Ventricular septal defect is the most common congenital cardiac defect accounting for more than 20% of all CHDs (median incidence of 2829 per 1 million live births). Reference Hoffman and Kaplan1 Since the first report by Lock et al Reference Lock, Block and McKay2 , transcatheter closure of ventricular septal defect has been attempted by a large number of different devices. Initially, these were mostly double-disc devices and required septal rims and an adequate-sized aortic rim, hence were not suitable for most perimembranous defects. Reference Lock, Block and McKay2–Reference Knauth, Lock and Perry5 Amplatzer asymmetrical membranous VSD device (St Jude Medical, St Paul, MN, USA) was specifically designed for perimembranous defects and was used extensively with mostly good results. Reference Hijazi, Hakim and Haweleh6–Reference Santhanam, Yang and Chen12 There was, however, an unacceptable risk of complete heart block, essentially abandoning its use. Reference Predescu, Chaturvedi and Friedberg13–Reference Yang, Kong and Sheng15 Nit-Occlud® Lê VSD-Coil (PFM Medical AG, Cologne, Germany) Reference Haas, Kock and Bertram16,Reference Kozlik-Feldmann, Lorber and Sievert17 and other Amplatzer family devices (AMVSD, ADO, ADOII, AVPII, pmVSO2) Reference Mijangos-Vázquez, El-Sisi and Sandoval Jones18–Reference Tzikas, Ibrahim and Velasco-Sanchez22 are currently being used with acceptable results. The devices of other manufactures (Cera VSD devices; LifeTech, Shenzhen, China, Occlutech muscular and membranous VSD Occluder; Helsingborg, Sweden, LEPU Medical Technology Co. Ltd, Beijing, China, and Shanghai pmVSD Occluder; Shape Memory Alloy Ltd, Shanghai, China) are also widely in use outside the USA. Reference Yang, Kong and Sheng15,Reference Yang, Yang and Wan23–Reference Zhou, Pan and Guan25 The anatomical variations, proximity to aortic and tricuspid valve, prolapse of right coronary cusp, and risk of complete heart block makes perimembranous VSD closure a complex interventional procedure and the perfect device is yet to be found.
LifeTechTM multifunctional Occluder (Konar-MF VSD Occluder) is soft, flexible, CE approved, and offers a hybrid design between single and double-disc devices, designed to conform to ventricular septal defect. Reference Schubert, Kelm and Koneti26 It can be delivered through a small sheath, can be screwed together at both sides which allows it to be placed in antegrade or retrograde way. In addition to that, its slim cable and flexible waist are expected to minimise damage to adjacent structures and reduce complications like complete heart block. At the moment, however, there is very limited data available on the outcome, efficacy, and safety of this device. Reference Schubert, Kelm and Koneti26–Reference Tanidir, Baspinar and Saygi28 The subgroup of patients with perimembranous outlet ventricular septal defect and right coronary cusp prolapse with none or trace to mild aortic regurgitation is potentially another indication for use of this device.
Materials and methods
Study design
This is a non-randomised clinical follow-up study to evaluate the feasibility and safety of LifeTechTM Konar-MF VSD Occluder used for patients with haemodynamicaly significant restrictive ventricular septal defect.
Patient population
All consecutive patients undergoing ventricular septal defect closure with the Konar-MFO device were prospectively enrolled at all participating institutions, from April, 2019 to March, 2020 and followed up until September, 2020. Written informed consent was taken and the study protocol was reviewed and approved by the institutional review boards of all participating hospitals.
Data collection
Pre-procedural data included patient demographics, baseline clinical characteristics, 12-lead electrocardiogram, a chest X-ray, and transthoracic echocardiography. The operator who was also the main interventionist in all the procedures performed transthoracic echocardiography at all centres. A detailed haemodynamic and defect morphological assessment was done.
