Currently, excellent long-term outcome is anticipated following repair of tetralogy of Fallot, where survival beyond 25 years exceeds 90%. Reference Park, Lee, Lim, Kim and Kim1 However, adults with repaired tetralogy of Fallot (denoted as tetralogy of Fallot throughout this paper) are exposed to continuing cardiovascular risk due to electrophysiological abnormalities and residual haemodynamic lesions, leading to increased risk of heart failure, arrhythmias, and sudden death. Reference Gatzoulis, Balaji and Webber2–Reference Valente, Gauvreau and Assenza4 Although patients with tetralogy of Fallot are typically defined under a single Adult Congenital Heart Disease (ACHD) category in reality, they constitute a complex and heterogenous population.
Previous studies have identified numerous risk factors for adverse outcome. These studies have varied in demographics, measured parameters, and outcome definitions, and not surprisingly different risk factors have emerged. For example, the evidence that ventricular function is important for survival has been widely replicated Reference Valente, Gauvreau and Assenza4–Reference Egbe, Kothapalli and Borlaug8 yet right ventricle (RV) volume, currently advocated as an important parameter when considering pulmonary valve replacement, Reference Stout, Daniels and Baoulhosn9 has not been consistently identified as a risk factor. Recent reports from the INDICATOR cohort have implicated RV hypertrophy and dysfunction, and not RV volume, as significant predictors of tetralogy of Fallot outcome Reference Valente, Gauvreau and Assenza4 and following pulmonary valve replacement. Reference Geva, Mulder and Gauvreau10
Surgical repair of tetralogy of Fallot has evolved. Repair is currently performed routinely in infancy, avoiding long-term shunt palliation. Importance is placed on preserving pulmonary valve function, reducing trans-annular patch rates, and minimising ventricular incisions. These technical modifications are anticipated to have beneficial long-term sequelae and improve adult outcomes. Reference Park, Lee, Lim, Kim and Kim1
Reintervention rates in this population are high in particular pulmonary valve replacement and increasingly, arrhythmia management strategies including electrophysiology studies +/− ablation, complex pacing, and automatic implantable cardiac defibrillator implantation. However, the indication, timing, and risk-benefit of these interventions continue to evolve.
As this population increases, it is important to describe the current status and to monitor change in outcome with evolving clinical practice.
The purpose of this study was to determine from a national cohort, managed through a single institution, current outcomes of adults with repaired tetralogy of Fallot, specifically, to determine clinical function, incidence of mortality and adverse events, and pertaining risk factors. Pulmonary valve replacement influence on outcome was also determined.
Patients and methods
In this retrospective cohort study, 341 adults with repaired tetralogy of Fallot who attended the national service within the period from April 2009 to September 2015 were analysed. Clinical events were recorded on an ACHD database, with total follow-up time of 1249 patient-years and median follow-up of 3.5 years (inter quartile range (IQR) 2.4–4.2). Baseline characteristics were defined by patient status at cohort entry. Clinical data available prior to the study period were used to describe patient baseline status (Table 1).
Table 1. Demographics and baseline parameters at study entry

LVEDVi = indexed LV end-diastolic volume; LVEF = LV ejection fraction; LVESVi = indexed LV end-systolic volume; PVR = pulmonary valve replacement; RVEDVi = indexed RV end-diastolic volume; RVEF = RV ejection fraction; RVESVi = indexed RV end-systolic volume; TOF = tetralogy of Fallot.
* Greater than 95th percentile, #less that 5th percentile of reference ranges obtained from healthy young adults by Le Ven et al. Reference Le Ven, Bibeau and De Larochelliere12
All-cause mortality was designated to avoid bias arising from incorrect classification. Atrial arrhythmia comprised atrial fibrillation, atrial flutter, and other forms of supraventricular tachycardia. Ventricular arrhythmia comprised non-sustained and sustained ventricular tachycardia and ventricular fibrillation. Clinical decisions on investigation and intervention were informed by guidelines. Reference Baumgartner, Bonhoeffer and DeGroot11 Pulmonary valve replacement was considered in asymptomatic patients with increasing indexed RV end-diastolic volume exceeding 150 ml/m2.
