekta, Farmanbar, Khalili and Atrkar Roushan 26 Adherence can be raised through different implementation programs.Reference Daniel, Booth, Ellis, Maher and Longmate 19 , Reference McLean, Jensen, Schroeder, Gibney and Skjodt 27 A systematic review identified education (eg, training sessions or development of concise summaries of the evidence) and execution strategies (eg, standardization of care processes and building redundancies into routine care) as strategies to enhance the adoption of VAP prevention measures.Reference Goutier, Holzmueller, Edwards, Klompas, Speck and Berenholtz 28 These authors also mentioned multidisciplinary teamwork, involvement of champions, and networking among peers as engagement strategies.Reference Goutier, Holzmueller, Edwards, Klompas, Speck and Berenholtz 28 Another systematic review showed that improvement in adherence to preventive measures occurred once audit and feedback of adherence rates with or without reminder systems were introduced in addition to organizational change efforts and education of frontline healthcare practitioners (HCPs).Reference Mauger, Marbella, Pines, Chopra, Black and Aronson 29 Generally, it is well accepted that theory-based implementation strategies are more effective in achieving sustained behavior changes.Reference Michie, Johnston, Francis, Hardeman and Eccles 30
In our hospital, a 9-element VAP bundle was designed in 2011 by an interprofessional working group. Implementation of the bundle began in 2013. In February 2015, the hospital infection prevention and control (IPC) team measured adherence to selected bundle elements and found suboptimal overall adherence rates. Therefore, the IPC team chose a mixed-methods approach to assess adherence to VAP prevention measures and identify barriers to and facilitators of protocol adherence.
Methods
Setting
The study took place at the University Hospital Zurich (UHZ), Zurich, Switzerland, a 900-bed tertiary-care teaching hospital featuring all medical specialties except pediatrics and orthopedics. In total, the study included 64 beds in 6 ICUS: medical ICU, general thoracic and transplant surgery ICU, trauma ICU, burn ICU, cardiac surgery ICU, and neurosurgery ICU.
The University Hospital Zurich (UHZ) VAP bundle
The UHZ VAP bundle was created by an interprofessional group comprising IPC team members, ICU nurses and physicians, and anesthesiologists. It included 9 elements: (1) continuous application of a sedation and weaning protocols with daily sedation interruptions; (2) head of the bed elevation of ≥30°; (3) oral decontamination with chlorhexidine mouth wash twice daily; (4) the use of endotracheal tubes with continuous subglottic secretion drainage; (5) hand hygiene according to the WHO Five Moments conceptReference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 31 ; (6) use of noninvasive ventilation (NIV) whenever feasible; (7) periodic changes of ventilator tubing and filters biweekly; (8) use of closed suction systems; and (9) daily evaluation of stress ulcer prophylaxis to limit its use. The bundle was enacted by the medical director in 2011, and the standard operating procedure (SOP) was made accessible via the hospital’s intranet. In 2013, the UHZ VAP bundle was formally implemented under the lead of ICU nurses by providing education and practical training. In June 2016, an ‘action month,’ 5 of the 9 bundle elements (ie, head of bed elevation, oral care with chlorhexidine, hand hygiene, NIV, closed suction system) were again addressed by providing education, practical training, contests, and posters and stickers as reminders. The elements were chosen by the ICU teams based on feedback adherence rates and the anticipated need for training.