The procedural details included procedure time, fluoroscopy time, mean pulmonary artery pressure, pulmonary-to-systemic blood flow ratio, angiographic, and transesophageal echocardiographic defect size, the size of the devices used during the procedure, complications, and aborted or failed implantations. Post-procedural follow-up data about rhythm disturbances, echocardiographic device position, residual leaks, and AV valve insufficiency (aortic and tricuspid valves) was collected. The data were recorded at the institutional level and then collated and analysed at The Children’s Hospital Lahore.
Inclusion criteria
Patients with a clinically relevant and haemodynamically significant ventricular septal defect were included. The significance was defined by the presence of LV volume overload. In the case of a perimembranous ventricular septal defect, the defect diameter (by two-dimensional echocardiography) of ≤ 10mm with an adequate sub-aortic rim (distance between the upper margin of the defect and the aortic valve ≥ 2.0 mm) was included. Patients with perimembranous ventricular septal defect and right coronary cusp prolapse were included only if the prolapse was mild, aortic regurgitation was mild or trivial, and an aneurysm was present for placement of the left ventricular disc of the device below the mildly prolapsed cusp.
Exclusion criteria
The following patients were excluded from the study: (a) perimembranous ventricular septal defect with more than mild aortic valve regurgitation; (b) sub-aortic rim ≤ 2 mm except in patients with an aneurysm or prolapse of right coronary cusp; (c) severe pulmonary artery hypertension; (d) presence of any other associated CHDs requiring cardiac surgery; (e) age <12 months or bodyweight <8 kg in view of vascular safety; (f) patients opting for surgery; or (g) not giving informed written consent for the procedure.
Device and device selection
The Konar-MF VSD Occluder is a soft woven mesh low-profile device made from 144 threads of 0.002-inch Nitinol wires. Reference Schubert, Kelm and Koneti26 This self-expanding device is a hybrid design with two discs joined by an articulated and expanding cone-shaped connecting waist. The device is delivered with SteerEase™ introducer (LifeTech, Shenzhen, China) with sheath sizes ranging from 5F to 7F. There are eight available sizes of the device, the waist of the four large models is securely sewn with polytetrafluoroethylene membrane using Nylon threads in order to increase its occlusion capacity, while the four smaller models have no membrane inside. Reference Schubert, Kelm and Koneti26–Reference Tanidir, Baspinar and Saygi28
In perimembranous ventricular septal defect, the aim was to completely occlude the left ventricle entry when sub-aortic rim length was adequate. In patients with deficient sub-aortic rim, the devices were chosen based on the diameter of the left retention disc with a preference for even sizes, whereas in those with sufficient sub-aortic rim, D2 guided the device selection. In defects with deep aneurysm, the device was slightly oversized (right ventricular side of the defect + 2mm), but if the device’s left ventricular disc (including rims) exceeded the size of the aneurysm, device size was decreased to the same size or +1 mm of the right ventricular disc. For muscular defects, the defect size +2 (right ventricle disc size) was usually enough.
Procedure
The procedure was performed under general anaesthesia in all patients. Transesophageal echocardiography was performed to reassess the defect size (left ventricle entry diameter and number and diameters of the right ventricle exit(s)), defect morphology, position, and the relationship and morphology of surrounding structures like aortic valve and tricuspid valve. Left ventriculography at 55–65° left anterior oblique and 20–30° cranial projections were used to profile the defect.
The decision of using the antegrade or retrograde approach was primarily based on the size and weight of the child and then on the sheath required from the arterial approach as per VSD size. If the VSD aneurysm is large and extends towards the tricuspid valve, a retrograde approach is preferred to be antegrade as the distance of the device to the tricuspid valve is increased. The arterial approach helped in reducing procedure time and cumulative radiation.
The procedures were performed as per standard guidelines either with retrograde or antegrade approach for perimembranous (Fig 1a–d) and high muscular defects (Fig 2a–f). Left ventriculogram and transesophageal echocardiography were performed before the release to confirm proper device position (Fig 2g–h). Post-procedure transesophageal echocardiography and left ventriculogram were performed in all cases to confirm results. Additional aortogram was performed in selected patients to check any aortic insufficiency.
