Introduction
The times at which we eat can impact on our health, with a comprehensive review by the American Heart Association revealing that eating later in the day increases risk of developing type 2 diabetes and obesity(Reference St-Onge, Ard and Baskin1). This is due to disruptions to the circadian system, which is coordinated by a ‘central clock’, found in the suprachiasmatic nucleus of the anterior hypothalamus of the brain, which receives signals of light and dark over 24 h(Reference Cagampang and Bruce2). The ‘central clock’ synchronises ‘peripheral clocks’ located in multiple sites such as the liver, pancreas and adipose tissue through hormonal, humoral and neuronal signals(Reference Cagampang and Bruce2). The synchrony between the external environment of light and dark with our internal central and peripheral clocks regulates behavioural cycles such that humans sleep at night, and wake and feed in the day. To match this behaviour, key metabolic processes also follow a circadian rhythm, which explains why glucose tolerance, insulin sensitivity and thermic effects of food are highest in the morning, and decrease as the day progresses into night(Reference Poggiogalle, Jamshed and Peterson3). Hence, a consequence of eating later into the night is poorer glycaemic control and a relative insulin resistance(Reference Leung, Huggins and Bonham4,Reference Sato, Nakamura and Ogata5) . Additionally, whilst light is the primary cue or zeitgeber for the central clock, peripheral clocks may be entrained by other zeitgebers such as body temperature and behavioural factors such as timing of food intake(Reference Cagampang and Bruce2). Thus, when feed–fast cycles are altered in relation to the light–dark cycle, peripheral clocks misalign in relation to the central clock, leading to circadian rhythm misalignment(Reference Baron and Reid6). This misalignment has been shown to raise glucose and insulin levels, blood pressure, and inflammatory markers in healthy adults(Reference Morris, Yang and Garcia7,Reference Morris, Purvis and Hu8) . Studies have also suggested that other temporal aspects of food intake, separate to meal timing, may impact on risk factors for chronic disease, including meal frequency (number of eating occasions in a day)(Reference St-Onge, Ard and Baskin1,Reference Bhutani and Varady9) and regularity (the consistency of frequency and spacing of eating occasions across the day)(Reference Leech, Worsley and Timperio10). Together, these studies fall within the emerging area of chrononutrition, which focuses on the effects of the timing, frequency and regularity of eating behaviour(Reference Almoosawi, Vingeliene and Gachon11) on health outcomes through circadian clock regulation of metabolism.
One factor that influences eating behaviour is an individual’s chronotype. Chronotype is an indicator of the phase, or timing, of one’s circadian rhythm in relation to the light–dark cycle(Reference Roenneberg and Merrow12). Chronotype markers include rhythms of physiological processes regulated by the central clock, such as core body temperature, plasma cortisol and melatonin(Reference Klerman, Gershengorn and Duffy13). For instance, melatonin levels are low in the day and rise at night, with production inhibited by bright light(Reference Cipolla-Neto and Amaral14). The rhythm of melatonin secretion under dim light conditions, or dim light melatonin onset (DLMO), is the most reliable marker of circadian phase(Reference Lewy and Sack15). However, DLMO collection can be expensive and burdensome. As such, questionnaires have been developed to identify actual or preferred times for daily activities, such as eating and sleeping, as behavioural indicators of chronotype(Reference Kantermann, Sung and Burgess16). For example, sleep and wake times relate to the period (cycle length) of the internal circadian clock(Reference Brown, Kunz and Dumas17). It is not surprising, therefore, that a typical population consists of a range of morning to evening chronotypes; the former population tends to wake and sleep early, while the latter group wakes and sleeps late(Reference Wittmann, Dinich and Merrow18). Evening chronotypes commonly experience circadian misalignment because of their propensity toward nocturnal behaviours, which are out of synchrony with the light–dark cycle. These behaviours are associated with higher odds of hypertension(Reference Merikanto, Lahti and Puolijoki19), diabetes and metabolic syndrome compared with morning types after adjustment for confounding variables(Reference Yu, Yun and Ahn20). Therefore, identifying the chronotype of an individual has relevance in understanding their health outcomes.
A recent scoping review reported that evening chronotypes across Europe, Asia, and North and South America have poorer diet quality, including lower vegetable intake and greater intake of sweet food/beverages and alcohol(Reference Mazri, Manaf and Shahar21,Reference Reutrakul, Hood and Crowley22) , increasing cardiometabolic disease risk(Reference Lichtenstein, Appel and Brands23). With a chrononutrition lens, we argue that it is pertinent to investigate whether not only diet quality but also food timing may contribute to increased health risk for those with later chronotype. To do so, it is important to first identify all time-related factors of eating, hereon referred to as temporal patterns of eating, in a range of chronotypes, and compare trends between chronotypes. Two recent reviews have considered chronotype in relation to diet (Mazri and colleagues(Reference Mazri, Manaf and Shahar21) and Almoosawi et al.(Reference Almoosawi, Vingeliene and Gachon11)). While both reviews discussed some evidence of the relationship between late chronotype and meal timing (e.g. delayed meals, breakfast skipping), the main focus was on the relationship between chronotype and food/nutrient intake, and cardiometabolic health, respectively. As such, it was not within the scope of these reviews to cover all temporal patterns of eating or discuss in detail the methodology of collecting data on chronotype and food timing. This highlights the need for further studies and, in particular, a systematic approach to establishing consistent and valid methods to capture chronotype and food timing.
Further, a recent position statement from the American Heart Association highlighted the translational importance of research in this area. This expert opinion statement, for the first time, made recommendations regarding the importance of taking into consideration temporal aspects of eating on cardiometabolic health, and recommended that being mindful of the timing and frequency of food intake may alleviate cardiometabolic health risks(Reference St-Onge, Ard and Baskin1). To assist with the translation of this advice into practice, this review will focus on understanding the temporal patterns of eating of different chronotypes, as well as identifying and assessing the tools used by studies in collecting data on temporal patterns of eating and chronotype, to enable identification of strengths, weaknesses and gaps. Recommendations on ways to improve data collection of chronotype and temporal patterns of eating will be made based on findings from this review. This will pave the way for improved collection of data on temporal patterns of eating and chronotype and a greater understanding of how chronotype may influence temporal patterns of eating and, possibly, health outcomes.
Methods
This scoping review was conducted according to the standard process outlined in Arksey et al.(Reference Arksey and O’Malley24). According to Arksey and colleagues, the aim of a scoping review is not to synthesise evidence like a systematic review; rather, scoping reviews have an analytical structure, whereby themes are identified, creating a narrative of the existing literature. The strength of a scoping review lies in its rigor and transparency in mapping the area of research in question.
A systematic literature search was conducted via the electronic databases Medline, Embase, Emcare, PsycInfo, Cochrane Library, Web of Science and Scopus for articles published from earliest to 23 June 2020. MeSH headings and keywords were initially identified in Medline, and re-run on the other databases with modifications to accommodate to each database where necessary. Extra headings identified on other databases were included across all databases and searches rerun for consistency (Supplementary material: Search terms). In Covidence(Reference Kellermeyer, Harnke and Knight25), two independent reviewers screened identified articles. Initial screening based on title and abstracts was conducted by Y.Y. and A.C./M.H./M.B., with disagreements resolved by J.D., and subsequent screening based on full-text assessment was conducted by Y.Y. and M.H., with disagreements resolved by J.D./A.C./M.B. The reference lists of reviews and full-text articles were searched for relevant publications, which equally underwent title, abstract and full-text screening of eligibility. Articles were excluded if they were non-human studies, were reviews, or were not in English.
The inclusion criteria were developed from the Joanna Brigg’s Institute Reviewer’s Manual for Scoping Reviews(Reference Peters, Godfrey, McInerney, Munn, Tricco and Khalil26).