Of the 341 patients in this cohort, 219 (64.2%) underwent at least 1 CMR scan during the study period. CMR was performed at 1.5 Tesla (Siemens AG, Erlangen, Germany). Ventricular volumes were indexed to body surface area and compared with reference ranges obtained from a health population with comparable age and gender distribution. Reference Le Ven, Bibeau and De Larochelliere12 Ventricular volumes and ejection fraction were considered normal if they were between 5th and 95th percentile of these reference ranges.
One hundred and eight-two patients (53%) underwent a minimum of one cardiopulmonary exercise testing during the study period. Cardiopulmonary exercise testing was performed on a bicycle ergometer with a progressive 10–20 Watt per minute incremental workload to a symptom-limited maximum. Maximum oxygen uptake (peak VO2) was defined as the highest recorded value of VO2 obtained during the last minute of exercise and expressed as percentage of predicted peak VO2 by comparison with individuals matched for age, gender, height, and weight. Reference Wasserman13 The ventilation per unit of carbon dioxide production (VE/VCO2 slope) was derived from linear regression of the minute ventilation versus carbon dioxide production assessed throughout the period of exercise testing. Reference Clark, Poole-Wilson and Coats14
Statistical analysis
Categorical data were described as frequency and percentages. Continuous data were summarised as mean ± SD if normally distributed or median and IQR otherwise. Survival analyses were applied using age as the time variable, rather time from the index procedure. Reference Korn, Graubard and Midthune15
Univariate analysis was undertaken to establish risk factors for adverse outcome, pulmonary valve replacement, and cardiac device implantation. As event rates for single adverse outcomes were low, a composite outcome comprising death or arrhythmia (ventricular or atrial) was analysed as the primary outcome. A multivariable Cox proportional hazards model was fitted to analyse the association between risk factors and primary outcome. A manual backward selection process was carried out with gender, shunt history, genetic syndrome, tetralogy subtype, age at repair and reintervention prior to cohort entry, and indexed RV end-diastolic volume, VE/VCO2 slope and QRS interval at baseline as covariates considered in the starting model. Covariates were sequentially excluded based on their p-value (at 5% significance level) to obtain a final model. A further time-varying model was undertaken to establish the association between pulmonary valve replacement and the primary endpoint.
Continuous variables were dichotomised to define thresholds predicting adverse outcome. Thresholds were determined by maximising sensitivity and specificity simultaneously using the R package “OptimalCutpoints.” Reference López-Ratón, Rodríguez-Álvarez, Cadarso-Suárez and Gude-Sampedro16
The standardised mortality ratio was determined to compare mortality in the study population with that expected in an age- and gender-matched general Scottish population. Reference Finkelstein, Muzikansky and Schoenfeld17,Reference Kahn and Sempos18 The data were used at face value without imputation for missing data. Analyses were performed in R version 3.4.0. 19
Individual patients were de-identified and the need patient consent was waived.
Results
Baseline characteristics of the cohort (n = 341) are presented in Table 1. Three hundred and eight patients (91%) had standard tetralogy of Fallot morphology with 30 patients (9%) having other tetralogy of Fallot morphology variants. Thirty-seven patients (10.9%) had a known genetic syndrome. Prior to database entry, 91 patients had undergone either surgical (n = 80, 24%) or catheter-based re-intervention (n = 23, 7%). Surgical procedures included pulmonary valve replacement, aortic root replacement (n = 2), and various tetralogy of Fallot revisions (n = 17). Catheter intervention was predominately dilation/stenting of pulmonary arteries.
Thirteen patients (4%) had a cardiac-implanted electronic device comprising pacemaker (n = 8) and automatic implantable cardiac defibrillator (n = 5). Twenty-nine patients had a history of prior arrhythmia comprising atrial (n = 21, 6.2%) and ventricular (n = 11, 3.2%), with some patients experiencing both.
CMR and cardiopulmonary exercise testing
Summary of the cardiopulmonary exercise testing and CMR data are presented in Table 1. In females, indexed RV end-diastolic and end-systolic volumes were larger than the reference range and RV ejection fraction within the low-normal range. Reference Le Ven, Bibeau and De Larochelliere12 In males, indexed RV end-diastolic volume remained within upper normal range, but indexed RV end-systolic volume was larger and ejection faction lower than reference range. Reference Le Ven, Bibeau and De Larochelliere12 Indexed left ventricle (LV) end-diastolic volume and LV end-systolic volume and LV ejection fraction were within normal ranges for both sexes. Compared with males, females had significantly smaller indexed RV and LV volumes (Table 1).
VE/VCO2 slope was substantially impaired compared with expected values from published normal population, Reference Shen, Zhang and Ma20 with females performing worse than males. The cohort’s %pVO2 = 70% lay within the lower 25% percentile of a referenced normal population. Reference Rapp, Scharhag, Wagenpfeil and Scholl21
Adverse events
The survival curves for death, arrhythmia, pulmonary valve replacement, and Implantable cardioverter defibrillator (ICD) are shown in Fig 1. Event rates of individual adverse events are listed in Table 2
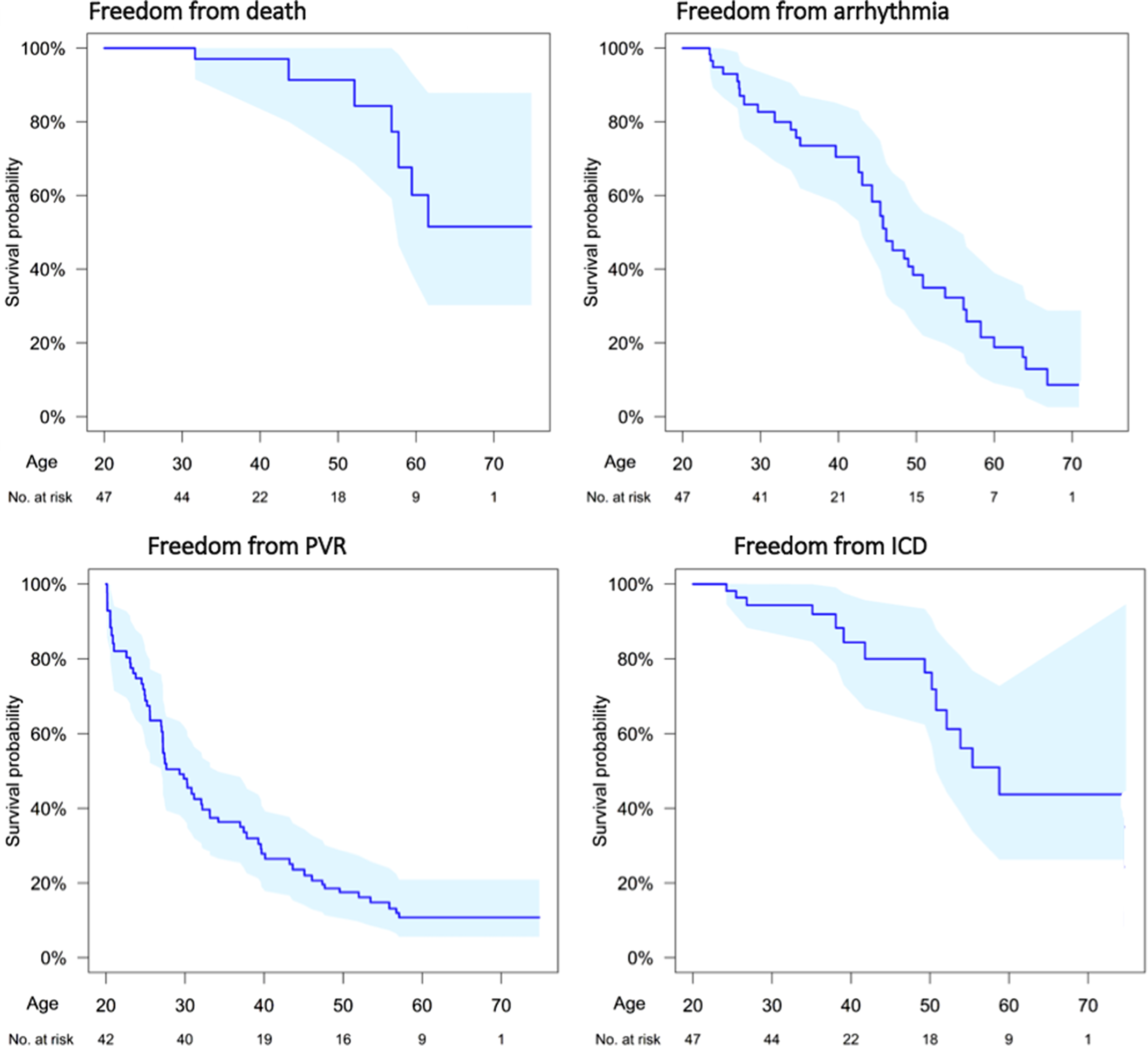
Figure 1. Age-based survival analysis for: death; arrhythmia (ventricular or atrial); pulmonary valve implantation; and implanted cardiac device (pacemaker or defibrillator). Shaded area represents the 95% confidence limits.
Table 2. Adverse events occurring during study period
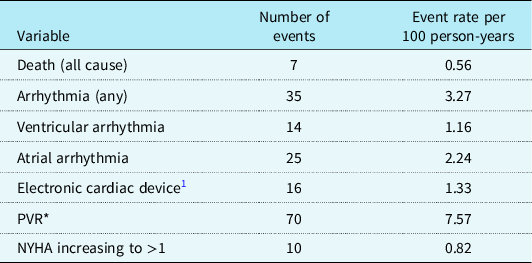
PVR = pulmonary valve replacement.
* Seventy patients underwent new PVR with an additional 17 patients who underwent repeat PVR during study period.
During the study period, seven patients died (2%; 5 males:2 females) at 56.9 years (IQR: 47.9–58.6). Freedom from death declined particularly beyond 50 years of age (Fig 1). Thirty-five patients (event rate (ER): 3.27 per 100 patient-years) experienced new-onset episodes of ventricular and atrial arrhythmic events during the study period. The freedom from arrhythmia decreased linearly with age (Fig 1). Compared with patients less than 40 years, patients over 40 years had significantly increased incidence of death/arrhythmia: (<40 versus >40 years, event rates 2.20 versus 8.17 respectfully, p = 0.004).
The univariate association between potential risk factors for primary outcome (death or arrhythmia) is listed in Table 3. Increasing indexed RV systolic and diastolic volumes were associated with increased risk of adverse event, while indexed LV end-diastolic volume less than 82 ml/m2 and VE/VC02 slope less than 34 were associated with reduced risk. Multivariate analysis identified female gender and increasing RV end-diastolic volume to be significantly associated with primary outcome.
Table 3. Univariate association between predictors and primary outcome

LVEDVi = indexed LV end-diastolic volume; LVEF = LV ejection fraction; LVESVi = indexed LV end-systolic volume; PVR = pulmonary valve replacement; RVEDVi = indexed RV end-diastolic volume; RVEF = RV ejection fraction; RVESVi = indexed RV end-systolic volume; TOF = tetralogy of Fallot.
Standardised mortality ratio forest plot is depicted in Fig 2. The overall cohort had a significantly higher mortality compared with a matched Scottish population: standardised mortality ratio of 2.77 (95% CI: 1.11–5.70), with higher mortality in males compared to females. When stratified according to age, only those patients over 55 years of age demonstrated a significantly higher mortality, standardised mortality ratio of 6.12 (95% CI 1.64–15.66).

Figure 2. Standard mortality ratio. Values to the right of the vertical dashed line represent increased mortality compared to matched Scottish population. Values to the right of the vertical dashed line represent increased mortality compared to matched Scottish population.
Pulmonary valve replacement
Eighty-seven patients (26%) underwent pulmonary valve replacement (surgical 72 and percutaneous 15) during the cohort period: 70 new pulmonary valve replacement and 17 repeat pulmonary valve replacement. Compared with patients who did not undergo pulmonary valve replacement during the study period, patients who had pulmonary valve replacement were associated with: indexed RV end-diastolic volume > 150 ml/m2, p < 0.001; indexed RV end-systolic volume > 95 ml/m2, p = 0.004; RV ejection fraction < 50%, p = 0.05; pVO2 < 68%, p = 0.007; VE/VCO2 slope > 34, p = 0.016; and QRS > 180 ms, p = 0.003. There was one post-operative death (1.4%).
Pre- and post-pulmonary valve replacement CMR and cardiopulmonary exercise testing data were available in 21 (24%) and 28 (32%) patients, respectively. A group of tetralogy of Fallot patients who did not undergo pulmonary valve replacement but who had a baseline and repeat MRI (n = 18) and cardiopulmonary exercise testing (n = 29) during the study period provided a comparison (Table 4). Following pulmonary valve replacement, indexed RV end-diastolic and end-systolic volumes decreased. RV ejection fraction remained unchanged as did LV volumes. QRS interval duration decreased following pulmonary valve replacement. pVO2 and VE/VCO2 slope did not change significantly following pulmonary valve replacement. In the non-pulmonary valve replacement group, no significant change in RV or LV volumes occurred, and a modest decline in %pVO2 and an increase in NYHA class were observed.
Table 4. Influence of PVR on MRI-derived ventricular volumes, CPET, and QRS interval.

CPET = cardiopulmonary exercise test; LVEDVi = indexed LV end-diastolic volume; LVEF = LV ejection fraction; LVESVi = indexed LV end-systolic volume; PVR = pulmonary valve replacement; RVEDVi = indexed RV end-diastolic volume; RVEF = RV ejection fraction; RVESVi = indexed RV end-systolic volume.
Pulmonary valve replacement undertaken prior to cohort entry was not associated with an increased risk of primary endpoint (HR: 0.80, 95% CI: 0.31–2.09; p = 0.65). By contrast, in those patients who entered the cohort pulmonary valve replacement-free and who underwent subsequent pulmonary valve replacement during follow-up (n = 70) were at higher risk of primary endpoint (HR: 2.82, 95% CI: 1.36–5.86, p = 0.005). When the overall effect of pulmonary valve replacement was considered, irrespective of when it was performed, pulmonary valve replacement was found to be associated with an increased risk of primary endpoint (HR: 2.04, 95% CI: 1.04–4.01, p = 0.04).
Implantable cardiac electronic device
Sixteen patients (5%, ER: 1.33 per 100 patient-years) required cardiac-implanted electronic device during the study period: pacemaker, n = 5, and automatic implantable cardiac defibrillator, n = 11. Cardiac-implanted electronic device was significantly associated with indexed RV end-diastolic >150 ml/m2, p = 0.015, RV end-systolic volumes >95 ml/m2, p = 0.004, and QRS duration >180 ms, p = 0.003.
Discussion
This study identified that young adults with repaired tetralogy of Fallot, despite the majority functioning well, are exposed to a continuous late hazard of mortality, arrhythmia, and reintervention that increased with age. Survival was comparable to the general population up to 55 years but declined thereafter, particularly in males. Female gender and increasing right ventricular end-diastolic volume predicted adverse outcome comprising death or arrhythmia. Pulmonary valve replacement reduced right ventricular volumes and maintained exercise parameters but did not reduce the incidence of mortality and arrhythmia.
As previously reported, RV volumes were generally enlarged in this population, while LV volumes and ejection fraction were, on average, within normal limits. In males, although indexed RV end-diastolic volume was within upper limits of the normal range, indexed RV end-systolic volume was higher than the 95th percentile and RV ejection fraction was reduced, suggesting an RV contractile deficit. Males had larger LV and RV index volumes compared to females as previously reported in normal populations. Reference Le Ven, Bibeau and De Larochelliere12,Reference Petersen, Aung and Sanghvi22,Reference Kawel-Boehm, Maceira and Valsanggiacomo23 In the subgroup of patients with paired CMR who did not undergo pulmonary valve replacement, RV volumes and ejection fraction remained stable over the CMR interval period, as previously reported. Reference Quail, Frigiola and Giardini24,Reference Frigiola, Hughes and Turner25
Cardiopulmonary exercise testing has become an important adjunct in the assessment of tetralogy of Fallot. Reference Kempny, Dimopoulos and Uebing26,Reference Inuzuki, Diller and Borgia27 In this study, despite the majority of patients assessed as NYHA 1, significant impairments of pVO2 and VE/VCO2 slope were identified. The cohort’s % predicted pVO2 of 70% suggests a mild exercise limitation and similar to previous tetralogy of Fallot reports. Reference Valente, Gauvreau and Assenza4,Reference Kempny, Dimopoulos and Uebing26,Reference Inuzuki, Diller and Borgia27 However, the cohort’s mean VE/VC02 slope of 33 signifies an important functional compromise Reference Arena and Sietsema28 and more impaired than previously reported tetralogy of Fallot studies. Reference Kempny, Dimopoulos and Uebing26 In general populations and ACHD/tetralogy of Fallot cohorts, cardiopulmonary exercise testing performance, when assessed by pVO2, is higher in males compared with females Reference Rapp, Scharhag, Wagenpfeil and Scholl21,Reference Kempny, Dimopoulos and Uebing26 In the current study, males also had greater exercise capacity, but this was apparent only with VE/VCO2 slope parameter; by contrast, %pVO2 was similar between genders.
In the current study, VE/VCO2 slope (>34), but not %pVO2, was associated with increased risk of primary outcome. The superiority of VE/VCO2 slope compared with %pVO2 in predicting mortality has been previously reported in chronic heart failure Reference Shen, Zhang and Ma20,Reference Gitt, Wasserman and Kilowski29 and in non-cyanotic CHD. Reference Inuzuki, Diller and Borgia27,Reference Arena and Sietsema28 This may be because VE/VCO2, slope unlike pVO2, can be reliably determined from a sub-maximal effort test.
In the multivariable risk model, increasing indexed RV end-diastolic volume and female gender predicted primary endpoint. RV end-diastolic dilation has been previously reported as independent predictor for adverse event in tetralogy of Fallot. Reference Knauth, Gauvreau and Powell3,Reference Diller, Kempny and Liodakis5 . However, more recently, studies utilising the INDICATOR cohort have identified increased RV mass-to-volume ratio, and reduced RV ejection fraction predicted tetralogy of Fallot outcome, while increased RV volume per se did not. Reference Valente, Gauvreau and Assenza4,Reference Geva, Mulder and Gauvreau10 In these studies, the endpoint comprised death or ventricular tachycardia whereas the current study also included atrial arrhythmia, and this may account for the differences in RV volume association on outcome between studies. Females were associated with adverse outcome as they had a higher incidence of arrhythmia compared with males, countering the higher incidence of death in males. Other commonly reported risk factors for adverse tetralogy of Fallot outcome, including reduced LV ejection fraction, Reference Knauth, Gauvreau and Powell3–Reference Geva, Sandweiss, Gauvreau, Gauvreau, Lock and Powell7 prolonged QRS interval, and older age at initial repair, Reference Gatzoulis, Balaji and Webber2–Reference Egbe, Kothapalli and Borlaug8,Reference Mouws, Roos-Hesselink, Bogers and de Groot30 were not identified as risk factors in this study.
During the study period, there were seven deaths representing a mortality of 0.33% per year or an event rate of 0.57 per 100 patient-years, similar to previous studies. Reference Bokma, Winter and Vehmeijer31 Mortality was higher than age-/gender-matched Scottish population and comparable to other UK reported tetralogy of Fallot cohorts. Reference Diller, Kempny and Alonso-Gonzalez32 Age >55 years was the only age group that had a significantly higher mortality than control, with a sixfold increase compared to the general population. This finding that tetralogy of Fallot mortality diverges from expected with age greater than 50 years is consistent with previous reports. Reference Valente, Gauvreau and Assenza4,Reference Egbe, Kothapalli and Borlaug8,Reference Frigiola, Hughes and Turner25,Reference Diller, Kempny and Alonso-Gonzalez32 In Scotland, male life expectancy is reduced compared to females. Our finding of increased mortality in males may reflect this national demographic.
Over the recent two decades, the frequency of pulmonary valve replacement has increased and is being performed at a younger age. Reference O’Byrne, Glatz and Mercer-Rosa33,Reference Egbe, Vallabhajosyula and Connolly34 To date, no survival benefit of pulmonary valve replacement has been demonstrated, and the indication and timing remain uncertain. Reference Tretter and Reddington35–Reference Greutmann37 Previously, studies have focused on defining RV volume thresholds in which post-pulmonary valve replacement RV volume normalisation is likely to occur in the expectation that a normal RV volume will be less prone to arrhythmia and dysfunction. Reference Bokma, Winter and Oosterhof38–Reference Geva, Gauvreau and Powell41 In this study, pulmonary valve replacement was associated with mean reduction of 22% in both indexed RV end-diastolic volume and indexed RV end-systolic volume, while RV EF remained unchanged consistent with previous studies. Reference Quail, Frigiola and Giardini24,Reference Bokma, Winter and Oosterhof38–Reference Ferraz Cavalcanti, Oliveira Sá and Santos44 However, despite pulmonary valve replacement being performed below published threshold volumes, mean RV volume did not normalise following pulmonary valve replacementFootnote 2 Suboptimal remodelling may relate to impaired RV myocardium prior to pulmonary valve replacement Reference Geva, Gauvreau and Powell41 suggested by reduced RV EF and prolonged QRS duration present in the current cohort. In this study, neither LV volumes nor LV ejection fraction changed following pulmonary valve replacement. Previous studies have demonstrated small increases in indexed LV end-diastolic volume and ejection fraction following pulmonary valve replacement due to increased LV preloading or alteration in intraventricular geometry. Reference Bokma, Winter and Oosterhof38–Reference Heng, Gatzoulis and Uebing40,Reference Ferraz Cavalcanti, Oliveira Sá and Santos44,Reference Frigiola, Tsang and Bull45
In the current study, patients who underwent pulmonary valve replacement during the study period experienced an increased risk of primary outcome compared to those who did not undergo pulmonary valve replacement or who underwent pulmonary valve replacement prior to the study period. This association of pulmonary valve replacement and adverse outcome is likely to be confounded by the presence of concomitant risk factors including large RV volumes, long QRS interval, and impaired cardiopulmonary exercise testing, in the pulmonary valve replacement group. Previous studies comparing pulmonary valve replacement with propensity-matched non-pulmonary valve replacement cohorts have found that while pulmonary valve replacement is associated with RV volume reduction and improved symptoms and functional class, it did not reduce incidence of sudden death or sustained ventricular tachycardia. Reference Quail, Frigiola and Giardini24,Reference Gengsakul, Harris and Bradley43,Reference Harrild, Berul and Cecchin46,Reference Bokma, Geva and Sleeper47 It is possible that pulmonary valve replacement does not modify these outcomes because, despite reducing RV volume, pulmonary valve replacement may have little impact on RV ejection fraction, dyssynchrony, or interstitial fibrosis. In addition, cardiac surgery has risks of complication including mortality and persistent atrial arrhythmia. Reference Gengsakul, Harris and Bradley43,Reference Ferraz Cavalcanti, Oliveira Sá and Santos44 And finally, current guidelines on the timing and/or indication of pulmonary valve replacement based on RV end-diastolic volume may be insufficient to alter outcome. Reference Tretter and Reddington35–Reference Greutmann37 Geva et al. reported that pulmonary valve replacement is associated with an increased risk of death or ventricular tachycardia with age at pulmonary valve replacement ≥ 28 years, increased RV mass/volume ratio, and reduced ejection fraction. Reference Geva, Mulder and Gauvreau10 In the current study, the risk of adverse outcome following pulmonary valve replacement predominantly occurred when performed later, that is, during the cohort period compared to prior to cohort entry, suggesting that delaying pulmonary valve replacement or when performed at an older age might detrimentally affect outcome.
By contrast, Bokma et al reported that heart failure, atrial arrhythmia, and non-sustained ventricular tachycardia were increased with pulmonary valve replacement compared to a propensity-matched non-pulmonary valve replacement cohort, when pulmonary valve replacement had been performed “too early,” that is, where a conservative criterion for pulmonary valve replacement had not been met. Reference Bokma, Geva and Sleeper47 In the current study, because atrial arrhythmia was a dominant variable within the primary outcome, it is possible that the association between pulmonary valve replacement and primary outcome was strongly weighed by the new-onset atrial arrhythmia.
Arrhythmia and sudden death are common sequelae of tetralogy of Fallot, and pulmonary valve replacement by itself may be insufficient to reduce these adverse events. It has been proposed that concomitant atrial maze and ventricular isthmus ablation procedures, at the time of pulmonary valve replacement in appropriately selected patients, may be required to reduce arrhythmia burden and sudden death in tetralogy of Fallot. Reference Caldaroni, Lo Rito and Chessa48,Reference Therrien, Siu and Harris49
In this study, pulmonary valve replacement performed during the follow-up period was not associated with improved cardiopulmonary exercise testing parameters nor NYHA functional class, consistent with that reported by Heng et al., where pVO2 and VE/VCO2 slope remained unchanged following pulmonary valve replacement. Reference Heng, Gatzoulis and Uebing40 Frigiola et al. identified that VE/VCO2 slope significantly improved following pulmonary valve replacement only in patients younger than 17.5 years. Reference Frigiola, Tsang and Bull45 Numerous studies have demonstrated improved symptoms and NYHA class following pulmonary valve replacement justifying guideline indications for symptomatic patients. Reference Oosterhof, van Straten and Vliegen39,Reference Gengsakul, Harris and Bradley43–Reference Frigiola, Tsang and Bull45,Reference Therrien, Siu and Harris49,Reference Rotes, Eidem and Connolly50 In the current study, the majority of patients (95%) were assessed as NYHA 1 prior to pulmonary valve replacement; therefore, identifying an improvement would be unlikely. By contrast, the non-pulmonary valve replacement group was associated with modest decline in NYHA functional class and %pVO2 during the study period. Pulmonary valve replacement may therefore act to prevent, rather than improve, functional decline.
Limitations
This is a retrospective single-centre study and hence has certain inherent limitations. The finding of an association of an increased risk of adverse outcome with pulmonary valve replacement may be confounded by higher occurrence of other risk factors for outcome within this group. Ultimately, a prospective randomised controlled trial of pulmonary valve replacement is preferred to determine the influence of pulmonary valve replacement on outcome of adults with repaired tetralogy of Fallot. Although cardiopulmonary exercise testing and MRI were performed in a majority, these data were not available in all patients. It is possible that a selection bias based on symptoms or other risk factors could occur, and thus the cardiopulmonary exercise testing/CMR sample data may not be representative of the entire cohort.
Within this sizable data set, the episodes of death and ventricular tachycardia/ventricular fibrillation were infrequent, even though CMR testing and cardiopulmonary exercise testing demonstrated significant impairments. Consequently, the study employed a composite primary outcome consistent with previous adult tetralogy of Fallot studies. Reference Valente, Gauvreau and Assenza4,Reference Geva, Mulder and Gauvreau10,Reference Caldaroni, Lo Rito and Chessa48 Atrial arrhythmia was included as an adverse event because it is associated with right and left ventricular dysfunction in tetralogy of Fallot. Reference Khairy, Aboulhosn and Gurvitz51 As equal weighting is applied to the adverse events irrespective of type (death and arrhythmia), and as event rates for arrhythmia exceeded that of death, arrhythmic events provide greater impact on the risk model than death. Despite these potential limitations, the study provides a comprehensive description of the clinical and functional outcomes in a contemporary adult tetralogy of Fallot population.
Conclusions
This study identified that adults with repaired tetralogy of Fallot experience an ongoing risk of mortality and arrhythmia that increases with age. Survival was reduced compared to the general population, particularly in patients older than 55 years. In this study, female gender and increasing indexed RV end-diastolic volume were risk factors for adverse outcome. Pulmonary valve replacement reduced RV volumes and prevented exercise decline but did not reduce adverse outcome.
Acknowledgements
The authors have no acknowledgements to disclose.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethical standards
This study was registered and approved by the institution’s governance department.









