Introduction
Parasites manipulate the behaviour of their hosts to increase the probability of transmission to the next host (Holmes & Bethel, Reference Holmes, Bethel, Canning and Wright1972; Moore, Reference Moore2002). The beetle, Tenebrio molitor, becomes infected with Hymenolepis diminuta when it ingests rat faeces containing the eggs of this tapeworm. Thus, the success of this parasite depends upon consumption of the egg stage by the beetle. Recent evidence suggests that this passive type of transmission involves a mechanism that influences host behaviour to benefit the tapeworm (Evans et al., Reference Evans, Wong, Hardy, Currie and Vanderwel1998). Previous studies tested the faecal preference of groups of beetles by allowing them to forage in an arena containing rat faeces from uninfected rats and rat faeces from H. diminuta-infected rats. Starved beetles (Tribolium confusum) of mixed sex preferred infective faeces (Evans et al., Reference Evans, Hardy, Singh, Moodie and Cote1992). When beetles (Tenebrio molitor) of known sex, age and feeding history were tested, starved and fed female beetles preferred infective faeces while starved male beetles preferred infective faeces and fed males preferred uninfective faeces (Pappas et al., Reference Pappas, Marschall, Morrison, Durka and Daniel1995). Another study found more ambiguous results after analysing beetle (T. confusum) movement, concluding that beetle behaviour appeared ‘highly heterogeneous, both among individuals and by the same individual over time’ (Shostak & Smyth, Reference Shostak and Smyth1998). These experiments involved uninfected beetles, but in a natural setting, both infected and uninfected beetles can be found (Rau, Reference Rau1979) and infected beetles may behave differently, especially if there are costs associated with infection. This study assesses the relative importance of infection status on feeding preference by repeating preference experiments using infected individual beetles and groups of beetles (T. molitor) of known sex and age. Additionally, the novel approach of this study provides data to assess infection variance and bias, and challenge infections, which are of epidemiological interest.
Materials and methods
Pre-trial protocol
The ‘OSU Strain’ (Pappas & Leiby, Reference Pappas and Leiby1986) of H. diminuta was maintained in male Sprague–Dawley rats and beetles (T. molitor). Three rats were infected with 30 cysticercoids and maintained on commercial rodent chow and water. Three additional rats, obtained from the same commercial source and of identical age, were maintained under the same conditions as the infected rats to serve as the source of control (uninfective) faeces. Faecal pellets from infected rats were not used prior to 20 days post-infection. Rat cages were checked every 10 min on the morning of the trials, and faecal pellets were collected with forceps to minimize contamination by rat urine. After each trial, the infective faecal pellets were examined to verify the presence of H. diminuta eggs.
Beetles were maintained on wheat bran, and small pieces of potato were added to the cultures on a regular basis. Pupae were removed from the cultures, and the male and female pupae (Bhattacharya et al., Reference Bhattacharya, Ameel and Waldebauer1970) were placed in separate dishes containing wheat bran. Beetles that emerged during a 24-h period were collected, such that a daily cohort of beetles was maintained for both sexes. In previous studies, this method has resulted in groups of male and female beetles that did not differ significantly in weight (Shea, Reference Shea2005).
Individual trials
Fifteen to 17 days before the trial, male and female beetles (in groups of 10) were exposed to a consistent concentration of tapeworm eggs (1 g of air-dried apple scrapings mixed with a 0.05 ml solution of water and tapeworm eggs) for 24 h. This concentration resulted in an average infection intensity of 12.5 cysticercoids per beetle (n = 53, ranging from 1 to 41) and simulated Rau's (Reference Rau1979) field study, which found a cysticercoid load of 10.5 per beetle.
The beetle preference experiment consisted of 53 trials that tested 27 male and 26 female infected individuals in a small plastic arena divided down the middle. Pairs of fresh control and infective faecal pellets were matched according to time of defecation (within 1 h of each other) and used immediately as a paired ‘batch’. Each pellet was placed in its own plastic screw-top vial, labelled and covered so that the preference trial was conducted blind. Bait placement was assigned randomly at the start of each trial, and the arena was rotated by 90° after each trial. To ensure a homogeneous distribution of eggs within the faecal pellets, they were stirred for 1 min. From these paired batches, faeces, matched by weight (28–37 mg) to within 2 mg, were placed on cover slips, and one paired batch of faeces was used for no more than four trials. The positions of both baits were alternated in successive trials. Beetles of known sex, age (21–25 days) and feeding history (starved 2–3 days) were tested in a 15-min trial (after a 7-min acclimatization period). The side to which the beetle first moved, the time spent on each side, the time spent on each type of bait, the number of contacts with each type of bait, and the number of times the midline of the arena was crossed were recorded. Six days after the trial, each beetle was dissected and the number of each stage of cysticercoid was recorded. Voge & Heyneman (Reference Voge and Heyneman1957) showed that cysticercoids undergo five morphologically distinct stages while developing inside the beetle. Beetles with one or more stage 5 cysticercoids were considered infected at the time of the trial, and only these were included in the preference analysis. All other beetles, those with no cysticercoids or those with stages 1 through 4 cysticercoids only, were considered uninfected at the time of the trial. Thus, a beetle may have experienced two infections: an initial infection during the experimental infection period as indicated by the presence of stage 5 cysticercoids, and a secondary infection during the preference trial as indicated by the presence of early stage cysticercoids.
Data analysis
The proportion of time spent on the side containing the infective faeces was tested with a Wilcoxon Signed Rank test for equality to 50% for all male and female beetles.
The Fligner–Policello procedure tested for a median difference in the proportion of time spent on infective and control baits between male and female beetles that experienced both sides (Hollander & Wolfe, Reference Hollander and Wolfe1999). As paired data, the difference between the proportion of time spent on infective and control baits was tested for equality to 0 with a Wilcoxon Signed Rank test. Confidence intervals (95% except where indicated) were constructed about each median using a non-linear interpolation procedure (Minitab v. 13.3, State College, Pennsylvania, USA).
Because 15 of 26 males and 17 of 27 females spent no time on either the infective or control bait, the data were re-analysed with the same tests using the data for beetles that contacted both baits, and significance was adjusted to 0.025 according to the post hoc Bonferroni adjustment. A two-tailed Fisher exact test was used to evaluate the association between sex and beetles that contacted both baits and beetles that contacted only one bait. For beetles that contacted only one bait, the same test analysed the relationship between sex and beetles that contacted infective bait and beetles that contacted control bait. Finally, the number of bait contacts was analysed for all beetles using the Scheirer–Ray–Hare extension of the Kruskal–Wallis test (with a correction for ties) for ranked data in a two-way design with treatment and sex as factors (Sokal & Rohlf, Reference Sokal and Rohlf1995). Data for beetles that contacted both baits were analysed with the same test. Again, significance was adjusted to 0.025 for the question of treatment and to 0.0167 for the question of sex effects (because the Fisher Exact test asks this question three times) according to the post hoc Bonferroni adjustment. Also, for beetles that contacted both sides, the proportion of time spent on the infected bait was plotted against the number of cysticercoids. Finally, the Fligner–Policello procedure (Hollander & Wolfe, Reference Hollander and Wolfe1999) tested for differences in the medians between male and female cysticercoid loads without assuming equal variances.
The data were then divided on the basis of the beetles' infection status: those with successful initial infections (defined as those with stage 5 cysticercoids), those with successful secondary infections (defined as those with stages 1 through 4 cysticercoids), those with both, and those with neither. These data were then analysed with a two-tailed Fisher exact test.
Group trials
Seven to 9 days before the trial, male and female beetles (in groups of 10) were allowed to feed on 1 g of air-dried apple scrapings mixed with a 0.01 ml solution of water and tapeworm eggs for 24 h. Immediately before the trial, each beetle was marked with latex-based paint so individuals could be tracked through the trial. No food was provided 20–35 h before the beetles' respective trials. Eleven groups of males and females (due to mortality, there were between 8 and 10 beetles per group) were used in the preference trial. Each group was placed under a glass bowl in the centre of a plastic arena (which was scrubbed twice and rinsed in hot water for at least 30 min before each trial) under red light conditions. Beetles were provided with two types of bait – uninfective (control) or infective faeces – in an arena divided into four equal quarters. Two control faecal baits were randomly positioned at two opposing quarters of the arena. Two infective faecal baits were then positioned in the two remaining areas. The bait areas were large enough for all beetles to occur simultaneously. A paper towel was used to mash the faecal pellets on the squares to prevent their displacement by beetles during the trial. After 15 min, the bowl was removed and the beetles' movements were videotaped for 1 h under red light conditions. The video was played back and the square that each individual beetle was on was recorded at 1-min intervals as an occurrence. Six days after the preference trial, each beetle was dissected and the number of each stage of cysticercoid was recorded. Beetles with no cysticercoids or those with stages 1 through 4 cysticercoids were considered uninfected at the time of the trial.
Data analysis
Preference was measured as the difference between the number of occurrences at infective and uninfective baits and determined with the Mixed procedure in SAS (v. 8, Cary, North Carolina, USA) with sex and trials as class variables and sex nested in trial as a random variable. The response variable was defined as (number of occurrences at the infective bait) – (number of occurrences at the control bait). The analysis was repeated after including number of cysticercoids in the model. The Fligner–Policello procedure (Hollander & Wolfe, Reference Hollander and Wolfe1999) tested for differences in the medians between male and female cysticercoid loads without assuming equal variances. For each trial, the average cysticercoid infection level (both early stage and stage 5 cysticercoids) was plotted against its variance, and the slope was compared to 1 (Minitab v. 13.3) to assess its similarity to a Poisson distribution. The data were then divided on the basis of the beetles' infection status: those with successful initial infections (defined as those with stage 5 cysticercoids), those with successful secondary infections (defined as those with stages 1 through 4 cysticercoids), those with both, and those with neither. Their relative numbers were analysed with a two-tailed Fisher exact test.
Results
Individual trials
Of beetles that visited both sides, neither females nor males spent a greater proportion of time on the infective faeces than on the control faeces (table 1; Wilcoxon Signed Rank, W♀ = 179, power = 0.106, P = 0.667; W♂ = 174, power = 0.401, P = 0.127). Further, males did not differ significantly from females in the proportion of time spent on the control bait (table 1; Fligner-Policello, P = 0.3907) or infective bait (P = 0.0568) after a post hoc Bonferroni adjustment. Of beetles that contacted both baits, neither females nor males spent a greater proportion of time on the infective faeces than on the control faeces (W♀ = 24, n = 11, P = 0.45; W♂ = 19, n = 9, P = 0.722). Further, males did not differ from females in the proportion of time spent on the control bait (P = 0.374) or infective bait (P = 0.228). There were no treatment or sex differences for number of contacts with either baits when all beetles were considered or when beetles that contacted both baits were considered (table 2; Scheirer–Ray–Hare, P>0.3 for all parameters).
Table 1 Median proportion of time spent on baits when individual beetles, after infection, were allowed to feed on fresh infective or control faecal baits; one female beetle did not contact either bait. Sample sizes and 95% median confidence intervals (except where noted) are in parentheses. There were no significant median differences between treatments or between sexes. All P values >0.05 after a post hoc Bonferroni adjustment.
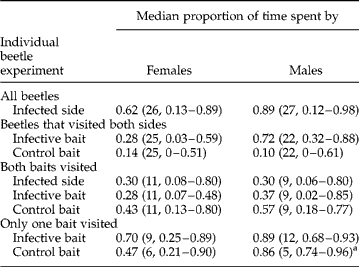
a 93.8% CI due to sample size.
Table 2 Median number of bait contacts for all beetles and for beetles that contacted both baits/cages in the individual beetle experiment. Sample sizes and 95% median confidence intervals are in parentheses. All P values >0.3 after a post hoc Bonferroni adjustment.

The median load of cysticercoids from the initial infection for males (median = 14) was significantly higher than the median load for female (median = 6) beetles (Fligner–Policello, n ♂ = 27, n ♀ = 26, U = 2.26, P = 0.0119). The median for each sex remained the same when data from the secondary infection were added, so that males still had the higher total median load of cysticercoids (fig. 1; Fligner–Policello, U = 2.078, P = 0.0189).
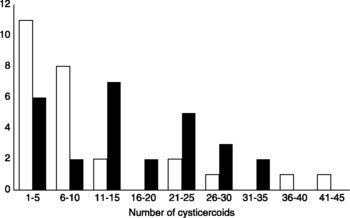
Fig. 1 Frequency distribution of the total number of cysticercoids dissected from female (□) and male (■) beetles at the completion of the individual beetle experiment. Males had the greater load of cysticercoids (Fligner–Policello test, P = 0.0189); total number of females was 26 and of males was 27.
The number of cysticercoids from the initial infection was not correlated with the proportion of time beetles spent on the infected bait for either males (r = 0.06, power = 0.049, P = 0.808) or females (r = 0.094, power = 0.067, P = 0.655). Further, there was no correlation between the number of cysticercoids from the initial and secondary infections for either male (r = − 0.12, power = 0.0052, P = 0.547) or female (r = − 0.25, power = 0.0007, P = 0.213) beetles.
The number of beetles with initial infections was independent of the number of beetles with secondary infections for females (two-tailed Fisher exact test, n = 29, P = 1.0), and for males (two-tailed Fisher exact test, n = 29, P = 0.069) at P = 0.05.
Group trials
Preference for infective faeces between infected male and female beetles did not differ (table 3; Mixed procedure, P = 0.626). The average difference in the total number of occurrences (infective bait occurrence – uninfective bait occurrence) of infected beetles did not differ from 0 for females ( − 1.55, P = 0.626) or males ( − 5.68, P = 0.332). Preference for infective faeces between infected male and female beetles was unrelated to the number of stage 5 cysticercoids in each beetle (P = 0.704). Again, the average difference in the total number of occurrences of infected beetles did not differ from 0 for females ( − 2.26, P = 0.712) or males ( − 5.34, P = 0.385). Of the 35 uninfected females in 11 trials, the average difference in the total number of occurrences did not differ from 0 ( − 4.7, n = 11, P = 0.641). Of the 15 uninfected males in 8 trials, the average difference in the total number of occurrences did not differ from 0 (8.37, n = 8, P = 0.285).
Table 3 Results of the Mixed procedure (SAS v. 8) of infected beetle preference for infective bait by sex (top), and for infective bait by sex with cysticercoid number (bottom) in the group trials. Estimates of the difference of the mean number of occurrences (infected – uninfected) for males and females are provided (n ♂=11, n ♀=11).

a For males and females, the mean difference equals total number of occurrences at the infective bait minus total number of occurrences at the control bait, such that a positive value indicates a preference for infective faeces.
A plot of the average number of early stage cysticercoids (from the secondary infection) against its variance resulted in a slope significantly greater than 1 for both male (b = 6.99, P < 0.001) and female (b = 3.93, P < 0.001) beetles (fig. 2). The same was true for a plot of stage 5 cysticercoids (from the initial infection) for males (b = 9.94, P < 0.001) and females (b = 3.0, P = 0.0042). When compared, the slope for males was greater for early stage cysticercoids (F 1,18 = 33.3, power = 0.045, P < 0.0001) and stage 5 cysticercoids (F 1,18 = 7.36, power = 0.702, P = 0.0143) than the slope for females.
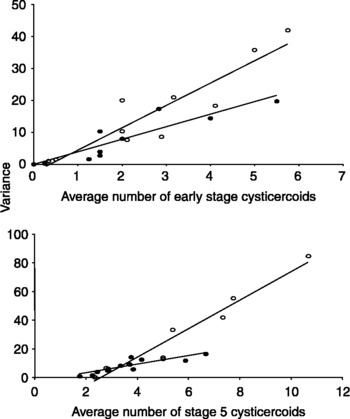
Fig. 2 Plots of the average number of early stage cysticercoids (top) and stage 5 cysticercoids (bottom) against their variance for both male (∘) and female (●) beetles of the group trial. All slopes were significantly different from 1 (t (0.975,9)>2.26, P < 0.01), and male slopes were steeper than female slopes (P < 0.05 for both graphs). In the top graph, females y = 3.9x–0.15 and r 2 = 0.83; males y = 7.0x–2.8 and r 2 = 0.86. In the bottom graph, females y = 3.0x–2.9 and r 2 = 0.74; males y = 9.9x–25.8 and r 2 = 0.95.
Median stage 5 and total cysticercoid distributions between males and females did not differ (Fligner–Policello, U = 0, P>0.15). There was no correlation between the number of cysticercoids from initial and secondary infections for either male (r = − 0.02, power = 0.016, P = 0.848) or female (r = 0.015, power = 0.033, P = 0.905) beetles.
The number of beetles with an initial infection was independent of the number of beetles with a secondary infection for females (two-tailed Fisher exact test, n = 105, P = 0.408), and for males (two-tailed Fisher exact test, n = 105, P = 0.409).
Discussion
Bait preference
Infected beetles of both sexes did not prefer one type of bait to another when tested individually. Additionally, infected beetles of both sexes did not exhibit a preference for either the infective or uninfective baits when tested in groups (table 3). A smaller sample size of uninfected male beetles behaved in the opposite (but not significantly different; table 4) way to infected beetles, which is consistent with the results of previous studies (Evans et al., Reference Evans, Hardy, Singh, Moodie and Cote1992; Pappas et al., Reference Pappas, Marschall, Morrison, Durka and Daniel1995). These previous studies demonstrated that uninfected beetles show a preference for infective faeces, and that sex plays a role in the beetle's preference. Also, parasite and host life histories play an important role in determining if and when parasite manipulation of host behaviour will occur (McCurdy et al., Reference McCurdy, Forbes and Boates1999). Although the powers of many tests are low, the cumulative non-significance of the results suggests that beetles, once infected, lose their faecal preference. This may be an adaptation on the part of the tapeworm to reduce competition for host resources. Alternatively, the lack of faecal preference in infected beetles may be a host adaptation to prevent a lethal infection level (Moore, Reference Moore2002). Both parasite manipulation and host adaptation may be occurring if the interests of host and parasite do not conflict. Further, data from the individual trials suggest that the proportion of time spent on the infective bait is not dose-dependent with regard to the infection load.
Table 4 Results of the Mixed procedure (SAS v. 8) of uninfected beetle preference for infective bait by sex in the group trials. Estimates of the difference of the mean number of occurrences (infected – uninfected) for males and females are provided (n ♂=8, n ♀=11).

a For males and females, the mean difference equals total number of occurrences at the infective bait minus total number of occurrences at the control bait, such that a positive value indicates preference for infective faeces.
Infection differences
Simultaneous tracking of initial and secondary infections allowed for an analysis of infection variance and bias, which are important epidemiological parameters.
If cysticercoid infection follows a Poisson distribution (Rau, Reference Rau1979), then a plot of mean infection against its variance should generate a slope equal to 1. However, plots of both initial and secondary infection generated slopes that are significantly greater than 1. This is true for both males and females, suggesting that some beetles are highly susceptible to infection while others are highly resistant to infection. Further, the slope for males was significantly steeper than the slope for females in both initial and secondary infections. Perhaps differences in male foraging activity leads to infection levels that are extremely high in some individuals and extremely low in others. If true, then females should have a more homogeneous distribution of cysticercoids than males. This is supported by the highly variable male distribution (fig. 2) and the male's higher median load of cysticercoids (fig. 1). However, there is no difference in bait contact between males and females (table 2) so activity level cannot explain the highly variable distributions in the infection data or the male infection bias. If males feed more than females (Shea, Reference Shea2005) and so differ in their susceptibility to infection, then natural selection can favour increased feeding activity in those males that are highly resistant to infection. This may also explain the large variation observed in the male infection distribution (fig. 2) as well as the male infection bias.
Yet, males of the group trials in this experiment did not show the infection bias observed in the individual trials, despite nearly identical infection procedures. Further, previous studies failed to find a difference in infection intensity between the sexes in Tenebrio beetles infected by feeding on gravid proglottids (Hurd & Arme, Reference Hurd and Arme1987) or in natural populations (Rau, Reference Rau1979). On the other hand, when beetles are fed after being exposed to infective rat faeces for 1 h, more cysticercoids are recovered from male than from female beetles (Pappas et al., Reference Pappas, Marschall, Morrison, Durka and Daniel1995). Faecal exposure time, along with the interaction of the nutritional status of males and females with the presence or absence of conspecifics, may explain why male infection bias is observed in some of the previously described studies.
Insight into host resistance may be obtained by examining the relationship between initial and secondary infections. It is possible that beetles, once infected, develop resistance to secondary infections. This does not seem to be the case for beetles in the group experiment or for females and males (at the P = 0.069 level) in the individual trials. Neither the number of cysticercoids nor the presence of cysticercoids could be correlated between initial and secondary infections. This lack of correlation suggests that beetles are unable to initiate an immune response to re-infection during the course of the trial. However, because conditions for the initial and secondary infection differ, it is difficult to draw further conclusions from these results.
Both parasite manipulation and host adaptation may explain the lack of preference for infective faeces by infected beetles if this behaviour benefits both organisms. The mechanism responsible for the altered behaviour of H. diminuta-infected beetles is not known, but is a promising research direction. The male infection bias observed in this experiment cannot be explained by differences in activity levels, but is consistent with the highly variable infection distribution observed in males. Although the initial and secondary infection protocols differed, the lack of correlation between initial and secondary infection levels suggests that infected beetles are no more or no less resistant to re-infection. Future studies using consistent infection and re-infection protocols may help explain the observed male infection bias.
Acknowledgements
I thank Jerry Downhower, Peter Pappas, Tom Waite, Larry Phelan and the anonymous reviewer for their helpful comments and suggestions in the preparation of this manuscript. I especially thank Peter Pappas for the use of his laboratory equipment. Finally, I thank Yimei He for her statistical advice.