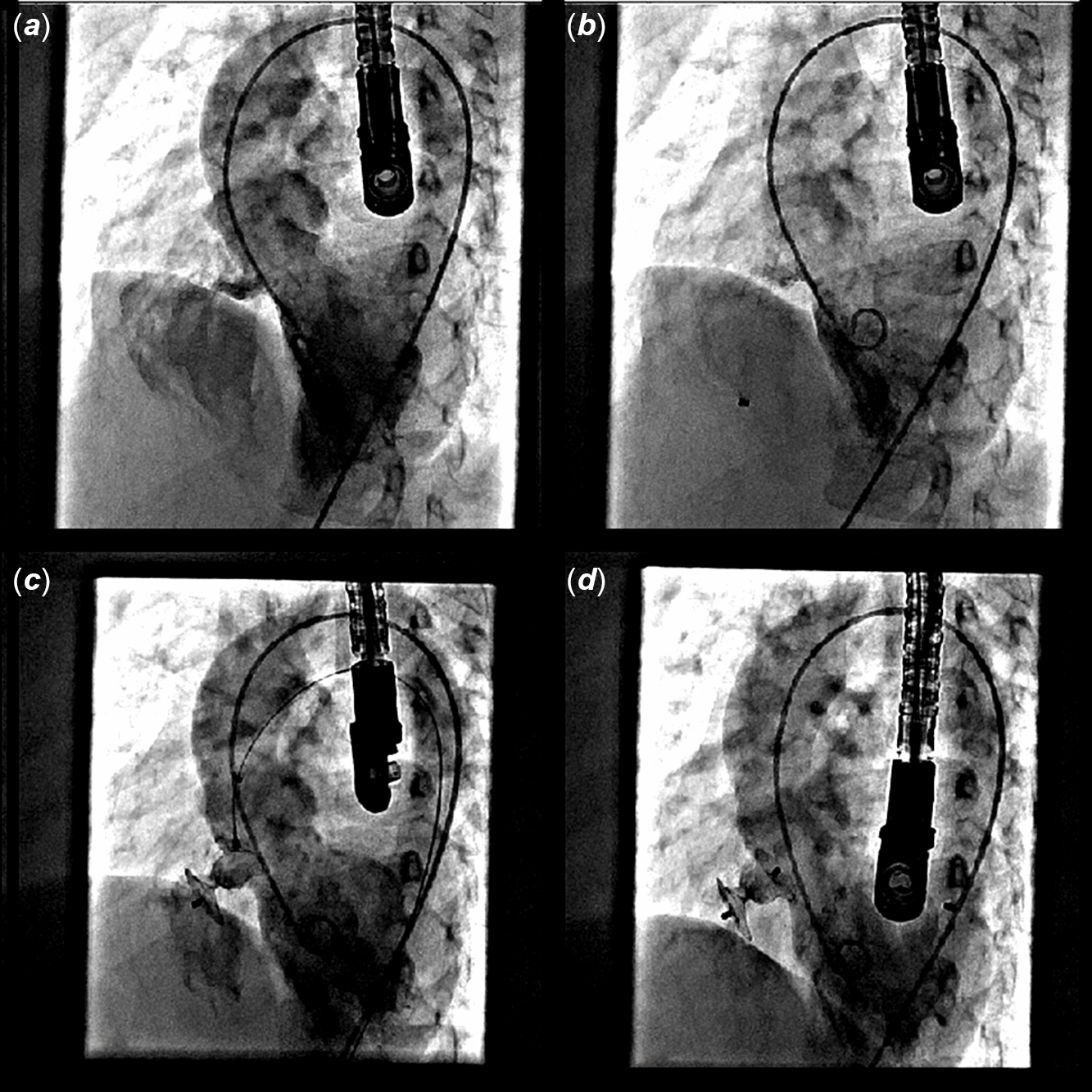
Figure 1. Device closure of a perimembranous defect using retrograde approach. ( a ) Left ventriculogram showing membranous septal defect. ( b ) Delivery sheath passed across VSD into RV. ( c ) RV end of device being released in RV, while LV end still attached to delivery cable within delivery sheath. ( d ) Final position of device after deployment completely occluding the defect.