Adherence measurements
Adherence to VAP prevention elements was assessed by overt, nonparticipatory observations in February 2015 (measurement 1), in August 2015 (measurement 2), in July 2016 (measurement 3) and continuously from July to September 2017 (measurement 4). Investigators visited all ICUs once or twice daily during weekdays between 8 am and 6 pm. We evaluated 4 bundle elements whose execution relied on HCP decision making rather than on standardized workflows. (1) Daily sedation interruption was assessed by reviewing the handwritten tracking sheet and by oral confirmation by the responsible nurse. We excluded patients with severe hemodynamic shock, a subset of specified intracranial injuries or neurologic diseases that challenge cerebral perfusion, extracorporeal membrane oxygenation, neuromuscular blocking agents, and therapeutic hypothermia, as well as those in need of a kinetic bed system. (2) Head of bed elevation was measured using a Smartphone application for angle measurement (Mammut Safety APP, version 1.0, Mammut Sports Group AG, Seon, Switzerland). We excluded patients with hemodynamic instability, instable spinal or pelvic fracture, and specific intracranial injuries or neurologic diseases that impair cerebral perfusion pressure. (3) Twice-daily oral care with chlorhexidine was assessed by review of the electronic patient record. (4) Continuous subglottic secretion drainage was assessed by direct observations. We excluded patients with endotracheal or tracheostomy tubes not featuring a suction port. In measurement 2, sedation interruption and subglottic drainage were not assessed. In measurement 4, subglottic drainage was not assessed. Good, intermediate, and poor adherence were defined as ≥80%, ≥50%–80%, and <50% adherence, respectively.
Theoretical framework
The behavior change wheel (BCW) is a theoretical framework that incorporates several existing behavior-change frameworks into a behavioral system (COM-B) composed of 3 ‘sources of behavior’: capability, opportunity, and motivation.Reference Michie, van Stralen and West 32 The BCW was chosen to guide the analysis in this study because it is an overarching framework that considers the influence of context on individual behavior. A further advantage of the BCW is that it links barriers and facilitators identified within the COM-B system to proposed interventions that may be effective in addressing deficits (Table 1).
Table 1 Links Between Components of the ‘COM-B’ Model of Behavior and Intervention FunctionsFootnote a

a Table reproduced with permission from Michie et al.Reference Michie, van Stralen and West 32
b Physical capability can be achieved through physical skill development, which is the focus of training, or potentially through enabling interventions such as medication, surgery or prostheses.
c Psychological capability can be achieved through imparting knowledge or understanding; training emotional, cognitive, and/or behavioral skills; or through enabling interventions such as medication.
d Reflective motivation can be achieved through increasing knowledge and understanding or through eliciting positive (or negative) feelings about behavioral target.
e Automatic motivation can be achieved through associative learning that elicits positive (or negative) feelings and impulses and counterimpulses relating to the behavioral target, imitative learning, or habit formation that directly influences automatic motivational processes (eg, via medication).
f Physical and social opportunity can be achieved through environmental change.
Focus group interviews—Data collection and analysis
The 6 focus group interviews, 1 per ICU, were conducted between May 4 and June 5, 2015. We used a purposeful criterion sampling approach,Reference Palinkas, Horwitz, Green, Wisdom, Duan and Hoagwood 33 and we sought to include both nurses and physicians from each of the 6 ICUs to gather a broad range of experiences related to VAP prevention. Beyond these criteria, participants included convenience samples of ICU nurses and physicians on duty who were available when the group interview took place. They represent a subset of the observed HCP. All semi-structured interviews were conducted by the same IPC nurse (M.T.M.). The interview guide is shown in Table 2. Written informed consent was obtained from all interviewees. Interviews were held in Swiss-German, audiotaped, and transcribed verbatim. Following a grounded theory approach, initial data analysis was conducted inductively by 2 investigators (A.W. and M.T.M.) to summarize interview content and to inform the ongoing data collection.Reference Strauss and Corbin 34 Following data collection, the same 2 investigators (A.W. and M.T.M.) deductively coded all identified barriers and facilitators according to the BCW behavioral system components: capability, opportunity, or motivation.Reference Michie, van Stralen and West 32 Any discrepancies in coding were resolved by a health psychologist (L.C.). Using the same approach, participant ideas for better bundle implementation approaches were deductively coded according to the intervention functions of the BCW with its 9 components: education, persuasion, incentivization, coercion, training, restriction, environmental restructuring, modeling, and enablement. These participant suggestions were then compared with the intervention functions proposed by the BCW for addressing identified barriers and facilitators (Table 1).