-
Participants: adults ≥18 years
-
Concept: chronotype; and dietary behaviours related to timing of food and energy intake, including studies on meal skipping, meal frequency, meal regularity, duration of eating window, and duration between meals, or meals and wake/sleep times
-
Context: nil.
-
Types of evidence sources: randomised controlled trials, non-randomised controlled trials, before and after studies, prospective and retrospective cohort studies, cross-sectional studies, and case–control studies
Results
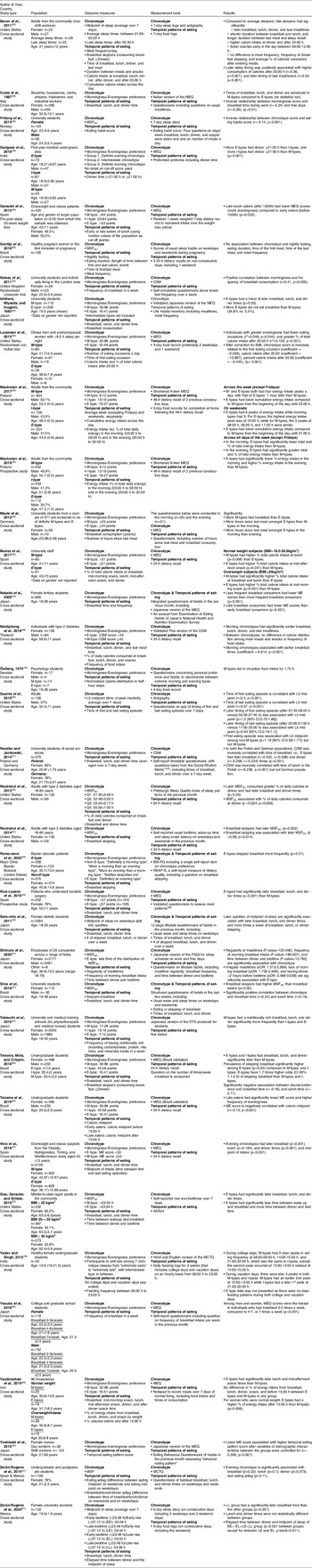
Fig. 1 represents a PRISMA extension for scoping reviews (PRISMA-ScR) flow diagram of study selection. From an initial search where 2150 articles were retrieved, screening yielded 36 studies to include in this review (Table 1).

Fig. 1. PRISMA flow chart of the review progress. Thirty-six papers were identified as having met the study criteria.
Table 1. Summary of study characteristics

g, grams; MEQ, Morningness-Eveningness Questionnaire; M-type, morning chronotype; E-type, evening chronotype; MSFSC, mid-sleep time on free days self-corrected for sleep debt; I-type, intermediate chronotype; CSM, Composite Scale of Morningness; MSF, mid-sleep time; BiB-PQ, Bipolar Biobank Patient Questionnaire; REAP-S, Rapid Eating Assessment for Participants – Shortened Version; PSQI, Pittsburgh Sleep Quality Index; aOR, adjusted odds ratio; DTS, Diurnal Type Scale; ASA24, Automated Self-Administered 24-h Dietary Assessment Tool.
Demographics
Study dates ranged from 1973 to 2020, with 89% published after 2010. Studies were mainly conducted in Europe (n = 13)(Reference Costa, Lievore and Ferrari28,Reference Friborg, Rosenvinge and Wynn29,Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Halsey, Huber and Low33,Reference Maukonen, Kanerva and Partonen36–Reference Munoz, Canavate and Hernandez39,Reference Östberg42,Reference Randler and Jankowski44,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Vera, Dashti and Gómez-Abellán56,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) , followed by Asia (n = 11)(Reference Gangwar, Tiwari and Rawat30,Reference Ishihara, Miyasita and Inugami34,Reference Nakade, Takeuchi and Kurotani40,Reference Nimitphong, Siwasaranond and Saetung41,Reference Sato-Mito, Sasaki and Murakami50,Reference Shimura, Sugiura and Inoue51,Reference Takeuchi, Yamazaki and Oki53,Reference Yadav and Singh58–Reference Yoshizaki, Kawano and Noguchi61) , North America (n = 7)(Reference Reutrakul, Hood and Crowley22,Reference Baron, Reid and Kern27,Reference Lucassen, Zhao and Rother35,Reference Quante, Mariani and Weng43,Reference Reutrakul, Hood and Crowley46,Reference Romo-Nava, Blom and Guerdjikova47,Reference Xiao, Garaulet and Scheer57) and South America (n = 6)(Reference Gontijo, Cabral and Balieiro32,Reference Silva, Mota and Miranda52,Reference Teixeira, Mota and Crispim54,Reference Teixeira, Barreto and Mota55,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) . Most were cross-sectional (n = 31)(Reference Reutrakul, Hood and Crowley22,Reference Baron, Reid and Kern27–Reference Gangwar, Tiwari and Rawat30,Reference Gontijo, Cabral and Balieiro32,Reference Ishihara, Miyasita and Inugami34,Reference Maukonen, Kanerva and Partonen36,Reference Meule, Roeser and Randler38–Reference Randler and Jankowski44,Reference Reutrakul, Hood and Crowley46,Reference Romo-Nava, Blom and Guerdjikova47,Reference Sato-Mito, Sasaki and Murakami50–Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , with two randomised trials(Reference Halsey, Huber and Low33,Reference Lucassen, Zhao and Rother35) , two prospective studies(Reference Maukonen, Kanerva and Partonen37,Reference Ruiz-Lozano, Vidal and de Hollanda48) and a single pre/post study(Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31). A majority of the studies included tertiary students (n = 17)(Reference Friborg, Rosenvinge and Wynn29,Reference Gangwar, Tiwari and Rawat30,Reference Halsey, Huber and Low33,Reference Ishihara, Miyasita and Inugami34,Reference Meule, Roeser and Randler38,Reference Nakade, Takeuchi and Kurotani40,Reference Östberg42,Reference Randler and Jankowski44,Reference Sato-Mito, Sasaki and Murakami50,Reference Silva, Mota and Miranda52–Reference Teixeira, Barreto and Mota55,Reference Yadav and Singh58,Reference Yasuda, Asako and Arimitsu59,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , followed by community-dwelling adults (n = 7)(Reference Baron, Reid and Kern27,Reference Costa, Lievore and Ferrari28,Reference Maukonen, Kanerva and Partonen36,Reference Maukonen, Kanerva and Partonen37,Reference Quante, Mariani and Weng43,Reference Xiao, Garaulet and Scheer57,Reference Yazdinezhad, Askarpour and Aboushamsia60) , and workplace employees, which included non-shift workers (n = 2)(Reference Munoz, Canavate and Hernandez39,Reference Shimura, Sugiura and Inoue51) and shift workers (n = 1)(Reference Yoshizaki, Kawano and Noguchi61). The remaining studies included individuals with medical conditions or requirements, such as people with type 2 diabetes (n = 3)(Reference Reutrakul, Hood and Crowley22,Reference Nimitphong, Siwasaranond and Saetung41,Reference Reutrakul, Hood and Crowley46) , people impacted by overweight and obesity (n = 3)(Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Lucassen, Zhao and Rother35,Reference Vera, Dashti and Gómez-Abellán56) , pregnant women (n = 1)(Reference Gontijo, Cabral and Balieiro32), bariatric surgery patients (n = 1)(Reference Ruiz-Lozano, Vidal and de Hollanda48) and individuals with bipolar disorder (n = 1)(Reference Romo-Nava, Blom and Guerdjikova47). Across all studies, there were 27 685 participants, ranging in age from 18 to 85 years. Seven studies included only female participants(Reference Gontijo, Cabral and Balieiro32,Reference Nakade, Takeuchi and Kurotani40,Reference Sato-Mito, Sasaki and Murakami50,Reference Yadav and Singh58,Reference Yazdinezhad, Askarpour and Aboushamsia60,Reference Yoshizaki, Kawano and Noguchi61,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , with the rest including a mix of genders.