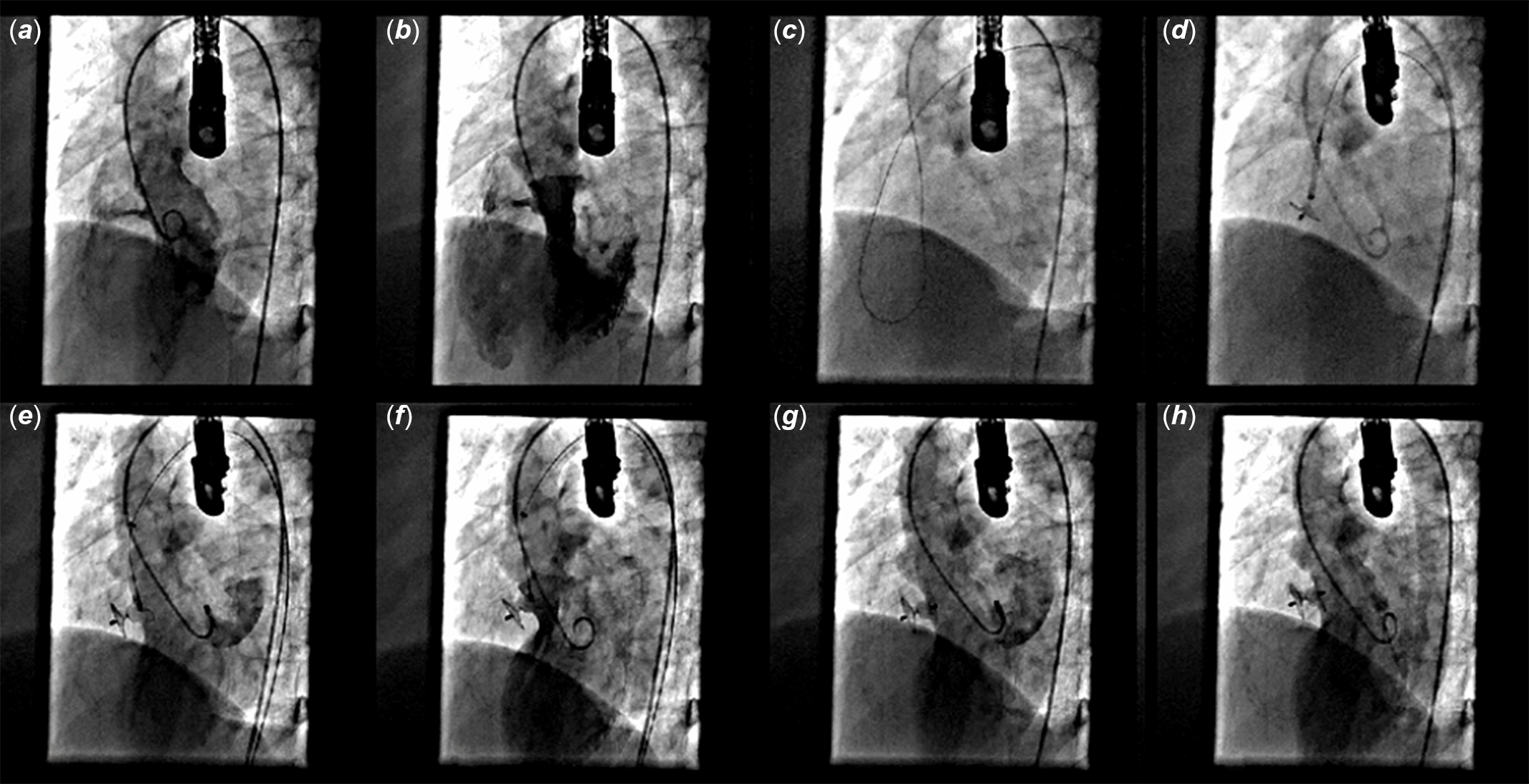
Figure 2. Device closure of a high muscular defect using retrograde approach. ( a ) Left ventriculogram showing high muscular VSD. ( b ) LV outflow angiogram showing high muscular VSD. ( c ) Crossed through retrograde approach with glide wire and engaged in main pulmonary artery. ( d ) Device's RV disc released in RV. ( e ) LV disc released in LV. ( f ) Final position checked prior to release. ( g ) Final result confirmed on LAO 45° projection ventriculogram. ( h ) Final result confirmed on LAO 60° projection ventriculogram.