Table 2 Semi-Structured Interview Guide
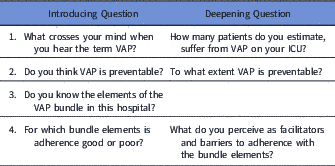
Note. VAP, ventilator-associated pneumonia.
Results
Adherence measurements
Data regarding adherence rates are shown in Table 3. Adherence was poor for head of bed elevation (27%; 95% confidence intervals [CI], 23%–31%) and oral care (41%; 95% CI, 36%–45%), though it was good for daily sedation interruption (81%; 95% CI, 74%–85%) and subglottic suctioning (88%; 95% CI, 83%–92%). A considerable number of patients had a contraindication for head of bed elevation (27%; 95% CI, 23%–31%) and sedation interruption (41%; 95% CI, 36%–46%). Also, 41% (95% CI, 36%–46%) of patients did not have a tube with a suction port for subglottic suctioning.
Table 3 Quantitative Adherence Rates to VAP Prevention Measures

Note. CI, confidence interval; N/A, not applicable.
a “Contraindication” for subglottic suctioning: no tube with suction port.
Focus group interviews
Overall, 42 nurses and 4 physicians participated in the interviews (Table 4); of these, 17 were male (37%). One participant withdrew consent because the interview was audiotaped. The interviews lasted between 35 and 45 minutes. We determined that data saturation was achieved (ie, no new barriers and facilitators were being identifiedReference Saunders, Sim and Kingstone 35 after the fourth focus group), but we continued data collection based on our criterion sampling strategy until all ICUs had been included.
Table 4 Focus Group Interview Participants

Note. ICU, intensive care unit.
Barriers and facilitators for adherence to the VAP bundle according to the BCW sources of behavior
At the center of the BCW framework lie the ‘sources of behavior’, 6 essential components that shape behavior: physical and psychological capability, reflective and automatic motivation, and physical and social opportunity.Reference Michie, van Stralen and West 32 The interviewee’s statements about barriers and facilitators for adherence to the prevention measures were coded according to these components (Table 5). A total of 104 statements were coded: 79 (76%) referred to barriers and 25 (24%) to facilitators. The most commonly coded components were ‘physical opportunity’ with 49% of statements (n=51: 47 barriers and 4 facilitators), followed by ‘reflective motivation’ with 21% (n=22: 9 barriers and 13 facilitators), ‘automatic motivation’ with 12% (n=12: 11 barriers and 1 facilitator), and ‘psychological capability’ with 10% (n=10: 5 barriers and 5 facilitators) of statements, respectively. ‘Social opportunity’ and ‘physical capability’ appeared rarely, in 7% (n=7) and 2% (n=2), respectively.
Table 5 Barriers and Facilitators Identified From the Focus Group Interviews Mapped According to the Behavior Change Wheel Framework
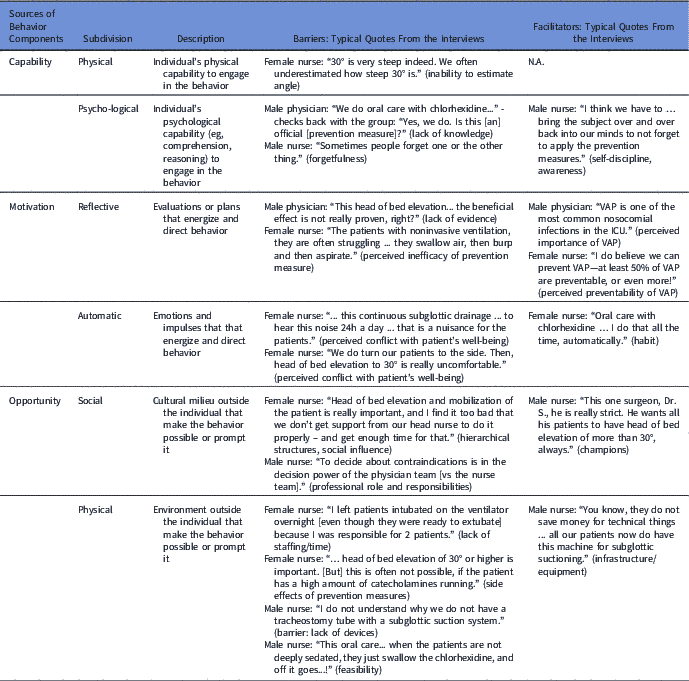
Note. ICU, intensive care unit; VAP, ventilator-associated pneumonia.
Physical and psychological capability
‘Physical capability’, which is defined as the individual’s physical capacity to engage in the activity, was brought up as a barrier once, referring to the inability to estimate the correct angle of head of bed elevation by eye. ‘Psychological capability,’ like self-discipline, was mentioned as a facilitator. Lack of knowledge and forgetfulness were brought up as barriers several times.
Reflective and automatic motivation
Most facilitators were related to the ‘reflective motivation’ BCW component, defined as ‘evaluations and plans that energize and direct behavior.’ Interviewees were aware of the frequency and consequences of VAP and generally considered prevention measures useful to lower VAP rates. Some HCPs, however, mentioned doubts about the effectiveness of certain prevention measures like head of bed elevation and noninvasive ventilation, which may have presented a barrier to adherence. Numerous barriers belonged to the ‘automatic motivation’ component, that is, emotions and impulses that direct behavior. HCPs were concerned about prevention measures affecting the patient’s well-being, such as disturbance by the noise of the subglottic suctioning device, uncomfortable body position due to head of bed elevation, and unpleasant taste of the chlorhexidine mouth wash.
Physical and social opportunity
Most barriers were assigned to the group of ‘physical opportunity’ and, less commonly, ‘social opportunity’—factors that lie outside the individual HCP. Three points emerged as being most important: (1) lack of equipment (eg, tracheal tubes with suction port for subglottic suctioning, chlorhexidine gel instead of mouth wash, beds with appropriate angle measurement devices), (2) lack of adequate staffing or time for patient care, and (3) competing priorities of patients and prevention measure (eg, head of bed elevation not possible due to the increased need for catecholamines or sedation interruption increasing intracranial pressure). Only rarely did HCPs mention the available infrastructure as a facilitator for bundle adherence. For ‘social opportunity,’ or the cultural milieu, champions were brought up as facilitators and lack of hierarchical support was mentioned as barrier for bundle adherence.
Intervention ideas according to the BCW intervention functions
The spontaneously mentioned ideas to improve bundle adherence were provision or improvement of equipment, which we mapped to the ‘environmental restructuring’ component. The introduction of a device to indicate head of bed elevation and tracheal tubes with ports for subglottic suctioning were most often mentioned. Second, alarm systems as reminders were mentioned several times, predominantly in the context of head of bed elevation, which we mapped to the components ‘environmental restructuring’ and ‘HCP enabling.’ Third, introduction of protocols and checklist for the bundle in general and for sedation interruption specifically were brought up, which belong to the components ‘restriction’ and ‘HCP enabling,’ respectively. Table 6 outlines observed adherence measures, self-reported adherence rates, and barriers, facilitators, and intervention ideas according the BCW for every VAP bundle component.
Table 6 Outline of Self-Reported Versus Measured Adherence Rates, Mapped Barriers and Facilitators, and Intervention Opportunities
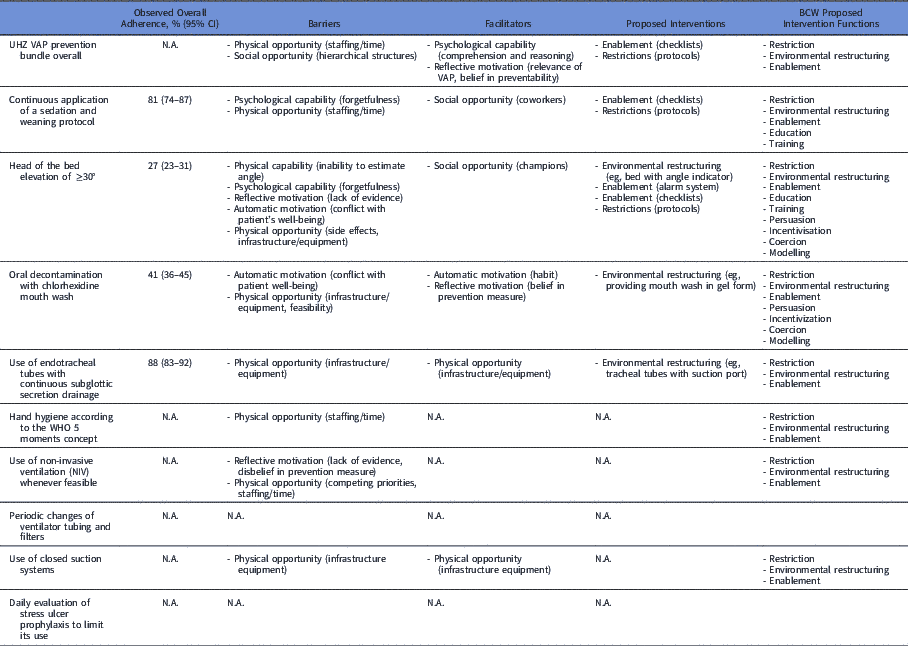
Note. BCW, behavior change wheel; N.A., not applicable. This table summarizes the adherence rates assessed by observation and reported barriers, facilitators, and proposed interventions mapped according to intervention functions of the BCW framework.Reference Michie, van Stralen and West 32
Discussion
This mixed-method study measured adherence to 4 VAP prevention measures and found poor adherence to head of bed elevation and oral care and good adherence to sedation vacation and subglottic suctioning. Corresponding to the BCW ‘sources of behavior,’ facilitators for adherence belonged primarily to the component ‘reflective motivation’: perceived seriousness of VAP and self-efficacy to prevent VAP. Barriers mainly belonged to the BCW component ‘physical capability’: lack of equipment and staffing and side-effects of prevention measures. To improve adherence, both HCPs and the BCW framework suggested 2 main interventions, ‘restructuring the environment’ and ‘enablement of HCP.’
VAP prevention measures were shown to be poorly executed by other investigators: Adherence to head of bed elevation or to daily sedation interruption, for example, was reported to be 25%–35%,Reference Llaurado-Serra, Ulldemolins and Guell-Baro 36 – Reference DuBose, Inaba and Shiflett 39 and 29%–56%,Reference Crunden, Boyce, Woodman and Bray 40 , Reference Mendez, Lazar and Digiovine 41 respectively. In our study, we found good adherence for sedation vacation, but adherence rates may have been overly optimistic because a considerable percentage of patients were judged to have contraindications for this prevention measure and because some of these contraindications might have been relative. On the other hand, the interviews revealed that adherence to oral care with chlorhexidine was low due to poor documentation rather than missing execution. For the other 3 measured bundle elements, self-assessment of interviewees corresponded largely with the measured adherence. For another 3 of the 5 bundle elements not measured (ie, hand hygiene, NIV, and daily evaluation of stress ulcer prophylaxis), adherence was considered improvable by the interview participants (data not shown).
Semi-structured interviews identified 2 main facilitators for bundle adherence, both belonging to the BCW component ‘reflective motivation.’ First, VAP was perceived as a serious and common problem among ICU patients. Many interviewees, especially nurses, estimated VAP incidence at 50% of ventilated patients, corresponding to VAP rates of around 40% of studies applying the Clinical Pulmonary Infection Score (CPIS) criteria.Reference Ego, Preiser and Vincent 3 , Reference Pugin 42 Second, HCPs perceived prevention measures to lower VAP rates by as much as 30%–80%, corresponding to 52% and 55% in scientific reports.Reference Umscheid, Mitchell, Doshi, Agarwal, Williams and Brennan 23 , Reference Lambert, Silversmit and Savey 24 These noticeably accurate estimations might represent important prerequisites for protocol adherence.
Many of the barriers belonged to the BCW component ‘physical capability.’ HCPs were not satisfied with the quality of the equipment or regretted lack of specific devices (eg, tracheal tubes with a suction port) and staffing levels. From the existing literature, the unavailability of resources is a well-known barrier.Reference Ricart, Lorente, Diaz, Kollef and Rello 43 , Reference Rello, Lorente, Bodi, Diaz, Ricart and Kollef 44 On the other hand, many HCPs raised concern over side effects of prevention measures (eg, head of bed elevation leading to increased need for catecholamines, belonging to component ‘physical capability’). Some HCPs were subjectively concerned about the patient’s well-being (eg, perception that head of bed elevation is uncomfortable for the patient, belonging to the component ‘automatic motivation’). These findings have not been described elsewhere in the literature.
The BCW framework offers specific interventions to change behavior by linking sources of behavior to intervention functions (Table 1).Reference Michie, van Stralen and West 32 Notably, the proposals of interviewees matched those found in the BCW (Table 5). To approach the 2 main barrier components ‘physical opportunity’ and ‘automatic motivation,’ the BCW proposes ‘environmental restructuring’ and ‘enablement’; the latter is considered to ‘going beyond education and training and beyond environmental restructuring.’Reference Michie, van Stralen and West 32 This is consistent with our finding that HCPs asked for better equipment, checklists, and alerts. Of special interest, both the interviewees and the BCW proposed almost exclusively technical solutions. The benefit of technical solutions is supported by Cafazzo’s ‘hierarchy of intervention effectiveness.’Reference Cafazzo and St-Cyr 45 This management theory promotes system-focused or technological interventions over interventions that require conscious effort and change of behavior because the latter are notoriously more difficult to implement and sustain.Reference Cafazzo and St-Cyr 45 , Reference McHugh 46 Concretely, our study revealed that the HCPs in our ICUs need (and request) a restructured work environment that provides forcing functions and automated or computerized processes.
Our study has several limitations. While we included all participating ICUs, the individual participants were recruited from a convenience sample of HCPs with an oversampling of nurses, and we cannot fully exclude the possibility that some opinions may have been missed. The quantitative measures were conducted in a pragmatic quality improvement context and included measurement points with small numbers of observations. Observations were not covered and adherence might have been overestimated because individuals modify their behavior when being observed (ie, the Hawthorne effect). Because this was a single-center study, the findings might not be generalizable to other settings. However, it covered 6 self-contained ICUs of different specializations and cultures.
In conclusion, adherence to 2 of 4 assessed prevention measures of our VAP bundle was assessed to be improvable, and barriers for adherence predominantly belonged to external reasons such as lack of adequate equipment or staffing or side effects of prevention measures. Mapping the inductively identified themes against the BCW framework pinpointed the need to ‘restructure the environment’ and to ‘enable HCPs.’ These findings were underpinned by the proposals of the interviewees, who also predominantly advocated for technical solutions to improve their adherence to VAP prevention measures. The BCW-informed mixed-method approach is an effective means for guiding infection prevention efforts. Further research is needed to assess the impact of these interventions on adherence rates.
Acknowledgments
We thank the HCPs of the 6 ICUs at University Hospital Zurich for their participation in the study and the University Hospital Zurich Infection Prevention and Control Team for collecting the adherence data.
Financial support
This study was partially funded by the Swiss National Science Foundation (grant no. 32003B_149474, principal investigator, Hugo Sax). A.W. is supported by the academic career program “Filling the Gap” of the Medical Faculty of the University of Zurich.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.