Assessment of chronotype
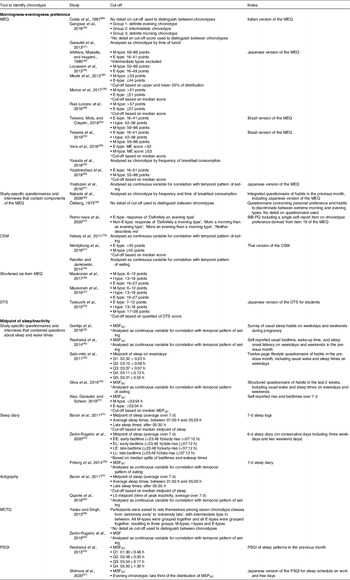
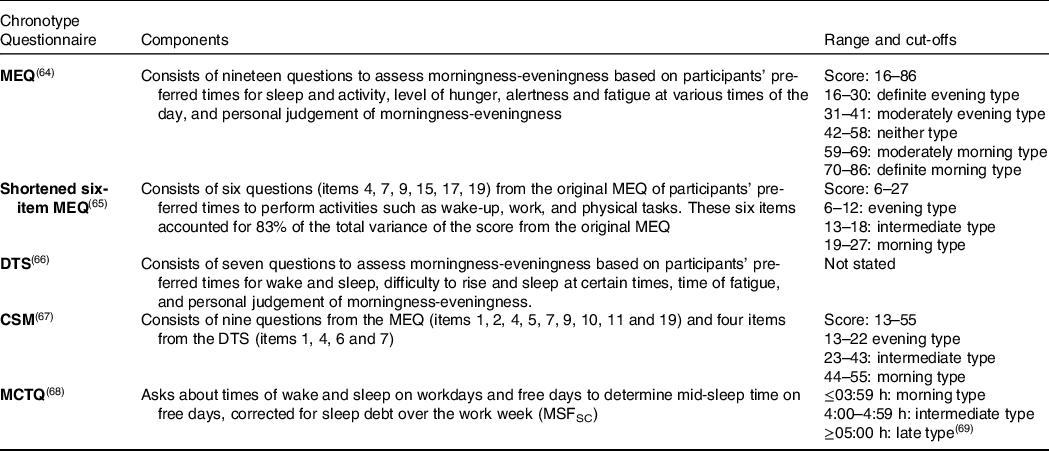
Details of the questionnaires used to capture chronotype in the studies are presented in Table 2. These instruments record behavioural indicators of chronotype through preferred or actual timing of daily activities such as sleep and wake. In addition to the variety of methods used to chronotype, studies also used different cut-off points to differentiate between chronotypes (Table 3).
Table 2. Details of chronotype questionnaires and original cut-offs points to determine chronotype
MEQ, Morningness-Eveningness Questionnaire; DTS, Diurnal Type Scale; CSM, Composite Scale of Morningness; MCTQ, Munich Chronotype Questionnaire; MSFSC, mid-sleep time on free days self-corrected for sleep debt.
Table 3. Summary of methods and cut-off points studies used to determine chronotype of individuals

MEQ, Morningness-Eveningness Questionnaire; M-type, morning chronotype; E-type, evening chronotype; I-type, intermediate chronotype; BiB-PQ, Bipolar Biobank Patient Questionnaire; CSM, Composite Scale of Morningness; DTS, Diurnal Type Scale; MSFSC, mid-sleep time on free days self-corrected for sleep debt; MCTQ, Munich Chronotype Questionnaire; MSF, mid-sleep time on free days; PSQI, Pittsburgh Sleep Quality Index.
Twenty-three studies estimated chronotype by ‘morningness-eveningness preference’, using the Morningness Eveningness Questionnaire (MEQ)(Reference Kantermann, Sung and Burgess16) (n = 14)(Reference Costa, Lievore and Ferrari28,Reference Gangwar, Tiwari and Rawat30,Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Ishihara, Miyasita and Inugami34,Reference Lucassen, Zhao and Rother35,Reference Meule, Roeser and Randler38,Reference Munoz, Canavate and Hernandez39,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Teixeira, Mota and Crispim54–Reference Vera, Dashti and Gómez-Abellán56,Reference Yasuda, Asako and Arimitsu59–Reference Yoshizaki, Kawano and Noguchi61) , study-specific questionnaires and interviews containing MEQ components (n = 3)(Reference Nakade, Takeuchi and Kurotani40,Reference Östberg42,Reference Romo-Nava, Blom and Guerdjikova47) , the Composite Scale of Morningness (CSM)(Reference Levandovski, Sasso and Hidalgo70) (n = 3)(Reference Halsey, Huber and Low33,Reference Nimitphong, Siwasaranond and Saetung41,Reference Randler and Jankowski44) , the shortened six-item MEQ (n = 2)(Reference Maukonen, Kanerva and Partonen36,Reference Maukonen, Kanerva and Partonen37) and the Diurnal Type Scale (DTS) (n = 1)(Reference Takeuchi, Yamazaki and Oki53) (Table 3).
Other quantitative instruments included those based on ‘midpoint of sleep’ or inactivity to estimate melatonin onset as an indicator of circadian timing(Reference Terman, Terman and Lo71). The Munich Chronotype Questionnaire (MCTQ) estimates chronotype using the midpoint between sleep and wake time on free days, corrected for sleep debt over the work week (MSFSC)(Reference Roenneberg72). The resulting time estimate can be categorised as representing morning, intermediate or evening chronotype based on cut-off values(Reference Juda, Vetter and Roenneberg69). MSFSC has shown to be a good proxy of DLMO(Reference Nováková, Sládek and Sumová73), that is, better than the MEQ(Reference Kantermann, Sung and Burgess16). An extension of the MCTQ for shift workers (MCTQShift) allows MSF calculation for morning, evening and night shifts, with the recommendation that the evening shift calculation best represents chronotype in shift workers(Reference Juda, Vetter and Roenneberg74). Thirteen additional studies estimated chronotype based on ‘midpoint of sleep’ or inactivity, using a range of study-specific questionnaires and interviews about sleep and wake times (n = 5)(Reference Gontijo, Cabral and Balieiro32,Reference Reutrakul, Hood and Crowley46,Reference Sato-Mito, Sasaki and Murakami50,Reference Silva, Mota and Miranda52,Reference Xiao, Garaulet and Scheer57) , sleep diaries (n = 3)(Reference Baron, Reid and Kern27,Reference Friborg, Rosenvinge and Wynn29,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , actigraphy (n = 2)(Reference Baron, Reid and Kern27,Reference Quante, Mariani and Weng43) , the MCTQ (n = 2)(Reference Yadav and Singh58,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) and the Pittsburgh Sleep Quality Index (PSQI) (n = 2)(Reference Reutrakul, Hood and Crowley22,Reference Shimura, Sugiura and Inoue51) (Table 3). To chronotype, studies used either mid-sleep time across the week or only on free days, with or without correcting for sleep debt over weekdays (Table 3).