Follow-up
Follow-up evaluation was done at 1, 3, 6, and 12 months’ post-procedure. Physical examination, transthoracic echocardiography, electrocardiogram, and chest X-ray (if needed) were performed. Holter monitoring (24-hour) was performed only when judged clinically indicated. Aspirin 5 mg/kg per day was administered for 6 months in all patients.
Statistical analysis
Descriptive statistics of demographic and clinical characteristics are presented as frequency and percentage for categorical variables, and mean and standard deviation or median and interquartile range depending on the distributional assumption for continuous variables using Shapiro–Wilk test. Comparison between groups was assessed using chi-squared or Student’s t-test considering p < 0.05 as significant.
Results
Forty-four patients were enrolled in this multicentre study. The demographic details and pre-procedural echocardiographic details are given in Table 1. None of these patients had symptoms of heart failure, 12 (27.2%) had a recurrent respiratory infection, 15 (34.1%) failed to thrive, and the remaining were clinically asymptomatic. There was no significant difference between types of ventricular septal defect in either gender (p = 0.71). At the time of intervention, all patients had sinus rhythm except one with first-degree heart block. All the ventricular septal defects had restrictive physiology with left ventricular volume overload and no or mild pulmonary hypertension. The median cardiothoracic ratio on the chest radiograph was 0.53 (range 0.41–0.67).
Table 1. Demographic characteristics and pre-procedural details of patients.

LV = Left ventricle; RV = Right ventricle; VSD = Ventricular septal defect
The procedural details are shown in Table 2. An arterial (retrograde) approach was adopted in 39 patients (88.6%). Immediately after device release, 16 patients (36.4%) showed some foaming through the device while 9 patients (20.5%) had a small residual leak. The median hospital stay was 2 days (range 1–4 days). Six (13%) patients with perimembranous outlet ventricular septal defect and mild prolapse of the right coronary cusp also underwent device closure successfully. One patient with trace to mild aortic regurgitation had slightly increased (but still mild) aortic regurgitation and no progression was detected at follow-up.
Table 2. Procedural details.

LV = Left ventricle; Qp = Pulmonary blood flow; Qs = Systemic blood flow; PTFE = Polytetrafluoroethylene; RV = Right ventricle; VSD = Ventricular septal defect
Complications
Most of the adverse events were minor complications (11.4%). Three patients (7%) required device recapture and readjustment before final release while 2 patients (4.5%) required reloading a larger device. Major complications occurred in 2 (4.5%) patients. One device embolised to the pulmonary artery, which was successfully retrieved percutaneously and the ventricular septal defect was closed with a larger device. There was no late embolisation. One patient with perimembranous ventricular septal defect and mild prolapse of right coronary cusp had a slight increase in aortic regurgitation, which remained mild and unchanged till the latest follow-up. Two patients had a slight increase in tricuspid regurgitation. There were no deaths, disabilities, or any other major complications such as complete heart block (Table 3).
Table 3. Complications.

