I. INTRODUCTION
The thermoelectric material is a function material that can transfer thermal energy into electric energy or electric energy into thermal energy directly. The applications of thermoelectric materials in electricity generators or refrigeration devices have many advantages: small, cheap, lightweight, and quiet. The transfer efficiency is proportional to the value of figure of merit ZT = σS 2/κ (where S is the Seebeck coefficient, σ is the electrical conductivity, κ is the thermal conductivity of the material, and T is the temperature (K)) (Disalvo, Reference Disalvo1999; Tritt, Reference Tritt1999). Binary skutterudite compound CoSb3 has a cobalt cubic structure with antimony rings occupying six of the cells with two remaining empty. It possesses a large Seebeck coefficient and good electrical conductivity. However, its thermal conductivity is too high to make it an efficient thermoelectric material (Caillat et al., Reference Caillat, Kulleck, Borshchevsky and Fleurial1996). Two approaches are used to reduce the thermal conductivity of skutterudites. One is to form filled skutterudites by filling the open ‘cage’ with rare earth and/or other metallic atoms (Sales et al., Reference Sales, Mandrus and Williams1996; Viennois et al., Reference Viennois, Charar, Ravot, Haen, Manger, Bentien, Paschen and Steglich2005; Mi et al., Reference Mi, Zhu, Zhao and Ma2007a, Reference Mi, Zhao, Zhu, Tu and Caob). The “rattling” motion of the filled atoms can effectively scatter phonons and hence cause a significant decrease of the lattice's thermal conductivity (Sales et al., Reference Sales, Mandrus, Chakoumakos, Keppens and Thompson1997). Another approach is nanostructuring of the thermoelectric material (Alboni et al., Reference Alboni, Ji, He, Gothard, Hubbard and Tritt2007). The incorporated nanoparticles within the CoSb3 bulk material add scattering centers to affect the phonons (Hicks et al., Reference Hicks, Harman and Dresselhaus1993).
A wet-chemical route is a simple and effective way for preparation of nanostructure materials. Using a hydrothermal method, nanostructured NaFe4P12 skutterudite was synthesized (Liu et al., Reference Liu, Wang, Hu, Li, Gu, Zhao, Gu, Boughton and Jiang2002). Toprak et al. (Reference Toprak, Stiewe, Platzek, Williams, Bertini, Müller, Gatti, Zhang, Rowe and Muhammed2004) reported a novel chemical-alloying route for synthesis of nano-engineered skutterudite CoSb3 by obtaining the highest ZT value of 0.17 at 611 K. Mi et al. synthesized CoSb3 by a solvothermal route, in ethanol solution, at 250 °C for 72 h (Mi et al., Reference Mi, Zhao, Zhu, Tu and Cao2006). Here, we report the synthesis of uniform and nano-sized skutterudite Co1−xNixSb3 thermoelectric powders of spherical shape (10–20 nm) in triethylene glycol solution at 290 °C with a reaction time of 12 h. The substitution of Ni for Co in CoSb3 is expected to further reduce the thermal conductivity of skutterudite (Viennois et al., Reference Viennois, Charar, Ravot, Haen, Manger, Bentien, Paschen and Steglich2005; Zhang et al., Reference Zhang, Lu, Zhang, Wei, Liu and Liu2008).
II. EXPERIMENTAL
SbCl3, CoCl2·6H2O, NiCl2·6H2O, NaBH4, and triethylene glycol of commercial grade were used without further purification as the starting materials. Stock solutions of SbCl3 (0.6 mol/dm3), CoCl2·6H2O (0.2 mol/dm3), and NiCl2·6H2O (0.2 mol/dm3) were separately prepared, by dissolving the reagents in triethylene glycol. Typically, 100 ml of SbCl3 stock solution and various quantities of CoCl2 and NiCl2 stock solutions were taken, according to the designed formula Co1−xNixSb3 (x = 0, 0.05, 0.075, 0.125, 0.15, and 0.25), and were mixed. To this mixed solution, NaBH4 in excess dissolved in 160 ml of triethylene glycol was added under vigorous stirring for complete reduction of the metal ions. The reaction lasted for 20 min and within a few minutes the color of the solution turned dark. This suspension was transferred to an autoclave and heated to 290 °C, at a heating rate of 10 °C/min, and maintained at 290 °C for some time. After cooling down to room temperature, the resulting product was centrifuged and washed with distilled water, ethanol, and acetone, sequentially, and finally vacuum dried at 60 °C overnight.
The phase structures of the powders were analyzed by X-ray diffraction (XRD) using a Bruker D8 Advance 18 kW X-ray diffractometer with CuKα radiation (λ = 0.154178 nm). Topas 3.0 software was used for Rietveld refinement. The particle size, morphology, and composition of the powders were analyzed using a Hitachi S-4700 field emission scanning electron microscope (FESEM) with an energy-dispersive X-ray (EDX) spectrometer.
III. RESULTS AND DISCUSSION
A. Synthesis of CoSb3
Figures 1(a–d) show the XRD patterns for CoSb3 samples with a proper ratio before heating (a), synthesized at 290 °C for 1 h (b), 3 h (c), and 12 h (d) respectively. From these four figures, we can see that the sample before heating shows traces of the Sb phase and perhaps the Co phase without any intermetallic compound being formed. The chemical composition of this sample as determined by X-ray fluorescence spectrometer (XRF) is Co-84.92 wt.% Sb (Co-73.2 at.% Sb), very close to the prepared composition of CoSb3. It means that all the Sb3+ and Co2+ in the mixed solution were reduced after the addition of reductant NaBH4, under vigorous stirring for 20 min at room temperature. The particles of these two phases are very small hence their peaks are very low and wide. The temperature is too low for formation of Co–Sb intermetallic compounds.

Figure 1. XRD patterns for solvothermal synthesis powders of CoSb3 before heat treatment (a), synthesized at 290 °C for 1 h (b), 3 h (c), and 12 h (d).
For the sample which was synthesized at 290 °C for 1 h, the skutterudite CoSb3 becomes the main phase, together with some amount of Sb and CoSb2 phases [see Figure 1(b)] without any CoSb or Co phases. The quantitative Rietveld analysis by Topas 3.0 software, shown in Figure 2 and Table I, indicated that the percentages of CoSb3, CoSb2, and Sb phases in the sample are 50.7, 34.9, and 14.4 (in wt%) or 33.8, 32.8, and 33.5 (in at.%), respectively. The agreement factors for this Rietveld refinement are R wp = 4.96%, R p = 3.95%, and R exp = 3.29%, respectively, with the goodness-of-fit (GOF) of 1.51, indicating that the Rietveld refinement is satisfactory. The molar ratio of CoSb2 and Sb phases is about 1:1. The pure CoSb3 phase should be obtained on completion of the synthesis reaction between CoSb2 and Sb. As the reaction time increases, the amount of skutterudite CoSb3 increases and reaches 71 wt% for the reaction time of 3 h due to the growth of CoSb3 in the reaction between CoSb2 and Sb. A single-phase skutterudite CoSb3 was obtained by solvothermal synthesis at 290 °C in 12 h [(Figure 1(d)], a much shorter time as compared with that required for the solvothermal synthesis of CoSb3 in ethanol, as reported by Mi et al. (Reference Mi, Zhu, Zhao and Ma2007a) (250 °C for 72 h).

Figure 2. Observed, calculated, and differential profiles by Rietveld refinement for the XRD pattern of the sample of CoSb3 powders synthesized at 290 °C for 1 h.
Table I. Rietveld structural refinement results for solvothermal synthesis powders of CoSb3 synthesized at 290 °C for 1 h.

B. Synthesis of Co1−xNixSb3
Based on the work of the preparation of single-phase CoSb3, we have further synthesized the single-phase Ni-doped Co1−xNixSb3 skutterudites. Figure 3 shows the XRD patterns for the Ni-doped Co1−xNixSb3 skutterudites with x = 0, 0.05, 0.075, 0.125, 0.15, and 0.25, synthesized at 290 °C for 12 h. They are all patterns of pure skutterudites, indicating the Ni atoms are already doped into the CoSb3 lattice, forming a solid solution up to x = 0.25. The unit-cell parameters for Co1−xNixSb3 were refined by Topas 3.0 software. A representative refinement of results (x = 0.15) is shown in Figure 4 and Table II, indicating the refinement is satisfactory. The unit-cell parameters for Co1−xNixSb3 increase linearly from a = 0.90440 to 0.90650 nm as x increases from 0 to 0.25. It is an evidence of the substitution or filling of Ni atom into the CoSb3 lattice.