Assessment of temporal patterns of eating
Amongst the studies reviewed, temporal patterns of eating were identified and pooled into eight categories (Supplementary material: Eight categories of temporal patterns of eating). They include (i) meal timings (n = 22)(Reference Reutrakul, Hood and Crowley22,Reference Baron, Reid and Kern27,Reference Costa, Lievore and Ferrari28,Reference Gangwar, Tiwari and Rawat30–Reference Gontijo, Cabral and Balieiro32,Reference Ishihara, Miyasita and Inugami34,Reference Lucassen, Zhao and Rother35,Reference Nakade, Takeuchi and Kurotani40,Reference Nimitphong, Siwasaranond and Saetung41,Reference Quante, Mariani and Weng43,Reference Randler and Jankowski44,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Sato-Mito, Sasaki and Murakami50,Reference Silva, Mota and Miranda52,Reference Teixeira, Mota and Crispim54,Reference Vera, Dashti and Gómez-Abellán56–Reference Yadav and Singh58,Reference Yazdinezhad, Askarpour and Aboushamsia60,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , (ii) meal skipping (n = 14)(Reference Baron, Reid and Kern27,Reference Friborg, Rosenvinge and Wynn29,Reference Halsey, Huber and Low33,Reference Ishihara, Miyasita and Inugami34,Reference Meule, Roeser and Randler38,Reference Nakade, Takeuchi and Kurotani40,Reference Reutrakul, Hood and Crowley46,Reference Romo-Nava, Blom and Guerdjikova47,Reference Sato-Mito, Sasaki and Murakami50–Reference Teixeira, Mota and Crispim54,Reference Yasuda, Asako and Arimitsu59) , (iii) energy distribution across the day (n = 9)(Reference Reutrakul, Hood and Crowley22,Reference Baron, Reid and Kern27,Reference Lucassen, Zhao and Rother35–Reference Maukonen, Kanerva and Partonen37,Reference Munoz, Canavate and Hernandez39,Reference Nimitphong, Siwasaranond and Saetung41,Reference Östberg42,Reference Yazdinezhad, Askarpour and Aboushamsia60) , (iv) meal frequency (n = 6)(Reference Baron, Reid and Kern27,Reference Friborg, Rosenvinge and Wynn29,Reference Gontijo, Cabral and Balieiro32,Reference Ishihara, Miyasita and Inugami34,Reference Lucassen, Zhao and Rother35,Reference Nimitphong, Siwasaranond and Saetung41) , (v) time interval between meals, or meals and wake/sleep times (n = 5)(Reference Baron, Reid and Kern27,Reference Meule, Roeser and Randler38,Reference Shimura, Sugiura and Inoue51,Reference Xiao, Garaulet and Scheer57,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , (vi) midpoint of food/energy intake (n = 3)(Reference Teixeira, Barreto and Mota55,Reference Vera, Dashti and Gómez-Abellán56,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) , (vii) meal regularity (n = 2)(Reference Shimura, Sugiura and Inoue51,Reference Yoshizaki, Kawano and Noguchi61) and (viii) duration of eating window (n = 1)(Reference Gontijo, Cabral and Balieiro32).
The dietary assessment tools used to capture these temporal patterns of eating include study-specific questionnaires and interviews (n = 18)(Reference Costa, Lievore and Ferrari28–Reference Gangwar, Tiwari and Rawat30,Reference Halsey, Huber and Low33,Reference Ishihara, Miyasita and Inugami34,Reference Meule, Roeser and Randler38,Reference Nakade, Takeuchi and Kurotani40,Reference Quante, Mariani and Weng43,Reference Randler and Jankowski44,Reference Romo-Nava, Blom and Guerdjikova47,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Sato-Mito, Sasaki and Murakami50–Reference Silva, Mota and Miranda52,Reference Yadav and Singh58,Reference Yasuda, Asako and Arimitsu59,Reference Yoshizaki, Kawano and Noguchi61,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) , dietary recalls (24–48 h) (n = 11)(Reference Reutrakul, Hood and Crowley22,Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Gontijo, Cabral and Balieiro32,Reference Maukonen, Kanerva and Partonen36,Reference Maukonen, Kanerva and Partonen37,Reference Munoz, Canavate and Hernandez39,Reference Nimitphong, Siwasaranond and Saetung41,Reference Reutrakul, Hood and Crowley46,Reference Teixeira, Mota and Crispim54–Reference Vera, Dashti and Gómez-Abellán56) and food records (3–7 d) (n = 7)(Reference Baron, Reid and Kern27,Reference Lucassen, Zhao and Rother35,Reference Maukonen, Kanerva and Partonen36,Reference Östberg42,Reference Xiao, Garaulet and Scheer57,Reference Yazdinezhad, Askarpour and Aboushamsia60,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) ; one study did not state the method used(Reference Takeuchi, Yamazaki and Oki53). Of the studies that used study-specific questionnaires, eleven studies did not state the period of recall(Reference Costa, Lievore and Ferrari28–Reference Gangwar, Tiwari and Rawat30,Reference Halsey, Huber and Low33,Reference Ishihara, Miyasita and Inugami34,Reference Meule, Roeser and Randler38,Reference Romo-Nava, Blom and Guerdjikova47,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Shimura, Sugiura and Inoue51,Reference Yadav and Singh58,Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) ; the remaining seven studies specified a period of the last 1 week(Reference Randler and Jankowski44), 2 weeks(Reference Quante, Mariani and Weng43,Reference Silva, Mota and Miranda52) or 1 month(Reference Nakade, Takeuchi and Kurotani40,Reference Sato-Mito, Sasaki and Murakami50,Reference Yasuda, Asako and Arimitsu59,Reference Yoshizaki, Kawano and Noguchi61) . Only three studies used a single study-specific questionnaire that captured both chronotype and temporal patterns of eating together(Reference Nakade, Takeuchi and Kurotani40,Reference Sato-Mito, Sasaki and Murakami50,Reference Silva, Mota and Miranda52) .
Nine studies described their definition of meals. Teixeira, Mota, and Crispim(Reference Teixeira, Mota and Crispim54), Zerón-Rugerio(Reference Zeron-Rugerio, Longo-Silva and Hernaez63), and Baron(Reference Baron, Reid and Kern27) et al. allowed classification of breakfast, lunch and dinner to be based on participants’ perception, while Takeuchi et al.(Reference Takeuchi, Yamazaki and Oki53) defined them as nutritionally rich meals (including carbohydrates, protein, vitamins and minerals). Reutrakul and colleagues defined breakfast and dinner meals as entries including at least one food item (i.e. excluding drink-only entries) and late evening snacks as any caloric intake between last meal and sleep onset(Reference Reutrakul, Hood and Crowley22). Nimitphong et al. defined last mealtime as the latest food intake of the day(Reference Nimitphong, Siwasaranond and Saetung41). However, these studies did not specify a minimum calorie requirement for consideration of a meal, which was done by three other studies. Teixeira et al.(Reference Teixeira, Barreto and Mota55) defined an eating episode as ≥21 kJ, while Lucassen et al.(Reference Lucassen, Zhao and Rother35) ≥84 kJ, and Gontijo et al.(Reference Gontijo, Cabral and Balieiro32) ≥209 kJ. The latter two studies additionally stipulated a time gap between eating occasions; ≥30 min by Lucassen et al.(Reference Lucassen, Zhao and Rother35) and ≥15 min by Gontijo et al.(Reference Gontijo, Cabral and Balieiro32). Despite not providing definitions of meals, the majority of the studies used conventional meal labels, such as breakfast, lunch and dinner, with only five studies(Reference Baron, Reid and Kern27,Reference Gontijo, Cabral and Balieiro32,Reference Lucassen, Zhao and Rother35,Reference Quante, Mariani and Weng43,Reference Xiao, Garaulet and Scheer57) using neutral labels like first and last eating episode/meal/occasion.
Temporal patterns of eating in relation to chronotype
Findings from the eight categories of studies described above (meal timings; meal skipping; energy distribution across the day; meal frequency; time interval between meals, or meals and wake/sleep times; midpoint of food/energy intake; meal regularity; and duration of eating window) are considered in relation to chronotype, in turn, in the following section.
Meal timings
Twenty-two studies considered aspects of meal timings; twenty compared mealtimes between chronotypes, while two compared mealtimes of chronotypes between day type (weekday versus weekend; college days versus vacation days). Seventeen studies used conventional meal labels (i.e. breakfast, lunch dinner), whereas five used non-conventional labels such as first/last meal or eating occasions.
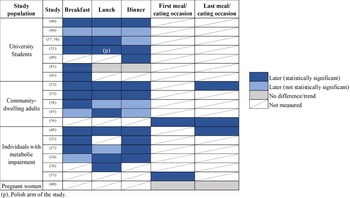
Nineteen of the twenty studies showed one or more meals were consumed later during the day among evening types; the exception was the study by Gontijo et al.(Reference Gontijo, Cabral and Balieiro32) in pregnant women (Fig. 2). Eight studies involved university students, of which evening types had later breakfast times in seven studies, later lunch times in five studies, and later dinner times in six studies. Five other studies included community-dwelling adults outside the student population, and consistently found evening types had later times of food intake across the day(Reference Baron, Reid and Kern27,Reference Costa, Lievore and Ferrari28,Reference Quante, Mariani and Weng43,Reference Xiao, Garaulet and Scheer57,Reference Yazdinezhad, Askarpour and Aboushamsia60) . Six studies included individuals with type 2 diabetes, or who were overweight or obese; in all of them, evening types had later times of one or more main meals than morning types(Reference Reutrakul, Hood and Crowley22,Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Lucassen, Zhao and Rother35,Reference Nimitphong, Siwasaranond and Saetung41,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Vera, Dashti and Gómez-Abellán56) . Fig. 3 illustrates timing of eating occasions for morning, intermediate and evening chronotypes (data from studies including clock times).

Fig. 2. Studies that examined mealtimes amongst chronotypes; presented by mealtimes that were statistically significantly later (dark blue), not statistically significantly later (light blue), or had no difference/trend (grey) amongst evening types compared with morning types. A strikethrough indicates mealtimes that were not measured.

Fig. 3. Studies that reported clock times of eating occasions of morning, intermediate and evening chronotypes over 24 h. Squares depict main meals, which include first eating occasion, breakfast, lunch and dinner; circles depict snacks, which include morning tea, afternoon tea, supper and last meal. Empty squares/circles represent morning chronotypes, filled squares/circles represent evening chronotypes, and shaded squares represent intermediate chronotypes.
Two studies compared differences in times of food intake between weekdays and weekends (i.e. breakfast/lunch/dinner jetlag, or meal lag)(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62), or college and vacation days(Reference Yadav and Singh58). Zerón-Rugerio et al.(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) found evening types to be exposed to meal lag across all three meals, while Yadav and Singh reported no clear pattern in times of food intake between chronotypes between college and vacation days(Reference Yadav and Singh58).
In summary, evening chronotypes tend to have later times of breakfast, lunch and dinner, as well as first and last eating meals/occasions.
Meal skipping
Fourteen studies investigated meal skipping. Six studies categorised subjects on the basis of whether breakfast was skipped or consumed, while eight studies examined the frequency of occurrence of main meal (breakfast, lunch and/or dinner) skipping.
Five out of six studies reported that breakfast skippers tend to be evening chronotypes(Reference Ishihara, Miyasita and Inugami34,Reference Meule, Roeser and Randler38,Reference Reutrakul, Hood and Crowley46,Reference Silva, Mota and Miranda52,Reference Teixeira, Mota and Crispim54) , with one study showing no difference in whether evening and morning chronotypes were breakfast skippers(Reference Baron, Reid and Kern27). These studies segregated breakfast eaters from skippers based on one day’s worth of food intake using a 24-h recall of dietary intake(Reference Reutrakul, Hood and Crowley46) and a questionnaire(Reference Meule, Roeser and Randler38) (n = 2), breakfast skippers as eating breakfast ≤2 times a week based on a 7-d food record(Reference Baron, Reid and Kern27) and a question on habitual intake(Reference Teixeira, Mota and Crispim54) (n = 2), and questionnaires on habits of breakfast skipping or consumption, with no details stated on how that is defined(Reference Ishihara, Miyasita and Inugami34,Reference Silva, Mota and Miranda52) (n = 2).
Eight studies extended the tendency to skip main meals to include frequency of occurrence of main meal skipping. This was either based on a Likert scale rating (e.g. always, often, rarely, never) (n = 3)(Reference Nakade, Takeuchi and Kurotani40,Reference Romo-Nava, Blom and Guerdjikova47,Reference Shimura, Sugiura and Inoue51) or on number of times a week (n = 4)(Reference Friborg, Rosenvinge and Wynn29,Reference Halsey, Huber and Low33,Reference Sato-Mito, Sasaki and Murakami50,Reference Takeuchi, Yamazaki and Oki53,Reference Yasuda, Asako and Arimitsu59) . Altogether, evening chronotypes tend to skip breakfast, lunch and/or dinner at a greater frequency compared with other chronotypes(Reference Friborg, Rosenvinge and Wynn29,Reference Halsey, Huber and Low33,Reference Nakade, Takeuchi and Kurotani40,Reference Romo-Nava, Blom and Guerdjikova47,Reference Sato-Mito, Sasaki and Murakami50,Reference Shimura, Sugiura and Inoue51,Reference Takeuchi, Yamazaki and Oki53,Reference Yasuda, Asako and Arimitsu59) , although frequency of lunch and dinner skipping was only examined in three studies(Reference Friborg, Rosenvinge and Wynn29,Reference Sato-Mito, Sasaki and Murakami50,Reference Takeuchi, Yamazaki and Oki53) . It must, however, be noted that, in Friborg and colleagues’ study, frequency of meal skipping is interpreted based on a combined ‘lower eating habit score’ with the number of meals a person has in a day(Reference Friborg, Rosenvinge and Wynn29). Thus, their results on meal skipping may be skewed by meal frequency, and future tools should segregate collection of these data.
Energy distribution across the day
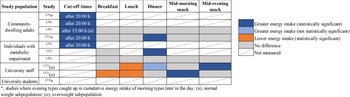
Nine studies assessed energy distribution across the day (Fig. 4).

Fig. 4. Studies that examined energy intake either after study-specified cut-off times during the day (i.e. after 15:00 h or 20:00 h) or at meal/snack times amongst chronotypes; presented as energy intake that was statistically significantly greater (dark blue), not statistically significantly greater (light blue), statistically significantly lower (orange), or had no difference (grey) after the cut-off times or at mealtimes amongst evening types compared with morning types. A strikethrough indicates mealtimes that were not measured.
Five studies analysed energy distribution based on cut-off times of 20:00 h (n = 4)(Reference Baron, Reid and Kern27,Reference Lucassen, Zhao and Rother35–Reference Maukonen, Kanerva and Partonen37) or 15:00 h (n = 1)(Reference Yazdinezhad, Askarpour and Aboushamsia60); they all found later chronotypes to have a greater energy intake than earlier chronotypes after the stipulated cut-off times, with the exception of the overweight subpopulation of Yazdinezhad and colleagues’ study(Reference Yazdinezhad, Askarpour and Aboushamsia60).
Five studies reported energy distribution across main meals and/or snacks, with results across the studies shown to be inconsistent. Reutrakul et al.(Reference Reutrakul, Hood and Crowley22) and Baron et al.(Reference Baron, Reid and Kern27) found later chronotypes had significantly greater energy intake at dinner, while Nimitphong et al.(Reference Nimitphong, Siwasaranond and Saetung41) and Yazdinezhad et al.(Reference Yazdinezhad, Askarpour and Aboushamsia60) both found no differences in energy distribution amongst breakfast, lunch, dinner and snacks between morning types and evening types. In a study where participants were separated on weight status, normal weight evening types were found to have a significantly lower percentage of total energy intake at lunch, but more at mid-evening snack compared with morning types, while overweight evening types had a significantly lower percentage of total energy intake at breakfast and lunch, but higher at mid-morning snack(Reference Munoz, Canavate and Hernandez39).
Of the three studies examining cumulative energy intake across the day, evening types were consistently shown to catch up in energy intake later in the day compared with morning types(Reference Baron, Reid and Kern27,Reference Maukonen, Kanerva and Partonen36,Reference Östberg42) . In summary, evening chronotypes distributed their energy intake towards later times of the day compared with morning chronotypes, although the trend of energy distribution between meals and snacks was inconsistent.
Meal frequency
Five studies looked at daily meal frequency (number of meals in a day) in relation to chronotype. Results were inconsistent, with three studies reporting no difference between chronotypes in frequency of food intake(Reference Baron, Reid and Kern27,Reference Gontijo, Cabral and Balieiro32,Reference Nimitphong, Siwasaranond and Saetung41) and two reporting that evening chronotypes had fewer meals(Reference Friborg, Rosenvinge and Wynn29,Reference Lucassen, Zhao and Rother35) . Of interest is that, of the studies where evening chronotypes had fewer meals, one consisted of individuals who were obese(Reference Lucassen, Zhao and Rother35), while the other based this outcome on an ‘eating habit score’, a sum score of the number of meals a day and the number of days a week that participants ate their main meals(Reference Friborg, Rosenvinge and Wynn29). Hence, these results may be skewed by population-specific traits and other eating habits, respectively.
Time interval between meals, or meals and wake/sleep times
Two studies investigated time intervals between meals. Baron et al.(Reference Baron, Reid and Kern27) found that evening types had shorter durations between breakfast and lunch, as compared with morning types. Meule and colleagues surveyed individuals in the morning (08:00–11:00 h) and the evening (16:00–19:00 h), regarding the number of hours that have passed since their last meal. When surveyed in the morning, evening types had significantly more hours since last meal compared with morning types, suggesting a longer gap between dinner and breakfast time(Reference Meule, Roeser and Randler38). However, when surveyed in the evening, there was no difference in time from last meal between both chronotypes, suggesting similar lunch times.
Four studies examined time intervals between meals and wake/sleep times. Xiao, Garaulet and Scheer(Reference Xiao, Garaulet and Scheer57) found that evening types had less time between awakening and breakfast and, along with three other studies of community-dwelling adults, university students and company employees consistently reported that evening chronotypes had longer intervals between dinner/time of last meal and bedtime/midpoint of sleep(Reference Baron, Reid and Kern27,Reference Shimura, Sugiura and Inoue51,Reference Xiao, Garaulet and Scheer57,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) .
In summary, these studies show evening chronotypes tend to have longer durations between dinner/time of last meal and bedtime/midpoint of sleep. However, there are insufficient studies to identify trends in times intervals between wake times and breakfast or between meals amongst chronotypes.
Midpoint of food/energy intake
Three studies examined midpoint of food/energy intake – defined as either the time point between first and last eating episodes, or the median point of energy intake in a day, which suggests at the spread of food/energy intake in the day. Two studies reported that evening types had significantly later midpoint of intake than morning types(Reference Teixeira, Barreto and Mota55,Reference Vera, Dashti and Gómez-Abellán56) , whereas Zerón-Rugerio et al. examined the difference between midpoint of food intake on weekends compared with weekdays, described by the authors as eating jetlag, and found that, compared with morning types, evening types had a greater difference in time of midpoint of intake on weekends compared with weekdays(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62).
Meal regularity
Two studies in Japan examined meal regularity, using a questionnaire about habitual food intake, in relation to chronotype. In Shimura and colleagues’ study, compared with morning types, evening types had higher odds of reporting irregular mealtimes(Reference Shimura, Sugiura and Inoue51). This was supported by Yoshizaki and colleagues’ study, which found more irregular meal timing for those who are more evening types(Reference Yoshizaki, Kawano and Noguchi61).
Duration of eating window
Only one study investigated chronotype in relation to duration of eating window. This study, in pregnant women, showed no association between duration of eating window and chronotype(Reference Gontijo, Cabral and Balieiro32).
Discussion
Of the thirty-six studies included in this review, thirty-two were published in the last 10 years – evidence of an increased interest in meal timing and health. This review found that evening chronotypes had later timing of meals (either conventional or first/last eating occasions) in nineteen studies and distributed a greater amount of energy and nutrient intake to the later part of the day, especially after 20:00 h, indicating a propensity for energy loading later in the day. Apart from eating later, it was consistently demonstrated that evening chronotypes also had a greater tendency to skip breakfast, lunch and dinner than other chronotypes. Less convincing were data that reported on meal regularity, midpoint of food/energy intake, meal frequency, intervals between meals or meals and sleep/wake times, and duration of eating window owing to a smaller number of included studies. In terms of identifying chronotype, the morningness-eveningness questionnaire was the preferred tool. On the other hand, temporal patterns of eating were primarily captured through study-specific questionnaires/interviews, followed by validated dietary assessment tools such as 24-h dietary recall and food diaries.
Temporal patterns of eating amongst chronotypes: implications for future studies and recommendations
Evening chronotypes have nocturnal lifestyle habits, and this is supported by their temporal patterns of eating. Apart from their greater likelihood and frequency of breakfast skipping, they also show signs of greater frequency of lunch and dinner skipping, which could explain their later timing of main meals (Fig. 3), energy loading towards the latter half of the day, and a longer interval between time of last meal and bedtime. These findings are of relevance as there are epidemiological data that show an association between breakfast skipping and/or late meals and cardiometabolic health(Reference St-Onge, Ard and Baskin1), although the relationship of interval between time of last meal and bedtime on health outcomes has not been well studied(Reference Maw and Haga75). Importantly, these temporal aspects of eating may all be measured using a single method – the actual timing of meals (clock time), rather than patterns of meal skipping, or intervals relative to wake or sleep times. Collecting data on meal timing enables data to be scrutinised, allowing the generation of meal pattern analyses that can then be linked to health outcomes. Using this approach, future studies can easily identify temporal patterns or cut-off times after which food intake may be detrimental to health of particular chronotypes. Additionally, they provide insight into the duration of eating window, the importance of which is discussed next.
Only one study examined participants’ duration of eating window in relation to chronotype, and this was in pregnant women. Duration of eating window is important given that metabolic processes such as glucose tolerance and insulin sensitivity peak in the morning and decrease towards the night(Reference Poggiogalle, Jamshed and Peterson3), and night-time eating is associated with perturbed glucose and lipid metabolism(Reference Leung, Huggins and Bonham4,Reference Bonham, Kaias and Zimberg76) , thus increasing risk of cardiovascular disease and type 2 diabetes(Reference St-Onge, Ard and Baskin1,Reference Zhang, Wu and Na77) . Meanwhile, minimising eating occasions by reducing the duration of eating period through time-restricted feeding has beneficial implications for metabolic health even in the absence of weight loss(Reference Sutton, Beyl and Early78,Reference Hutchison, Regmi and Manoogian79) . Research has shown how limiting the eating window through time-restricted feeding trials have benefits on fat oxidation, blood pressure, glucose levels and inflammation(Reference Sutton, Beyl and Early78,Reference Chung, Chou and Sears80–Reference Jamshed, Beyl and Della Manna82) . Failure to account for one’s duration of eating window may explain why studies of other temporal aspects of eating such as breakfast skipping have found conflicting results on cardiometabolic health outcomes(Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Deshmukh-Taskar, Nicklas and Radcliffe83,Reference Bonnet, Cardel and Cellini84) . Additionally, as evening chronotypes have late wake and sleep times, exploring if a late restricted feeding window provides health benefits may prove to be a feasible strategy to suit their lifestyle. Conversely, if the metabolic benefits of time-restricted feeding are only seen with earlier (relative to body clock timing) eating windows, this is important information for health recommendations for those with greater eveningness(Reference Rynders, Thomas and Zaman85).
Energy distribution across the day has implications for metabolic health because consuming food at night can exacerbate circadian misalignment(Reference Damiola, Le Minh and Preitner86), which results in impaired glucose tolerance, inflammatory markers and blood pressure in healthy adults(Reference Morris, Yang and Garcia7,Reference Morris, Purvis and Hu8,Reference Scheer, Hilton and Mantzoros87) . Whilst five studies in this review suggest that evening chronotypes distribute a greater percentage of their energy intake towards later parts of the day(Reference Maukonen, Kanerva and Partonen36,Reference Maukonen, Kanerva and Partonen37,Reference Quante, Mariani and Weng43,Reference Xiao, Garaulet and Scheer57,Reference Yazdinezhad, Askarpour and Aboushamsia60) , three did not present data on dinner intake(Reference Maukonen, Kanerva and Partonen36,Reference Maukonen, Kanerva and Partonen37,Reference Quante, Mariani and Weng43) , which means food intake at this time of the day could have consisted of a large dinner meal or may have been smaller snacks. Regardless, this behaviour of night-time eating poses health concerns as intervention studies have demonstrated that individuals who consumed dinner later rather than earlier had higher triacylglycerol and cholesterol levels(Reference Yoshizaki, Tada and Hida88), as well as raised glucose levels after breakfast the next day(Reference Sato, Nakamura and Ogata5,Reference Tsuchida, Hata and Sone89) . At the same time, a review found that individuals who consume food late in the night tend to choose foods rich in carbohydrates, including refined sugars(Reference Gallant, Lundgren and Drapeau90), while a separate study found that foods consumed between 22:00 h and 01:59 h to be the most energy dense of the day(Reference de Castro91), suggesting at the poor quality of food consumed in the late night. Strategies to minimise these risks include modifying the energy content or macronutrient composition of food consumed at night. This was demonstrated in studies by Jakubowicz et al., where limiting dinner intake to 837 kJ at 19:00 h(Reference Jakubowicz, Wainstein and Ahren92), or between 18:00 h and 21:00 h(Reference Jakubowicz, Barnea and Wainstein93), has beneficial effects on weight outcome and glycaemic control. Similarly, consuming a high-protein meal compared with a standard meal at night alleviated increases in postprandial glucose levels(Reference Davis, Bonham and Nguo94). Therefore, providing guidance to evening chronotypes on limiting energy intake at night and careful consideration of food choice may be helpful.
A small number of studies included in this review explored factors such as meal regularity and variability in weekday and weekend food intake. Evening types were found to have more irregular meal timings(Reference Shimura, Sugiura and Inoue51,Reference Yoshizaki, Kawano and Noguchi61) , previously defined as a generally inconsistent frequency and spacing of eating occasions across the day(Reference Leech, Worsley and Timperio10). As meal irregularity has been associated with increased risk of metabolic syndrome, and increased body mass index (BMI) and waist circumference(Reference Sierra-Johnson, Unden and Linestrand95,Reference Pot, Hardy and Stephen96) , the ability to identify irregular from regular meal eaters is crucial. Whilst regularity of meals has been linked to frequency, we found no clear relationship between chronotype and meal frequency. As the evidence linking meal frequency with cardiometabolic health status has been inconsistent(Reference St-Onge, Ard and Baskin1), a key question lies in whether meal frequency is a relevant temporal pattern of eating that warrants further investigation, and inadvertently, does meal frequency mask relevant details such as the aforementioned duration of eating window, spacing between meals, or regularity. Because of the lack of detail data on meal frequency provides, these other temporal factors should take precedence over data on meal frequency, or at least be analysed in relation to it. In terms of variability between weekday and weekend food intake, only two studies looked at it in terms of mealtimes and meal frequency. Compared with morning chronotypes, evening chronotypes had a larger difference between weekdays and weekends in terms of meal times (later meals on weekends)(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) and frequency (trend of greater frequency on weekends)(Reference Lucassen, Zhao and Rother35). Firstly, this highlights a large gap where future studies may investigate differences between weekday and weekend habits; if temporal patterns of eating are found to be worse on certain days of the week, intervention studies may then be targeted towards addressing them first. Secondly, later meal times on weekends have been associated with greater social jetlag(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62) (the difference between midpoint of sleep on work and work-free days(Reference Roenneberg, Allebrandt and Merrow97)), while also suggesting at meal irregularity across the week. Therefore, future studies should also consider the implications of sleep regularity, including timing and duration of sleep, on meal timing and regularity.
Inclusion of other study populations
Whilst the health implications that morning and evening chronotypes face as a result of their mealtimes are apparent, results are not easily generalisable across populations because of cultural differences in food and eating patterns(Reference Park, Freisling and Huseinovic98). In this review, almost half the studies conducted in Europe practice siesta(Reference Costa, Lievore and Ferrari28,Reference Garaulet, Gomez-Abellan and Alburquerque-Bejar31,Reference Munoz, Canavate and Hernandez39,Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Vera, Dashti and Gómez-Abellán56,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , but none highlighted how the presence of a siesta influences dinner times. From this review, in Spain, while evening chronotypes were found to still have later dinners than morning chronotypes(Reference Ruiz-Lozano, Vidal and de Hollanda48,Reference Vera, Dashti and Gómez-Abellán56) , the negative consequences to health attributed to evening types for their late meals may also be shared by morning types in this population, whose dinner times were even later than that of evening types in the other study populations (refer to Ruiz-Lozano(Reference Ruiz-Lozano, Vidal and de Hollanda48), and Vera and colleagues’(Reference Vera, Dashti and Gómez-Abellán56) study in Fig. 3). This also applies to the religious practice of Ramadan, where Muslims fast between sunrise and sunset for a month, which may negate the difference usually observed in temporal patterns of eating between chronotypes. These cultural and religious differences reinforce the need for such studies to be conducted across populations, and for authors to identify and comment on cultural traits that could potentially diminish or amplify the differences in temporal patterns of eating between chronotypes, which could then alter effects on their health outcomes.
Assessment of chronotype: implications for future studies and recommendations
The majority of studies in this review used questionnaires that identify ‘morningness-eveningness preference’ as an indicator of chronotype instead of ‘midpoint of sleep’. While the former, which includes the MEQ, CSM and DTS, represents a psychological indicator of chronotype(Reference Di Milia, Adan and Natale99), the latter, which includes the MCTQ, represents a behavioural indicator of chronotype. The strengths and weaknesses of each of these questionnaires lie beyond the scope of this review, and have been comprehensively detailed in an earlier review paper(Reference Di Milia, Adan and Natale99).
A challenge for the field is that, despite multiple studies using the same tool, studies in this review employed a myriad of cut-off points in categorising chronotypes. Most studies used population specific cut-offs such as median, tertiles and quartiles, while few used the original thresholds suggested by questionnaire authors. This resulted in great variation in cut-off points where evening types were defined by an almost 2·5-h difference in mid-sleep time(Reference Baron, Reid and Kern27,Reference Xiao, Garaulet and Scheer57) . A previous review has concluded that percentile-based cut-offs applied to the population are preferable as they reflect the spread of chronotypes specific to the population and its culture(Reference Caci, Deschaux and Adan100). However, the lack of standardisation in cut-off methods employed amongst studies in this review reduces comparability of outcomes.
A further challenge for studies that extrapolate concepts or components of original questionnaires such as the MEQ and the MCTQ into their study-specific questionnaires is the accuracy of data provided to chronotype participants. In this review, some study-specific questionnaires were used that asked participants to subjectively report sleep and wake times(Reference Gontijo, Cabral and Balieiro32,Reference Reutrakul, Hood and Crowley46,Reference Sato-Mito, Sasaki and Murakami50,Reference Silva, Mota and Miranda52,Reference Xiao, Garaulet and Scheer57) . While the MCTQ requests the same information, it has been validated as a whole, through correlation with sleep diaries, actigraphy, and melatonin rhythms(Reference Roenneberg, Kuehnle and Juda101). Furthermore, some studies(Reference Gontijo, Cabral and Balieiro32,Reference Silva, Mota and Miranda52,Reference Xiao, Garaulet and Scheer57) had a recall period of sleep and wake habits that were shorter than the recall period of 1 month used by the MCTQ. Out of thirteen studies, five did not chronotype based on MSFSC, and instead used mid-sleep time on weekdays(Reference Sato-Mito, Sasaki and Murakami50), mid-sleep time averaged across a 7-d week(Reference Baron, Reid and Kern27,Reference Quante, Mariani and Weng43,Reference Zeron-Rugerio, Longo-Silva and Hernaez63) , or MSF without correcting for sleep debt(Reference Zeron-Rugerio, Hernaez and Porras-Loaiza62), which can skew chronotype estimates. This raises questions about the validity of these study-specific questionnaires for estimation of chronotype, and applies equally to study-specific questionnaires that incorporate only an item from the MEQ as an indicator of chronotype(Reference Romo-Nava, Blom and Guerdjikova47). Limitations arising due to deviation from originally validated instruments could be overcome by further studies validating alternative, briefer instruments. Care must be taken to accurately capture the concept of chronotype using validated measures.
Lastly, for the purpose of identifying chronotype in relation to temporal patterns of eating, ‘midpoint of sleep’ may be a better choice compared with ‘morningness-eveningness preference’ as the latter considers personal preferences for activities at various points of the day that do not represent differences in individual circadian rhythm cycle. This shortcoming is addressed by the MCTQ, by factoring in and accounting for differences in sleep–wake patterns on work and work-free days(Reference Levandovski, Sasso and Hidalgo70). As results of the MCTQ are a point in time instead of a score on preference, it is a continuous trait(Reference Juda, Vetter and Roenneberg69), which makes it more adaptable for evaluation in relation to time points of meals. At the same time, in the process of obtaining MSFSC, collection of data on wake and sleep times forms relevant datapoints to be analysed in relation to mealtimes or circadian phase.
Identifying temporal patterns of eating and creating the ideal questionnaire
Choice of dietary method should depend on study objectives, including but not limited to the dietary aspect of interest, the requirement for absolute versus relative intake data, the time frame of interest, the extent of specificity in dietary data required, and resource availability(Reference Biró, Hulshof and Ovesen102). In this review, a variety of dietary assessment tools were used; most commonly, study-specific questionnaires or interviews, followed by 24-h dietary recalls, and food records. Dietary recalls and food records both capture temporal patterns of food intake, with the latter being the preferred tool as it captures differences in food intake across the days of the week and minimises recall bias since food intake is recorded on consumption(Reference Shim, Oh and Kim103). However, it places a large burden on participants, who have to fill in details of food types and portion sizes, and training of both researchers and participants is required to ensure accurate data collection(Reference Livingstone and Black104). This renders food records to be a time-intensive form of dietary assessment, which, if not done properly, reduces the reliability of findings. To overcome these limitations, studies in this review used study-specific questionnaires that focused on obtaining the key points of interest – temporal patterns of eating, such as mealtimes or meal regularity. However, these questionnaires were not validated, and are lacking in their ability to capture all temporal patterns of eating. Forslund and colleagues recently developed a meal pattern questionnaire to collect data on frequency, type and time of meals(Reference Bertéus Forslund, Lindroos and Sjöström49). Whilst participants completed all the questionnaires that were returned, which suggests at the ease of filling in such a tool, it was not validated. Similarly, a Chrononutrition Profile Questionnaire (CP-Q) created by Veronda et al. identifies six components of chrononutrition likely to influence health(Reference Veronda, Allison and Crosby105). While its components were validated against the Automated Self-Administered 24-h Dietary Assessment Tool (ASA24), PSQI, and Night Eating Questionnaire (NEQ), it does not differentiate between workdays and work-free days across all temporal patterns of eating, nor does it collect information on chronotype. Making improvements to existing questionnaires to create a single instrument that captures all temporal patterns of eating in relation to chronotype and/or work schedules is relatively straightforward, and will result in a convenient tool that provides a wealth of information for use by future epidemiological studies in this area.
Further distinguishing factors between study-specific questionnaires include the presence or absence of a recall period, the duration of recall, and the definition used to define eating occasions. Amongst the questionnaires that stipulated a recall period, the majority used a duration of 1 month, which is helpful as a longer duration better reflects habitual intake. This duration also reflects the timeframe captured by the MCTQ, so a combination of the two will generate information on both chronotype and temporal patterns of eating. Questionnaires should also standardise the definition of meals between neutral labels (i.e., eating occasion) or conventional labels (i.e., breakfast, lunch, dinner and snack). Neutral labels are more all-encompassing compared with conventional labels, which may hold different meanings to individuals with different cultural backgrounds(Reference Mäkelä, Kjærnes and Pipping Ekström106) and work types (e.g., night shift workers may face difficulty in deciding which meal constitutes breakfast). Neutral labels have also been shown to best predict variance in total energy intake(Reference Leech, Worsley and Timperio107). Yet, meal size or caloric load consumed at midnight has been shown to impact on glucose response to breakfast the next morning(Reference Centofanti, Dorrian and Hilditch108). Hence, the ability to distinguish main meals from snacks as an indicator of size of meal or caloric load may prove to be useful when analysing the impact of timing of food intake on health outcomes. A method most commonly used in studies is the participant-identified method of distinguishing main meals from snacks(Reference Leech, Worsley and Timperio10), and would thus serve well for this purpose. Incorporating these elements to create a purposeful and customised validated questionnaire allows the collection of targeted and relevant information on temporal patterns of eating with ease, speed and convenience.
Conclusion
This is the first review using systematic search techniques to present data on the temporal patterns of eating in relation to chronotype. The interaction between chronotype and timing and patterns of meals is important due to the impact of these relationships on cardiometabolic disease risk, as demonstrated in Fig. 5. Although the body of literature is sufficient to indicate key directions for future research, conclusive statements regarding findings are limited due to the large methodological variation across studies, with clear opportunities indicated for standardisation and validation. As epidemiological studies show evening chronotypes face increased risk of obesity and chronic diseases, it is important to understand which aspects of timing are driving health risk and how this translates for individuals with anomalies in circadian timing, including late chronotypes and shift workers. In this review, evening chronotypes tend to skip meals more frequently, have later mealtimes, and distribute more of their energy intake towards later times of the day than morning chronotypes. Future studies should analyse meal frequency in relation to meal timing, meal regularity and duration of eating window, and further explore chronotype-related differences in meal regularity and duration of eating window. Lastly, tools to collect data on chronotype and temporal patterns of eating are varied; they should be unified into a single assessment tool so future studies may identify these outcomes in a standardised manner. This will enable the development of more comprehensive and concise guidelines to optimise health outcomes through temporal patterns of eating.

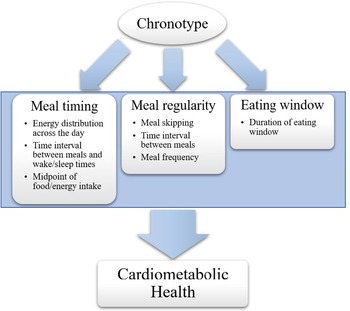
Fig. 5. The eight categories of temporal patterns of eating identified from the literature in this review, that is, (i) meal timings, (ii) meal skipping, (iii) energy distribution across the day, (iv) meal frequency, (v) time interval between meals, or meals and wake/sleep times, (vi) midpoint of food/energy intake, (vii) meal regularity, and (viii) duration of eating window, can be organised into three fundamental aspects of eating patterns that are influenced by chrononutrition and impact on cardiometabolic health outcomes: meal timing, meal skipping, and eating window. All of these aspects can be derived from a record of eating times. Creating a single, standardised, validated measure to investigate these factors in relation to chronotype will facilitate targeted recommendations for timing of food intake, tailored to individual body clock timing, in order to improve cardiometabolic health.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, or commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
Conceptualisation, Y.P., M.R., M.B., J.D. and A.C.; methodology, Y.P.; validation, M.R., M.B., J.D. and A.C.; data curation, Y.P.; writing – original draft preparation, Y.P.; writing – review and editing, Y.P., M.R., M.B., J.D. and A.C.; supervision, M.R., M.B., J.D. and A.C.; project administration, Y.P.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0954422421000123