RV = Right ventricle, VSD = Ventricular septal defect
Follow-up
Median follow-up was 13 months (range 5–18 months) with 97.7 % follow-up rate, (one patient lost to follow-up). The left ventricular diastolic dimension dropped from median +2.17 z score (range +0.48–+6.02z) to +0.87 z score (range −0.61 – +3.23z) at 3 months and +0.1 z score (range −1.9 – +1.37z) at final follow-up. The rate of improvement in left ventricular diastolic dimension was fastest in the first 3 months (median 10.1%, range 0–20.83 %) while median fall in left ventricular diastolic dimension at the final follow-up was 15.8% (range 5.9–25%) Figure 3.

Figure 3. Gradual normalisation of LV diastolic dimensions in 18 months’ follow-up.
At 3 months’ follow-up, residual leak persisted in 6 patients (13.6%). After 6 months, only 3 patients (6.3%) had a small residual leak. At the final follow-up (5–18 months), only 1 patient (2.3%) had a minimal residual leak with no patients showing any increase in aortic regurgitation. Mild neo-tricuspid regurgitation was persisted in one patient. No right ventricular or left ventricular outflow tract obstruction was noted (Table 4).
Table 4. Follow-up data.

LVOT = Left ventricular outflow tract obstruction; RVOT = Right ventricular outflow tract obstruction; VSD = Ventricular septal defect
A follow-up transthoracic echocardiography in a patient with ventricular septal defect and mild prolapse of RCC closed with a 10/8 MFO device (Fig 4) clearly shows that the device did not distort the mildly prolapsed cusp, and no progression of aortic regurgitation after 12 months was seen.

Figure 4. Echocardiographic picture of pmVSD with RCC prolapse at follow- up. ( a ) Parasternal long axis 2D. ( b ) Parasternal long axis with color Doppler. ( c ) Parasternal short axis 2D. ( d ) Parasternal short axis with color Doppler. ( e ) Parasternal short axis with 3D showing device in situ, no residual defect and no aortic insufficiency.
Discussion
Ventricular septal defect is the commonest of all CHDs and there has always been a lot of enthusiasm for device closure of the ventricular septal defect. The initial devices used for ventricular septal defect closure were not specifically designed for this purpose. Rashkind’s double umbrella device was designed for the closure of PDA and the Lock Clamshell device was designed for closure of ASD. Reference Lock, Block and McKay2,Reference Kalra, Verma and Dhall3 Detachable coils used for vessel embolisation was used for VSD closure. Reference Kalra, Verma and Dhall3 Sideris buttoned device was again designed for ASD and PDA occlusion. Reference Sideris, Walsh and Haddad4 Knauth AL and colleagues reported upon successive generations of the STARFlex® and CardioSEAL® devices (NMT Medical, Inc., Boston, Massachusetts, USA) for VSD closure. Reference Knauth, Lock and Perry5 These devices were cumbersome to use with large delivery sheaths, inability to recapture and reposition, structural failure, interference with the aortic valve and tricuspid valve, and a high rate of dislodgement, embolisation, and residual shunting. Reference Lock, Block and McKay2–Reference Knauth, Lock and Perry5
The first device designed for perimembranous ventricular septal defect closure was the Amplatzer asymmetrical membranous VSD device (AGA Medical Corporation, MN, USA) in which the aortic end of the left ventricular disc is short (0.5 mm). Reference Hijazi, Hakim and Haweleh6–Reference Santhanam, Yang and Chen12 However, anatomic proximity to the cardiac conduction system and high radial stress and clamp force led to an increased risk of a complete atrioventricular block (5–22%) essentially abandoning its use. Reference Predescu, Chaturvedi and Friedberg13–Reference Yang, Kong and Sheng15 The Nit-Occlud® VSD device (pfm – Produkte für die Medizin AG, Köln, Germany) was also developed for dedicated ventricular septal closure (aneurysmatic perimembranous and muscular). Reference Haas, Kock and Bertram16 No permanent heart block has been reported so far although severe tricuspid regurgitation needing surgery has been reported in 2.3% of patients. