Traumatic brain injury (TBI) is a significant cause of disease burden worldwide (James et al., Reference James, Bannick, Montjoy-Venning, Lucchesi, Dandona, Dandona and Zaman2019) and has both economic impact due to direct medical and rehabilitation costs as well as indirect social impacts related to disability and loss of function (Nguyen et al., Reference Nguyen, Fiest, McChesney, Kwon, Jette, Frolkis and Gallagher2016). Mild traumatic brain injury (mTBI) is the most common form of TBI and accounts for as much as 80% of all head-related injuries seen at Emergency Departments (Dewan et al. Reference Dewan, Rattani, Gupta, Baticulon, Hung, Punchak and Park2019; Faul, Xu, Wald & Coronado, Reference Faul, Xu, Wald and Coronado2010). This has led to extensive research in various populations, including paediatric mTBI and associated outcomes in adulthood (Crowe, Babl, Anderson & Catroppa, Reference Crowe, Babl, Anderson and Catroppa2009; Emery et al., Reference Emery, Barlow, Brooks, Max, Villavicencio-Requis, Gnanakumar and Yeates2016), blast-related mTBI in military settings (Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Schneiderman, Braver & Kang, Reference Schneiderman, Braver and Kang2008), chronic traumatic encephalopathy after repetitive sports-related concussion (Baugh et al., Reference Baugh, Stamm, Riley, Gavett, Shenton, Lin and Stern2012), and long-term risk of dementia after mid-life TBI (Godbolt et al., Reference Godbolt, Cancelliere, Hincapié, Marras, Boyle, Kristman and Cassidy2014; Livingston et al., Reference Livingston, Huntley, Sommerlad, Ames, Ballard, Banerjee and Mukadam2020). When specifically considering aging populations, previous research has predominantly focused on outcome following moderate–severe head trauma, noting an increasing risk of mortality with increasing age (Hashmi et al., Reference Hashmi, Ibrahim-Zada, Rhee, Aziz, Fain, Friese and Joseph2014; McIntyre, Mehta, Aubut, Dijkers & Teasell, 2013a). However, to date, there has been less focus on outcome associated with milder injury sustained in older age. Therefore, it is timely to evaluate the existing literature to understand the overall impact of mTBI in older age and to identify current gaps in research for this population.
The global trend towards aging communities (World Health Organisation, 2011) and noted increase in older age demographics presenting at Emergency Departments following traumatic injury (Mitra & Cameron, Reference Mitra and Cameron2012) highlight the need to focus on older age patients. Compared with younger cohorts, older people are at higher risk of mortality following moderate–severe TBI (Cheng et al., Reference Cheng, Lin, Lee, Hsu, Lee and Su2014; Dams-O’Connor et al., Reference Dams-O’Connor, Cuthbert, Whyte, Corrigan, Faul and Harrison-Felix2013; Gardner, Dams-O’Connor, Morrissey & Manley, Reference Gardner, Dams-O’Connor, Morrissey and Manley2018; Hukkelhoven et al., Reference Hukkelhoven, Steyerberg, Rampen, Farace and Habbema2003) and also possibly following mTBI (Susman et al., Reference Susman, Dirusso, Sullivan, Risucci, Nealon and Cuff2002). Explanations for this increased vulnerability may include age-related structural changes to the brain, such as brain volume shrinkage (Fjell & Walhovd, Reference Fjell and Walhovd2010) and the dura adhering more closely to the skull leading to stretching and weakening of bridging veins (Flanagan, Hibbard & Gordon, Reference Flanagan, Hibbard and Gordon2005; Karibe et al., Reference Karibe, Hayashi, Narisawa, Kameyama, Nakagawa and Tominaga2017; Thompson, McCormick & Kagan, Reference Thompson, McCormick and Kagan2006). Older age is also related to an increased risk of frailty or having at least one chronic health condition (Thompson, Dikeman & Temkin, 2012; Vogeli et al., Reference Vogeli, Shields, Lee, Gibson, Marder, Weiss and Blumenthal2007) which can produce symptoms, such as balance instability or visual deficits. These impairments can increase the risk of injury (Ambrose, Paul & Hausdorff, Reference Ambrose, Paul and Hausdorff2013; Rubenstein, Reference Rubenstein2006) and also the rate of recovery (Abdulle et al., Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018; Rapoport, McCullagh, Streiner & Feinstein, Reference Rapoport, McCullagh, Streiner and Feinstein2003). Comorbidities seen in older adults often require pharmacological intervention that can interact with trauma effects and subsequent management. For example, anticoagulants commonly prescribed to manage heart conditions in older adults may also increase the risk of a brain bleed if blunt trauma is applied to the head (Peck et al., Reference Peck, Calvo, Schechter, Sise, Kahl, Shackford and Blaskiewicz2014). This potential vulnerability to more problematic trauma outcome after mTBI has resulted in calls for more targeted research in older age populations (Kristman et al., Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014; Peters & Gardner, Reference Peters and Gardner2018).
Although there is some debate about the time course of recovery from mTBI, the general consensus remains that recovery on standard neuropsychological testing is expected within the first 3 months of injury for working-age adults, who present with no additional risk factors (Carroll et al., Reference Carroll, Cassidy, Peloso, Borg, von Holst, Holm and Pépin2004; Frencham, Fox & Maybery, Reference Frencham, Fox and Maybery2005; Karr, Areshenkoff & Garcia-Berrera, 2014; Rohling et al., Reference Rohling, Binder, Demakis, Larrabee, Ploetz and Langhinrichsen-Rohling2011). Nevertheless, in older people, differences in pre-injury cognitive reserve and presence of comorbidities may play an important role in outcome post-injury (Kumar et al., Reference Kumar, Juengst, Wang, Dams-O’Connor, Dikmen, O’Neil-Pirozzi and Wagner2018; Schneider et al., Reference Schneider, Sur, Raymont, Duckworth, Kowalski, Efron and Stevens2014), and it remains unclear whether the 90-day timeline for cognitive recovery holds true for older populations (Kinsella, Olver, Ong, Hammersley & Plowright, 2014a). Therefore, in older age cohorts, it is important to consider slower rates of recovery through extended follow-up of trauma outcomes.
Additionally, although cognition is an important measure of TBI outcome, those who are often considered to be cognitively “recovered” (i.e., demonstrate no deficits on objective neuropsychological assessment) may continue to report difficulties related to daily activities and low mood (Cassidy et al., Reference Cassidy, Cancelliere, Carroll, Côté, Hincapié, Holm and Borg2014). Indeed, cognitive recovery may not always be indicative of functional recovery, as it has been consistently shown that at least a small proportion of working-age adults show incomplete functional recovery from mTBI up to 12-month post-injury (De Koning et al., Reference De Koning, Scheenen, van der Horn, Hageman, Roks, Spikman and van der Naalt2017; Korley et al., Reference Korley, Diaz-Arrastia, Falk, Peters, Leoutsakos, Roy and Bechtold2017; McMahon et al., Reference McMahon, Hricik, Yue, Puccio, Inoue, Lingsma and Vassar2014; Nelson et al., Reference Nelson, Temkin, Dikmen, Barber, Giacino, Yuh and Zafonte2019; Scheenen et al., Reference Scheenen, Spikman, De Koning, van der Horn, Roks, Hageman and van der Naalt2017; van der Horn, Spikman, Jacobs & van der Naalt, Reference van der Horn, Spikman, Jacobs and van der Naalt2013).
To address this, Silverberg et al. (Reference Silverberg, Crane, Dams-O’Connor, Holdnack, Ivins, Lange and Iverson2017) propose a more global endpoint for recovery that includes other domains of outcome in addition to cognition, such as psychological health (e.g., anxiety, depression, post-concussion symptoms (PCSs), and posttraumatic stress) and life participation (e.g., recreational activities, community integration, quality of life, etc.). This more global approach in outcome measurement does start to address the potentially complex interactions between cognition, psychological status, and functional capacity. Although the most commonly used measure of functional recovery from injury remains the Glasgow Outcome Scale (original and extended versions; GOS/GOSE), some concerns have been raised about its use in determining outcome in older populations, where premorbid functioning and comorbidities may be misattributed to mTBI effects (Gardner et al., Reference Gardner, Dams-O’Connor, Morrissey and Manley2018). Nevertheless, at this stage, the GOS/GOSE provides the most extensive data on functional outcome.
The purpose of this review was to provide a systematic evaluation of the literature on mTBI sustained in older adulthood (i.e., ≥60 years of age). Outcome domains included cognition, psychological health, and life participation (including functional recovery based on the GOS/GOSE). Recovery was considered at various time points post-injury.
METHODS
This systematic review was conducted in accordance with the established PRISMA guidelines (Moher et al., Reference Moher, Liberati, Tetzlaff, Altman, Altman, Antes and Tugwell2009) and a search protocol was created and registered with PROSPERO (registration number: CRD42020139113).
Definition of mTBI
A problem that often pervades mTBI research is the inconsistency in both the reporting and operationalisation of mTBI (Kristman et al., Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014). The American Congress of Rehabilitation Medicine (ACRM) and the International Collaboration on mTBI Prognosis (National Center for Injury Prevention and Control, 2003; Kristman et al., Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014; Menon, Schwab, Wright & Maas, Reference Menon, Schwab, Wright and Maas2010) have proposed largely consistent guidelines to identify mTBI using four main criteria: (1) loss of consciousness and Glasgow Coma Scale (GCS) score; (2) posttraumatic amnesia; (3) alteration in mental state; and (4) focal neurological deficits or abnormalities. The International Collaboration on mTBI taskforce states that “mild TBI is an acute injury resulting from mechanical energy to the head from external physical forces” (Kristman et al. Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014, p. S266) and provides specific criteria to determine the presence or absence of injury (see Table 1).
Table 1. International Collaboration on mTBI prognosis case definition criteria for mild traumatic brain injury

For the purpose of this review, only articles that are consistent with (but not necessarily identical to) this definition of mTBI were included. As posttraumatic amnesia duration is often not reported in mTBI cases due to the transient nature of injury, this criterion was used as an indication of injury severity when reported, however, was not required for inclusion for review.
Search Strategy
In collaboration with search experts and researchers within the field of mTBI, a comprehensive search strategy for studies of mTBI outcomes was developed, focusing particularly on the outcome domains: (1) cognition; (2) psychological health; and (3) life participation (see Tables 2 and 3).
Table 2. General MeSH terms and key search terms related to mTBI and older adults that were used for the systematic search in MEDLINE (OVID)

*As age range included the younger age bracket of 60–65 years, the MeSH term “Aged” (referring to 65–79 years olds) as well as the key search word “Over 60” was included to ensure all participants 60+ years were captured.
Table 3. Additional MeSH terms and key search terms related to specific domains of function that were used for the systematic search in MEDLINE (OVID)

The electronic databases MEDLINE (OVID), Embase (OVID), CINAHL (EBSCO), and PsychINFO (OVID) were systematically searched up to March 23, 2020. There was no date restriction applied to database searches, as the aim was to achieve a comprehensive review of all literature within the field. However, it was noted that early research investigating mTBI was often prone to incomplete documentation of mTBI classification. To ensure consistency of injury type and severity, we used the current widely accepted diagnostic criteria to determine the presence of mTBI which resulted in the loss of some early research studies (see Figure 1).
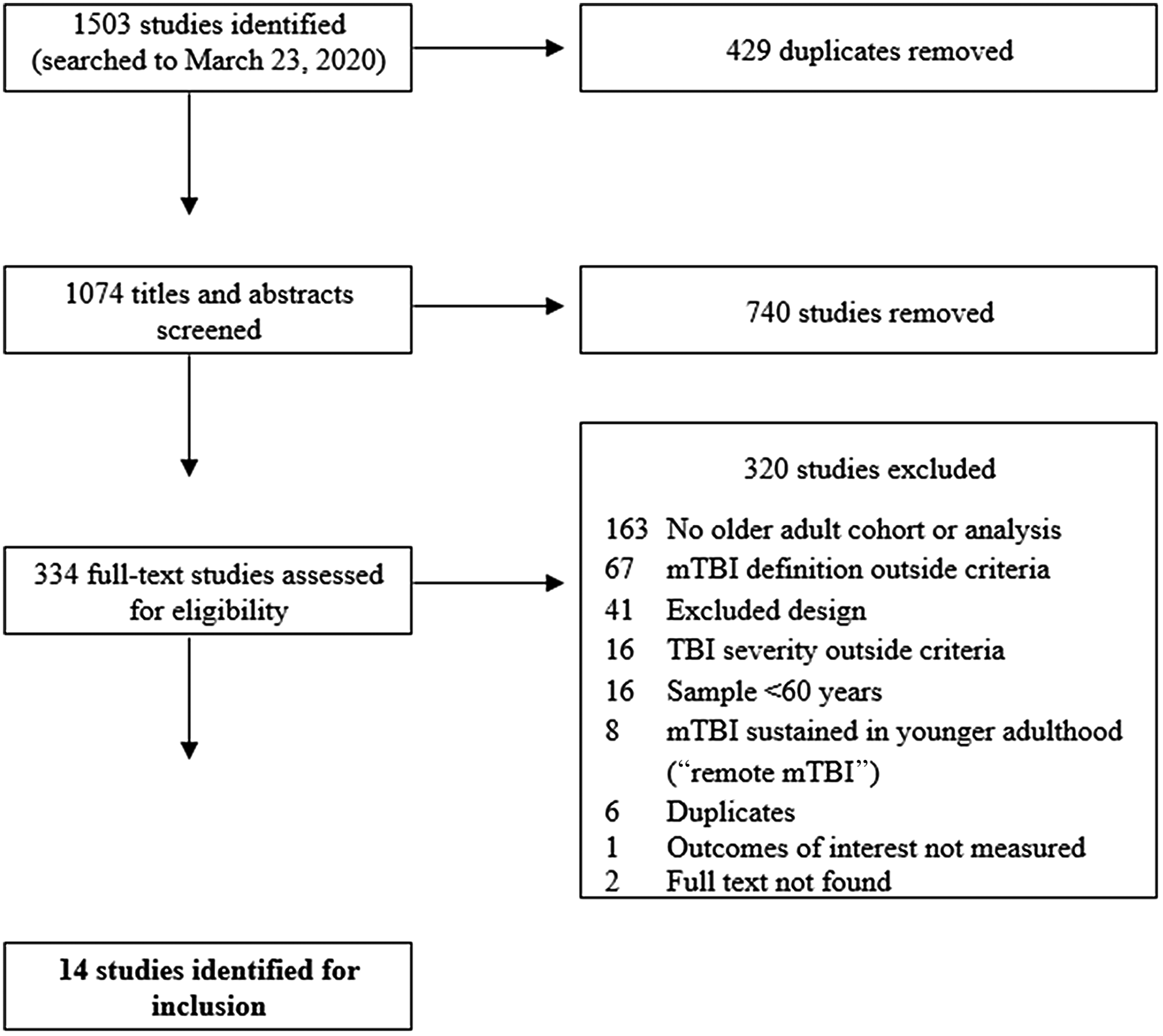
Fig. 1. Flowchart of study selection process.

Fig. 2. Forest plot depicting the proportion of older and younger adults recovered from mTBI at 6–12 months, based on GOS score. Note.* = 12-month follow-up.
Study Selection
Two independent reviewers were involved in screening studies for inclusion using a web-based software tool (Covidence; Veritas Health Innovation, www.covidence.org) which allowed reviewers to organise search results, efficiently manage the screening of titles and abstracts, and identify and locate full texts for inclusion. This software program is designed to follow the PRISMA guidelines for article screening, risk of bias assessment, and data extraction.
First, titles of all citations were retrieved from database searches and duplicates were removed. From 1503 titles identified, 429 duplicates were removed. Next, 1074 abstracts and titles were screened; those that were clearly not related to mTBI (e.g., severe TBI) or older adult populations (e.g., paediatric samples) were removed. Additionally, any titles and abstracts that violated the exclusion criteria listed below were also removed.
-
Samples representing populations <60 years (e.g., collegiate athletes), or adult samples not distinctly stratified into an older adult age group, or articles with no separate analysis of older adult cohorts
-
Samples that included injury severity greater than “mild” TBI (i.e., moderate–severe brain injury) or that combined different severities of TBI
-
Long-term outcome of mTBI sustained in younger adult (i.e., remote mTBI) in older populations
-
Insufficient detail to identify mTBI according to the operationalised criteria (Table 1)
-
Animal studies
-
Study designs and formats including letters, narrative reviews, reviews without data, theses, government reports, books and book chapters, case reports, and case series
From this, 334 full-text articles were obtained to assess full-text eligibility based on selection criteria below:
-
Full-text access and published in the English language
-
Identified acute mTBI in accordance with our criteria, including complicated mTBI but not those requiring neurosurgery
-
Sample or cohort analysis ≥60 years
-
Study designs and formats including meta-analyses, systematic reviews, randomised controlled trials, cohort studies, case–control studies, and cross-sectional studies
Any disagreement about inclusion of full-text articles was discussed by both reviewers and consensus was reached for all conflicts, without need for a third reviewer. Fourteen studies were identified for final inclusion (see Figure 1).
All identified papers were evaluated using an appropriate quality assessment tool based on research design (Quigley, Thompson, Halfpenny & Scott, Reference Quigley, Thompson, Halfpenny and Scott2019). The Quality Assessment Tool for Observational Cohort and Cross-sectional Studies (National Heart Lung and Blood Institute, 2017) comprises of 14 items, assessed as present or absent from study design. All 14 identified studies were evaluated as being of “fair” or “good” quality (see Supplementary Table 1). Four of the 14 studies were identified as having overlapping samples and therefore the effect sizes were selected only from studies that provided relevant information, or the most recent information (see Tables 4–7 for details). All data included in this manuscript were obtained in compliance with institutional/national research standards for human research and the Helsinki Declaration.
Table 4. Characteristics of identified studies
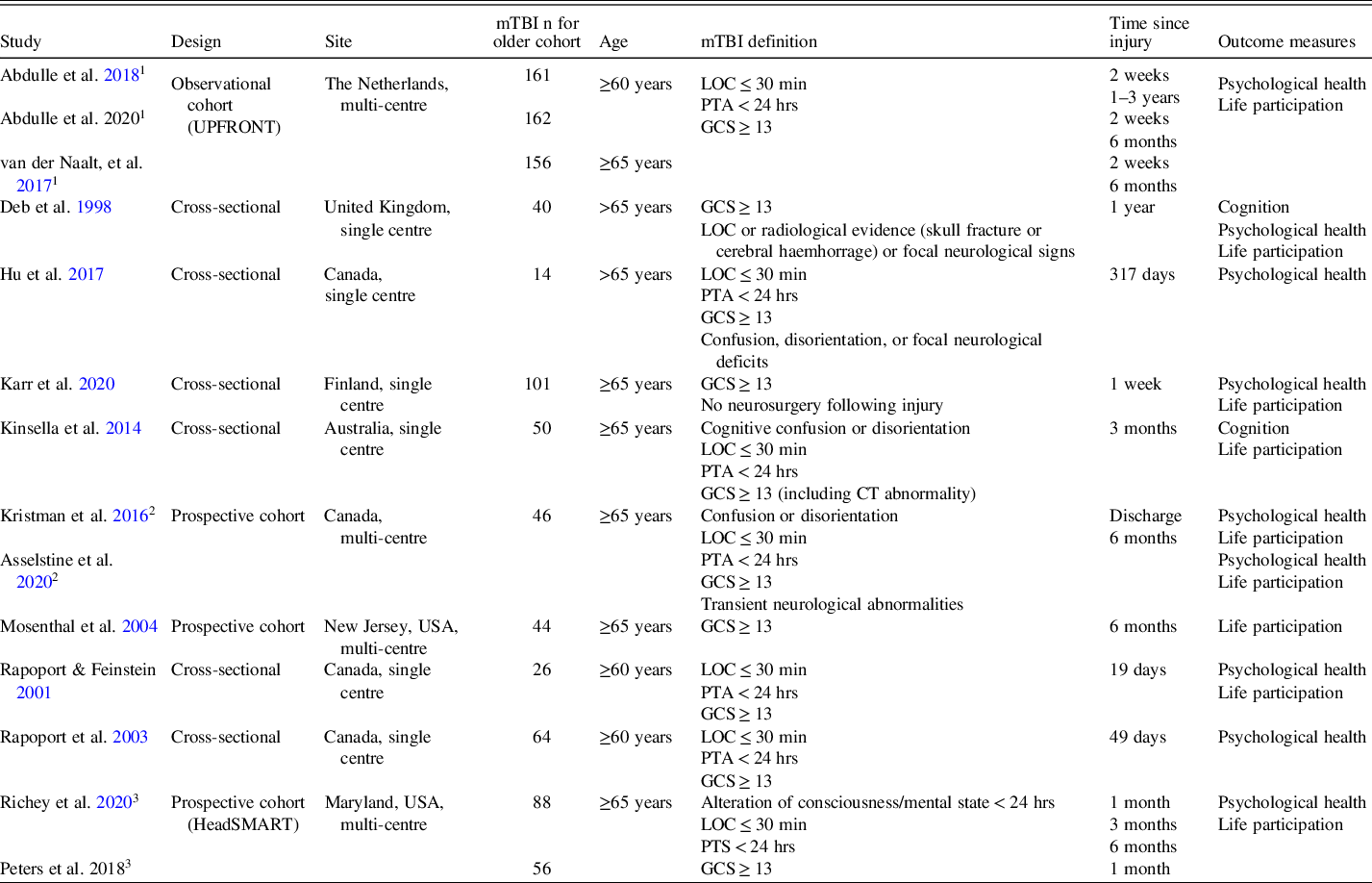
Note: “Cognition” refers to performance on standardized cognitive assessment; “Psychological Health” outcomes refer to self-reported depressive and anxiety symptoms, post-concussion symptoms (psychological and/or physical), or general mental wellbeing; “Life Participation” outcomes refer to self-reported health and general quality of life, functional status, and/or recovery from injury.
LOC = loss of consciousness; PTA = posttraumatic amnesia; GCS = Glasgow Coma Scale; PCS = post-concussion symptoms.
1 Sample from the UPFRONT observational study, the Netherlands.
2 Same sample used.
3 Sample from the HeadSMART prospective cohort study, USA.
Table 5. Cognitive outcomes

Table 6. Psychological health outcomes

1 sample from the UPFRONT observational study, Netherlands
Table 7. Life participation outcomes

Data Extraction and Analysis
A narrative synthesis was undertaken for all studies within the cognitive, psychological health, and life participation domains. A further meta-analysis was undertaken but only for studies using the GOS/GOSE at 6+ months post-injury. This narrower focus was necessary due to the large variety of outcome measures and comparison groups used between studies and at various time points, which prevented further quantitative analysis.
For quantitative analysis, the same two independent reviewers extracted effect size data from the papers with consensus reached on all included effects. Comprehensive Meta-Analysis software (Version 3; Borenstein, Hedges, Higgins & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2005) was used to calculate the estimated proportion of “complete” functional recovery, as shown by the GOS/GOSE 6+ months post-mTBI. Proportions from individual studies were then combined using random-effects models. Groups were split into subgroups of older adults (≥65 years) and younger adults (<65 years). The I2 statistic was used to determine heterogeneity of subgroups, with scores of .25, .50, and .75 corresponding with low, moderate, and high levels of heterogeneity, respectively (Higgins, Thompson, Deeks & Altman, Reference Higgins, Thompson, Deeks and Altman2003). As we did not have more than five studies in any subgroup, we did not conduct meta-regression moderation analysis to assess for differences on demographic information.
RESULTS
Cognition
Only two studies were identified, at any time points, that investigated cognition following mTBI in older adults using neuropsychological assessment or cognitive screening tools.
Acute outcome (≤1 month)
No studies meeting criteria were identified.
Short-term outcome (1–3 months)
Kinsella, Olver, Ong, Gruen, and Hammersley (2014b) assessed neuropsychological outcome 3 months following mTBI injury and compared test performances to a trauma (orthopaedic) and community control group. The mTBI group displayed deficits on tasks related to prospective memory and control of attention allocation (executive function) when compared to community controls. However, both trauma groups (mTBI, orthopaedic) were impaired on these tests when compared to community controls. This raises the issue of whether the noted cognitive deficits in the mTBI group were related to general trauma effects (e.g., posttraumatic stress) rather than specific brain injury effects. Potential for a mediating role of psychological distress in cognitive outcome was not addressed in this study and will be important to further explore. However, both trauma and community groups reported similar levels of mental well-being on the 12-item Short-Form Survey Version 2 (SF-12v2) suggesting that psychological health, as measured by a quality-of-life scale, was not associated with differences seen on neuropsychological testing. Although the severity of brain injury (presence of intracranial injury, i.e., complicated mTBI) and increasing age were identified in further analyses as significant predictors of cognitive outcome, it could be that some of the observed cognitive differences predated the traumatic injury for both trauma groups.
Long-term outcome (≥6 months)
Deb, Lyons, and Koutzoukis (Reference Deb, Lyons and Koutzoukis1998) rated older adult cognitive performance 1-year post-injury in comparison to a younger adult group, by using a cognitive screening tool (Mini Mental Status Examination; MMSE). Results suggested that 62% of older adults presented with “cognitive disability” (based on MMSE score <24) 1 year after sustaining a mTBI, compared to 8% of younger adults. The MMSE is a cognitive screening tool often used as the first step in further evaluation of cognitive status in older patients; as no further analysis was done to screen or control for an evolving comorbidity (e.g., dementia), it is possible that observed age differences on the MMSE identified premorbid and/or subsequent cognitive decline, rather than cognitive change related to mTBI.
Psychological Health
Acute outcome (≤1 month)
Karr et al. (Reference Karr, Luoto, Gilman, Berghem, Kotilainen and Iverson2020) examined PCSs 1-week post-mTBI, using the Rivermead Post-concussion Symptoms Questionnaire (RPQ). Participants were dichotomised into older (≥65 years) and younger (<65 years) age groups and included adults with premorbid functional impairment, neurological impairment, and/or dementia diagnosis, which were more likely to be present in older adults. Despite this, no group differences were found for PCS severity or total number of symptoms endorsed, indicating similar levels of PCS across age groups. However, younger adults were more likely to endorse particular PCS (i.e., headaches, noise and light sensitivity, irritability, frustration and impatience) and from a subset of participants reporting “new” onset of functional impairment after injury, younger adults reported greater PCS severity compared to older adults. This suggests that older adults report similar or fewer PCS soon after injury, although this does not necessarily translate to better functional outcome post-injury.
In a single-age cohort study of hospital admissions of older adults (≥60 years) following mTBI, Abdulle et al. (Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018) examined the proportion of older people endorsing “high” levels of anxiety and depressive symptoms (scores >8 on the Hospital Anxiety and Depression Scale; HADS) 2 weeks post-injury. Overall, only 16% endorsed anxiety symptoms and 14% endorsed high levels of depressive symptoms post-mTBI, which are slightly less or similar to normative levels for HADS anxiety and depression in a general nonclinical population (normative anxiety symptoms 33%, normative depressive symptoms 11%; Crawford, Henry, Crombie & Taylor, Reference Crawford, Henry, Crombie and Taylor2001). Nevertheless, when the older adults were dichotomised into “frail” or “non-frail” groups, a higher proportion of depressive symptoms were identified in “frail” compared to “non-frail” older people (26% and 7%, respectively, p = .001). The researchers suggest that although in the acute phase of recovery many older adults will not report elevated levels of anxiety or depression compared to general populations, the presence of comorbidities resulting in frailty will negatively impact psychological health post-injury.
PCS for this same sample was also examined (Abdulle et al., 2020) using the Head Injury Symptoms Checklist (HISC) and measures of posttraumatic stress and coping. Results indicated that 73% of older people endorsed at least one PCS 2 weeks after injury, with the most frequent complaints being dizziness, fatigue, and headache. Further analysis also revealed that endorsing higher levels of depressive symptoms and PCS were associated with slightly decreased odds of complete recovery, whereas coping style, posttraumatic stress, and other demographic variables were not. This suggests that greater PCS severity and depression may impede recovery from injury, rather than other personal or injury-related factors.
Using a referral clinic for trauma patients, Rapoport & Feinstein (Reference Rapoport and Feinstein2001) recruited a sample of older (≥60 years) and younger (18–59 years) participants. At the acute assessment stage (mean 19-day post-injury), using the General Health Questionnaire, older patients reported significantly less psychological impairment and distress than the younger patients. Older adults also reported significantly fewer psychosocial difficulties (as rated on the Rivermead Head injury Follow-up Questionnaire) and PCS (measured on the RPQ) than younger adults. However, after controlling for employment status, group differences were smaller (and mostly nonsignificant) across these outcome measures, leading to the suggestion that age differences in psychological health and PCS can be moderated by psychosocial variables, such as the stress related to early return to work as often experienced by younger patients.
A more recent large study (Richey et al., Reference Richey, Rao, Roy, Narapareddy, Wigh, Bechtold and Peters2020), based on Emergency Department admissions, used a prospective cohort design to examine age differences in recovery from mTBI at 1-, 3- and 6-month post-injury. Depressive symptoms were monitored using the Patient Health Questionnaire-9 (PHQ-9), where scores of >5 identified people with mild depressive symptomatology. This cutoff is lower than the recently recommended cutoff of ≥10 (Levis, Benedetti & Thombs, Reference Levis, Benedetti and Thombs2019); however, it appears to have acceptable sensitivity and specificity for TBI patients (Fann et al., Reference Fann, Bombardier, Dikmen, Esselman, Warms, Pelzer and Temkin2005). PCS were measured using the RPQ, whereby scores were dichotomised into “favourable” and “unfavourable” outcome based on the severity and number of symptoms endorsed. At the 1-month assessment, only 18.9% of older adults (65+ years) endorsed depressive symptoms and 14.8% endorsed PCS symptoms, compared with 43.8% and 51.0% of younger adults, respectively (18–59 years). In this study, sample size was substantially larger for the younger adult cohort, (n = 259 compared with n = 74 for older adults) and psychosocial variables, including employment status, differed significantly between age groups (and were not statistically controlled in analyses).
Short-term outcome (1–3 months)
In a further evaluation of patients attending a trauma clinic (see study description in acute findings above), Rapoport et al. (Reference Rapoport, McCullagh, Streiner and Feinstein2003) used the structured clinical interview (SCID-DSM-IV) to report that older adults had lower rates of major depression than younger adults (6.3% vs. 21.2%, respectively) at 1–3 months post-injury. They also had a lower relative risk of post-injury depression, even after accounting for history of substance abuse and previous/family history of depression.
As part of their cohort study (described in acute findings above), Richey et al. (Reference Richey, Rao, Roy, Narapareddy, Wigh, Bechtold and Peters2020) reported that older adults continued to endorse fewer depressive symptoms (23.1%) and PCS (21.2%) than younger adults (37.6% and 50.2%, respectively) 3-month post-mTBI.
Long-term outcome (≥6 months)
Six months following injury, in the same sample described above (Richey et al., Reference Richey, Rao, Roy, Narapareddy, Wigh, Bechtold and Peters2020), older adults continued to endorse lower levels of depressive symptoms (24.1%) and fewer PCS symptoms (18.6%) in comparison to younger adults (38.1% and 48.4%, respectively); at this time point, the younger adult group was almost twice as likely to endorse high levels of depressive symptoms and unfavourable PCS outcome compared to older adults. The researchers reported no change in depressive symptoms or PCS outcome for older adults across acute, short-term and longer-term time points.
A similar study (Asselstine, Kristman, Armstrong & Dewan, Reference Asselstine, Kristman, Armstrong and Dewan2020) used a prospective cohort of older adults (≥65 years) to examine PCS and depressive symptoms (using the Centre for Epidemiological Studies-Depression Scale; CES-D) at baseline (i.e., 10-day post-injury) and 6-month post-injury. By 6 months, older adults endorsed fewer symptoms of post-concussion (baseline M = 3.20 vs. 6 months M = 1.17), indicating some resolution of symptoms over time. Although no statistical group analysis was completed for depressive symptoms, results also indicated only small changes in mean scores across time (baseline M = 7.57 vs. 6 months M = 6.41) which are similar to normative levels for community-dwelling older adults (n = 1,005; M = 8.33; SD = 6.84; Lewinsohn, Seeley, Roberts & Allen, Reference Lewinsohn, Seeley, Roberts and Allen1997). Further predictive analysis indicated that older people with higher endorsement of PCS at baseline were twice as likely to have incomplete functional recovery (RR = 2.13; 95% CI 1.51, 6.07), and incomplete self-reported recovery (RR = 2.64; 95% CI 1.31, 8.98) 6-month post-mTBI. Although the attrition rate appeared adequate (17% of the sample), participants that were removed or lost to follow-up endorsed significantly higher levels of PCS (M = 14.4) at baseline compared to those who were included in final analysis (M = 3.2). Therefore, findings are likely to be an underrepresentation of PCS outcome in older people, but remain in line with results from other identified studies.
Another study (Hu, Hunt & Ouchterlony, Reference Hu, Hunt and Ouchterlony2017) examined PCS as measured by the RPQ in patients attending a head injury clinic, approximately 1-year post-injury. Participants were grouped by age, and similar to findings from previous time points, total PCS severity was significantly lower in the oldest participants (>65 years) compared to middle-aged groups (36–65 years). Additionally, several age differences for individual symptoms were identified, whereby middle-aged participants (aged 36–55 years) were significantly more likley to report greater severity of headaches, nausea and vomiting, irritability, poor concentration, and taking longer to think, compared to adults >65 years. Adults 46–55 years were also more likely to report greater sleep disturbance, blurry vision, and light sensitivity than older adults, and the 56–65 age group endorsed greater concentration issues compared with adults >65 years.
Finally, Deb et al. (Reference Deb, Lyons and Koutzoukis1998) used the Clinical Interview Schedule-Revised (CIS-R) to determine mental health in a sample of younger (18–65 years) and older adults (>65 years) 1 year following mTBI. Consistent with other time points, older adults were four times less likely to report significant psychological symptoms (scores >12 on the CIS-R) than younger adults (5.2% vs. 21%, respectively). This provides some evidence that older adults may continue to report lower levels of psychological distress up to 1 year following mTBI compared to younger adults.
Life Participation
Meta-analysis of long-term functional recovery using the GOS
From the identified studies, the GOS/GOSE was the only consistently used outcome measure that allowed for meta-analytic evaluation of functional outcome following mTBI. The GOS/GOSE measures global functional outcome following injury (or worsening of preexisting disability) using a 5- or 8-point rating system, whereby lower scores indicate greater disability or “incomplete” recovery, and a perfect score indicates “complete” or full recovery. Although there are limitations to the GOS/GOSE, this is currently the most widely used outcome measure following TBI and provides the most extensive data on functional outcome to date.
Five studies reported the proportion of “complete” recovery for older adults using the GOS/GOSE; four examined outcome at 6-month post-injury, whereas one study (Deb et al., Reference Deb, Lyons and Koutzoukis1998) observed outcome at 1-year post-injury. The paper by Abdulle & van der Naalt (Reference Abdulle and van der Naalt2020) was initially identified for inclusion; however, as this study dichotomised older age as ≥60 years and all other studies defined older age as ≥65 years, the van der Naalt et al. (Reference van der Naalt, Timmerman, de Koning, van der Horn, Scheenen, Jacobs, Hageman, Yilmaz, Roks and Spikman2017) data, which shared the same sample as Abdulle et al. (Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018, 2020) and reported proportion of recovery based on age ≥65 years, was used in preference. This also provided data for a further subgroup analysis (old vs. young), as two other studies reported proportion of recovery for younger adults aged between 18 and 64 years as well as their older age samples.
An examination of the data indicates that 6+ months after mTBI, 67.2% of older adults were considered functionally recovered, which represented a significant logit event rate of recovery, 95% CI 0.569, 0.761, p = .001 (See Figure 2). For younger adults, by comparison, a nonsignificant 56.2% of people had recovered from injury, 95% CI 0.420, 0.694, p = .392. However, subgroup analysis revealed that the proportion of recovered individuals did not differ between older and younger populations, Q = 1.629, p = .202. Heterogeneity of event rates was calculated using I2 and suggested moderate heterogeneity for the older adult subgroup but high heterogeneity for the younger adult subgroup which may partially explain nonsignificant findings in terms of recovery for the younger age group.
The result from Egger’s regression (p (two-tailed) = .179) confirmed that these findings were not significantly asymmetric and Duval and Tweedie’s trim and fill method indicated it was unlikely there were missing studies, which collectively suggests a low risk of publication bias. The finding of a significant proportion of older adults achieving recovery post 6 months also appears robust, with the fail-safe N statistic suggesting that another 35 studies with a logit event rate of zero would be required to render the current finding nonsignificant.
Additional studies of life participation
Several studies could not be included for quantitative analysis due to variability in timing of assessment or outcome measures, and therefore they are reviewed individually.
Karr et al. (Reference Karr, Luoto, Gilman, Berghem, Kotilainen and Iverson2020) investigated changes in functional status in older and younger adults using the Modified Rankin Scale. Findings (and our analysis of the data) indicated that older adults were 1.4 times more likely than younger adults to transition from no functional impairment prior to injury to functional impairment 1 week after mTBI (65.3% of older adults vs. 46.7% younger adults). At 1-month post-mTBI, however, Rapoport & Feinstein (Reference Rapoport and Feinstein2001) reported that although older adults ≥60 years who had sustained a mTBI showed slightly better functional recovery (mean GOS = 4.67) than younger adults (mean GOS = 4.03), small differences between age groups became nonsignificant when employment status was controlled.
Kinsella et al. (2014b) examined community integration [using the Community integration Questionnaire (CIQ)] and mental and physical quality of life (using the SF-12v2) 3-month post-injury. Findings demonstrated no significant differences in community integration between older adults who sustained mTBI and orthopaedic injury or community control groups suggesting that community integration is normative by 3-month post-injury. In contrast, physical quality of life was significantly lower for both trauma groups (mTBI, orthopaedic) but not mental quality of life, although small effects were found between trauma groups and community controls. Kristman, Brison, Bedard, Reguly & Chisholm (Reference Kristman, Brison, Bedard, Reguly and Chisholm2016) also investigated mental and physical quality of life in an older age cohort up to 6-month post-injury using the SF-12, as well as a single-item measure of recovery (labelled global self-reported recovery). Mean scores in both mental and physical quality of life significantly improved by 6-month post-injury from baseline levels. Self-reported recovery at hospital discharge was low (20.4%), however, also significantly increased to 73.5% by 6-month post-injury.
In comparison to younger adults, however, Mosenthal et al. (Reference Mosenthal, Livingston, Lavery, Knudson, Lee, Morabito and Coimbra2004) suggested that a greater percentage of older adults (34%) reported decreased functional outcome (using a modified version of the Functional Independence Measure) at 6-month post-injury compared to younger adults (11%; p = .02) even after accounting for preexisting impairment. Nevertheless, both age groups did show higher levels of functional independence 6-month post-injury when compared to discharge from hospital.
Deb et al. (Reference Deb, Lyons and Koutzoukis1998) also compared the rehabilitation status of older adults (>65 years) to younger adults (18–65 years), 1 year after mTBI to suggest that older adults were 1.7 times more likely to show disability as rated on the Edinburgh Rehabilitation Status Scale (ERSS) than their younger adult counterparts. Regression analysis indicated that cognitive function (based on MMSE score) was associated with ERSS scores, whereas age, gender, GCS score, estimated premorbid intelligence, and alcohol consumption were not. When cognition was controlled, increasing age became positively associated with increasing disability. However, the researchers note that some disability could have existed pre-injury which is an important consideration in ageing cohorts.
Finally, Abdulle et al. (Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018) examined recovery 1–3 years after mTBI (mean time since injury = 30.1 months) to show that 54% of older adults fully recovered (based on GOSE scores of 8) and a significantly lower percentage of frail older adults reported complete recovery (24% frail vs. 72% nonfrail, p < .01). However, due to the variability in follow-up time since injury it remains unclear whether “incomplete recovery” on GOSE captured post-injury function, or a worsening of new or non-injury-related problems.
DISCUSSION
This review aimed to identify mTBI outcomes for older people using multiple domains – cognition, psychological health, and life participation (including functional recovery). Overall, the current evidence suggests cautious optimism for older adults following mTBI, at least in terms of psychological health and longer-term functional recovery from injury.
Surprisingly, only two studies examining cognitive outcome post-mTBI in older adults met inclusion criteria (Deb et al., Reference Deb, Lyons and Koutzoukis1998; Kinsella, et al., 2014b). From this, the limited evidence suggests that older adults may still display specific cognitive deficits 3-month post-injury. However, whether this outcome is due to compromised premorbid cognitive functioning leading to increased risk of injury, or a generalised effect of trauma, cannot be determined yet and requires further investigation. In addition, longer follow-up assessments (6-month+) will determine if cognitive difficulties persist or recovery is generally achieved, albeit at a slower rate than expected for younger age cohorts following mTBI.
In terms of psychological health, there is emerging evidence that older adults consistently report less psychological distress, endorse less symptoms of depression and anxiety, and report less severity of PCS than younger adults, regardless of time since injury (Deb et al., Reference Deb, Lyons and Koutzoukis1998; Rapoport & Feinstein, Reference Rapoport and Feinstein2001; Rapoport et al., Reference Rapoport, McCullagh, Streiner and Feinstein2003; Richey et al., Reference Richey, Rao, Roy, Narapareddy, Wigh, Bechtold and Peters2020; Hu et al., Reference Hu, Hunt and Ouchterlony2017). Possible explanations for these older age benefits in psychological outcome have not been systematically addressed, although there is limited evidence to suggest that increased psychosocial stressors associated with younger age (e.g., employment demands) may moderate age differences in outcome.
Other explanations could be that better psychological health prior to injury acts as a protective factor, allowing for better psychological adjustment, and ensuring a return to “baseline” mental well-being soon after injury. Results from this review revealed that, in comparison to normative data, older adults who sustained a mTBI reported similar levels of depression and anxiety compared to general population samples (Abdulle et al., Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018; Asselstine et al., Reference Asselstine, Kristman, Armstrong and Dewan2020). More specifically, older adults reported generally low levels of psychological distress immediately following injury with little to no change over time (Kristman et al., Reference Kristman, Brison, Bedard, Reguly and Chisholm2016) and similar trajectories compared to younger adults (Richey et al., Reference Richey, Rao, Roy, Narapareddy, Wigh, Bechtold and Peters2020). Nevertheless, frailty has been identified as a factor associated with poorer psychological outcome in older adults (Abdulle et al., Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018) and there is some evidence that greater severity of PCS and depressive symptoms immediately after injury may decrease the likelihood of recovery (Abdulle et al., 2020; Asselstine et al., Reference Asselstine, Kristman, Armstrong and Dewan2020). In addition, recent research in younger adult cohorts (not part of this review) has emphasised the strong relationship between pre-injury characteristics and ongoing somatic post-mTBI complaints (Meares et al., Reference Meares, Shores, Taylor, Batchelor, Bryant, Baguley and Marosszeky2011; Ponsford et al., Reference Ponsford, Nguyen, Downing, Bosch, McKenzie, Turner and Green2019), highlighting the possible impact of premorbid psychological well-being on post-injury outcome. Although intuitively defensible, the evidence for these prognostic variables in older adult samples is generally based on single studies and requires further investigation and replication.
Alternative explanations for age effects on psychological well-being include generational differences surrounding perceived stigma of mental disorders (Conner et al., Reference Conner, Copeland, Grote, Koeske, Rosen, Reynolds and Brown2010), possibly resulting in an unwillingness to report (or even an inability to identify) symptoms of psychological distress (Wetherell et al., Reference Wetherell, Petkus, McChesney, Stein, Judd, Rockwell and Patterson2009; Andreas et al., Reference Andreas, Schulz, Volkert, Dehoust, Sehner, Suling and Härter2017). Older adults may be more likely to endorse more somatic symptoms, anhedonia, and cognitive complaints compared to younger adults, suggesting a different experience of psychological distress that may not be fully captured on current psychological measures of distress (Wuthrich, Johnco & Wetherell, Reference Wuthrich, Johnco and Wetherell2015; Fiske, Wetherell & Gatz, Reference Fiske, Wetherell and Gatz2009). Therefore, future research will need to consider the appropriateness of psychological health measures for older adult samples.
Evidence related to functional outcome and life participation varied across time points and comparison groups; however, based on findings from our meta-analysis using the GOS/GOSE, a significant proportion (67%) of older adults aged ≥65 years show full functional long-term recovery from injury post-mTBI, and this proportion of recovered individuals does not significantly differ and may even surpass the rate from younger adults (56%). By contrast, previous research that has investigated outcome following moderate–severe TBI suggests older age negatively predicts outcome and mortality (Flaada et al., Reference Flaada, Leibson, Mamdrekar, Diehl, Perkins, Brown and Malec2007; Gardner et al., Reference Gardner, Dams-O’Connor, Morrissey and Manley2018; Hashmi et al., Reference Hashmi, Ibrahim-Zada, Rhee, Aziz, Fain, Friese and Joseph2014; McIntyre et al., 2013a). Our more positive age-related findings after mTBI align with a previous meta-analysis (McIntyre, Mehta, Janzen, Aubut & Teasell, 2013b) that reported 80% of older adults had a favourable outcome after mTBI, compared to 32% for moderate and 8% for severe TBI.
Although not the focus of this review, our finding that 56% of younger adults showed complete recovery as measured on the GOS appears low given the consistent evidence that neuropsychological recovery, by contrast, is expected within 90 days of injury (Carroll et al., Reference Carroll, Cassidy, Peloso, Borg, von Holst, Holm and Pépin2004; Frencham et al., Reference Frencham, Fox and Maybery2005; Karr et al., Reference Karr, Areshenkoff and Garcia-Barrera2014; Rohling et al., Reference Rohling, Binder, Demakis, Larrabee, Ploetz and Langhinrichsen-Rohling2011). Nevertheless, reports of incomplete or unfavourable recovery from mTBI are not unusual for a proportion of younger adults, ranging anywhere between 23% and 53% of adults (De Koning et al., Reference De Koning, Scheenen, van der Horn, Hageman, Roks, Spikman and van der Naalt2017; Korley et al., Reference Korley, Diaz-Arrastia, Falk, Peters, Leoutsakos, Roy and Bechtold2017; McMahon et al., Reference McMahon, Hricik, Yue, Puccio, Inoue, Lingsma and Vassar2014; Nelson et al., Reference Nelson, Temkin, Dikmen, Barber, Giacino, Yuh and Zafonte2019; Scheenen et al., Reference Scheenen, Spikman, De Koning, van der Horn, Roks, Hageman and van der Naalt2017; van der Horn et al., Reference van der Horn, Spikman, Jacobs and van der Naalt2013). Additionally, although the GOS is a commonly used measure of recovery from injury, there is some criticism about its use as a dichotomous measure (McMillan et al., Reference McMillan, Wilson, Ponsford, Levin, Teasdale and Bond2016) and it may provide little information about current life participation or functional status compared to community-dwelling age-matched samples. To this end, we evaluated and described results from several identified studies that used outcome measures other than GOS rates of recovery, to determine outcome for life participation across several time points.
From these studies, the evidence was more mixed. One week after mTBI, older adults may show greater functional impairment than younger adults (Karr et al., Reference Karr, Luoto, Gilman, Berghem, Kotilainen and Iverson2020) and yet, it has been reported that within the first month following injury, older adults show similar functional recovery as compared to younger adults (Rapoport & Feinstein, Reference Rapoport and Feinstein2001). Additionally, older adults report similar levels of community integration 3-month post-injury compared to non-injured older adults (Kinsella, et al., 2014b), even though physical quality of life remained lower; and many older adults have been reported to perceive themselves as “recovered” by 6-month post-injury (Kristman et al., Reference Kristman, Brison, Bedard, Reguly and Chisholm2016). In contrast, there is some evidence to suggest that older adults show greater long-term disability compared to younger adults 6- to 12-month post-injury (Deb et al., Reference Deb, Lyons and Koutzoukis1998; Mosenthal et al., Reference Mosenthal, Livingston, Lavery, Knudson, Lee, Morabito and Coimbra2004), but it should be noted that whether these disabilities were not related to the actual trauma and were additional comorbidities has not been determined. Therefore, the need for ongoing investigation of life participation post-injury remains a priority.
Future Considerations for mTBI Research in Older People
Several limitations previously highlighted in mTBI research generally (Kristman et al., Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014) continue to pose unique challenges for mTBI research in older populations (Gardner et al., Reference Gardner, Dams-O’Connor, Morrissey and Manley2018; Peters & Gardner, Reference Peters and Gardner2018) and require consideration going forward. The first is that many mTBI studies use adult samples aged 18–90+ years to run prognostic analyses. Although useful, this requires large representative samples to allow for age comparisons and moderation analysis, as well as a need to account for potential age-related differences in psychosocial (e.g., return-to-work stress or carer responsibilities for younger adults) and biological (e.g., reduced cognitive reserve for older adults) factors. Thus, using a more focussed approach that specifically examines older cohorts may be more achievable and moves away from simply monitoring age (and ageist connotations) to allow for analysis of more targeted prognostic variables particularly relevant for older people (Romero-Ortuno & O’Shea, Reference Romero-Ortuno and O’Shea2013). Promisingly, more recent research (e.g., Abdulle et al., Reference Abdulle, De Koning, van der Horn, Scheenen, Roks, Hageman and van der Naalt2018; Asselstine et al., Reference Asselstine, Kristman, Armstrong and Dewan2020) has begun examining predictive factors (e.g., frailty, post-injury complaints, mood, PCS, etc.) that may impact outcome after injury in specifically older age cohorts.
Comparison Groups and Sample Recruitment
In this review, we focused on older adults as the primary population of interest and in doing so our results suggest that a large proportion of older adults do show functional recovery after mTBI and can expect similar (or even better) outcome in terms of psychological health and life participation as compared to younger adults. While it is useful to understand differences associated with age (younger vs. older age cohorts), appropriate age-matched control groups (e.g., orthopaedic trauma control groups, or healthy community control groups) and repeated measures designs with longer follow-up may provide more meaningful information and expectations about recovery specifically for older people.
Additionally, recruitment and sampling strategies used to select older adult participants following mTBI are important to consider. Clinical guidelines in many health settings recommend neuroimaging for all older people presenting with suspected head injury to manage risk of acute intracranial bleeding (National Institute for Health and Care Excellence, 2019). Therefore, older adults may be more likely to engage with health services following very mild injury which may inflate reported rates of recovery from injury. Additionally, stringent exclusion criteria often prohibit older adults with significant comorbidities (e.g., dementia diagnosis) from participating in major research trials. Although this may be necessary to control for confounding factors, recruited samples may risk being unrepresentative of older adult populations, thereby increasing the likelihood of a positive recovery. For example, a recent study examined mTBI functional outcome at discharge from hospital in a sample of older adults ≥75 years and reported that cancer or dementia diagnosis are significant predictors of outcome (Seno et al., Reference Seno, Tomura, Ono, Tanaka, Ikeuchi and Saitoh2019). The impact of culture may also emerge as a strong factor impacting prognostic models of outcome following TBI in older age. Often, there is an underrepresentation of ethnically diverse populations and samples (e.g., fluency in English language is commonly required for inclusion) and recommended interventions in response to TBI outcome (including mTBI) may depend on middle- to high-income country status (de Silva et al., Reference de Silva, Roberts, Perel, Edwards, Kenward, Fernandes and Patel2009).
Given the complexities and multifactorial nature of aging and health, future studies of mTBI in older age will need to account for a range of confounding variables (pre- and post-injury), thereby requiring large datasets. These large and varied cohorts across different cultures and societies may provide a deeper understanding of the issues confronting older people following a traumatic injury.
Age-appropriate Outcome Measures
Most studies included in this review investigated multiple domains of outcome following mTBI, which indicates a positive shift towards a more holistic view of TBI outcome and recovery. However, as highlighted by this review, the variation in outcome measures makes direct comparisons of results difficult and often limits interpretation to single samples. Additionally, several studies reported arbitrary (and varied) cutoff scores to dichotomise outcomes and many used single-item or modified outcome measures without reporting psychometric evaluation. For older age cohorts, age-appropriate outcome measures that can adjust for the impact of premorbid physical and medical comorbidities and provide community norms for older cohorts is particularly important.
From the identified research, the only consistently used outcome measure was the GOS/GOSE, which allowed for quantitative analysis of longer-term functional recovery. However, even for this well-established measure, there was variation in reporting of outcome, with some studies presenting mean GOS scores and others using varied cutoff scores to represent “good recovery” from injury. Additionally, concerns have been raised that the GOS/GOSE may be particularly insensitive in older adult populations, where compromised premorbid functional abilities due to comorbidities may be inaccurately attributed to injury (Gardner et al., Reference Gardner, Dams-O’Connor, Morrissey and Manley2018). Therefore, using consistent and age-appropriate measures for older adult populations is essential to allow for further systematic evaluation of outcome post-injury.
Recommendations
-
Although definitions of old age continue to vary across different cultures, it is recommended that age ≥65 years is used as a reference point for forming a sample of older adults, based on current global aging trends (World Health Organisation, 2011). This will allow for better comparison of research outcomes across studies. If the sample is sufficiently large, it is also recommended that diversity in old age is recognised by defining subgroups; for example, using youngest-old (65–74), middle-old (75–84), and oldest-old (85+) age ranges (see Lee, Oh, Park, Choi & Wee (Reference Lee, Oh, Park, Choi and Wee2018) for an application of these subgroups).
-
Include age-appropriate comparison groups, rather than relying on younger age comparisons. When possible, include both community and mild orthopaedic control groups as this may help to elucidate differences between pre-injury status or general trauma effects and mTBI-specific changes. This is especially relevant in older age where the impact of peripheral injuries resulting in chronic pain, medication use, or sleep disturbance may significantly impact cognition (Higgins, Martin, Baker, Vasterling & Risbrough, Reference Higgins, Martin, Baker, Vasterling and Risbrough2018; Ponsford, Hill, Karamitsios & Bahar-Fuchs, Reference Ponsford, Hill, Karamitsios and Bahar-Fuchs2008; Vincent, Horodyski, Vincent, Brisbane & Sadasivan, Reference Vincent, Horodyski, Vincent, Brisbane and Sadasivan2015).
-
It is recommended that research designs with older age populations include at least 6-month review, and preferably 12-month follow-up. In younger age cohorts, normative neuropsychological outcome is generally expected by 3-month post-injury. This has not been well established for older people and as neural recovery in older age maybe slower, at least a 6-month review is needed. Due to the higher risk of developing unrelated diseases and health conditions that frequently present in older age, high attrition rates in longitudinal studies may be expected and this should be factored into the initial design of the study.
-
Researchers should consider the appropriateness of outcome measures for older adults by adequately accounting for preexisting functional status and abilities. This will reduce the possibility of premorbid conditions being falsely attributed to brain injury. In relation to cognitive outcome, researchers should aim to use objective and detailed measures (e.g., reaction time, which is commonly measured using computerised tests) that go beyond limited screening tools and are based on age-appropriate normative data. Similarly, use psychological and functional outcome measures that have known validity for older age populations.
Limitations
This review used a stringent definition of mTBI based on widely accepted current criteria to best identify mTBI (Kristman et al., Reference Kristman, Borg, Godbolt, Salmi, Cancelliere, Carroll and Cassidy2014; Menon-et al., Reference Menon, Schwab, Wright and Maas2010; National Center for Injury Prevention and Control, 2003). Given that the definition of mTBI has been historically contentious (Raskin, Lovejoy, Stevens, Zamrozieqicz & Oakes, 2014), it was considered important to adopt present guidelines for operationalising mTBI. Nevertheless, several early studies of mTBI did not use these criteria and, therefore, could not be included for systematic evaluation. A small number of studies were not reviewed due to including participants that required neurosurgery for injury and therefore were considered to have experienced a moderate TBI.
This review also used a cutoff age of ≥60 years. This resulted in some early studies being excluded as older age was identified as ≥50 years, which is inconsistent with the widely accepted chronological age used to consider health and older age (World Health Organisation, 2011).
All included studies were deemed as fair to good quality evidence for cohort or observational studies; however, only three studies were prospective studies with appropriate follow-up. Most were cross-sectional in nature and therefore findings should be interpreted with some caution. This review provides a summary of the current “state of the evidence” but acknowledges these design limitations in many of the included studies.
CONCLUSION
There is reason for cautious optimism for older adults following mTBI, as positive outcomes for psychological health and life participation are common for older adults. Nevertheless, similar to the investigation of younger adults, further research is also needed to identify predictive factors (including pre-injury health) for subpopulations of older adults who do not recover fully from injury or continue to show cognitive deficits following mild traumatic injury (whether related to brain injury or general trauma). Using a focused approach that specifically examines outcomes in older cohorts will allow for analysis of individual prognostic variables particularly relevant for older people. As the research field continues to expand, this review highlights the critical need for adopting appropriate measures and comparison groups to examine multi-domain outcome following mTBI in older adults, as well as the continued challenges associated with this.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617721000795
Acknowledgements
The authors would like to acknowledge the contribution to the research by Monika Konjarski.
FINANCIAL SUPPORT
The authors declare that no funding was received.
CONFLICTS OF INTEREST
The authors have nothing to disclose.