Figure 3. XRD patterns for Co1−xNixSb12 powders with x = 0, 0.05, 0.075, 0.125, 0.15, and 0.25 synthesized at 290 °C for 12 h.

Figure 4. Observed, calculated, and differential profiles by Rietveld refinement for the XRD pattern of Co0.85Ni0. 15Sb3 powders synthesized at 290 °C for 12 h.
Table II. Rietveld structural refinement results for the Co0.85Ni0.15Sb3 compound.

C. SEM observation
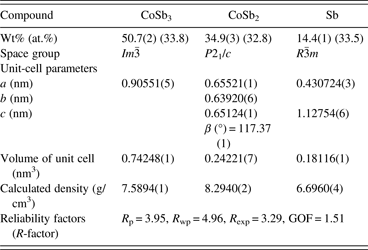
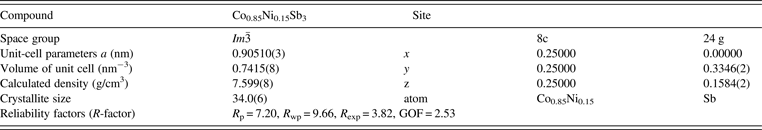
Figures 5(a) and 5(b) show the representative FESEM morphologies for the Ni-doped Co1−xNixSb3 powders with x = 0 and 0.15, solvothermally synthesized in triethylene glycol solvent at 290 °C for 12 h. The SEM figures reveal that the skutterudite powder consists of small and homogeneous particles size of 10 nm. The spherical shape of the particles suggests an isotropic crystal growth of skutterudite grains due to its cubic structure. The average particle size for undoped sample CoSb3 is about 10 nm. As the Ni content in Co1−xNixSb3 increases, the average particle size of skutterudite powders increases, and reaches about 30 nm for x = 0.25.

Figure 5. FESEM image for the Co1−xNixSb3 powders with (a) x = 0 and (b) 0.15 synthesized at 290 °C for 12 h.
The solvothermal syntheses in an ethanol medium for preparation of nano-sized skutterudite CoSb3 (Mi et al., Reference Mi, Zhao, Zhu, Tu and Cao2006) and CoSb3−xTex (Mi et al., Reference Mi, Zhao, Zhu, Tu and Cao2007b) were reported. To synthesize nano-sized skutterudite CoSb3 and CoSb3−xTex, reaction performed at 250 °C required a long reaction time of 72 h. The average particle size of CoSb3 and CoSb3−xTex were about 80 and 40 nm, respectively. In this work, the high boiling point of triethylene glycol enables the synthesis at a higher temperature of 290 °C. A much shorter reaction time of 12 h was needed for preparation of the nano-sized Ni-doped Co1−xNixSb3 skutterudite powders. From the kinetics point of view, higher reaction temperature is beneficial for both reaction and diffusion. The much smaller and more uniform particles synthesized in triethylene glycol, which is expected to be beneficial to the thermoelectric properties of the material, might not be only due to the shorter reaction time. Triethylene glycol may act as a surface active agent and/or promote nucleation, which leads to fine, uniform, and homogeneous Co1−xNixSb3 skutterudite particles.
IV. CONCLUSION
Nano-sized thermoelectric skutterudite Co1−xNixSb3 (x = 0, 0.05, 0.075, 0.125, 0.15, and 0.25) with a single phase was synthesized by the solvothermal method in triethylene glycol solution, performed at 290 °C for 12 h. High reaction temperature is an important factor to promote the kinetic process and reduce synthesis duration. The Co1−xNixSb3 powders synthesized in triethylene glycol solution in this work are small (10–30 nm), uniform, and homogeneous. As the replacement content of Co by Ni increases in Co1−xNixSb3, the lattice parameter a increases from 0.9044 to 0.9065 nm and the particle size increases from 10 to 20 nm.
ACKNOWLEDGEMENTS
The authors would like to acknowledge financial support from the National Natural Science Foundation of China (No. 51171117), Shenzhen Science and Technology Research Grant (Nos. CXB200903090012A and JC200903120109A).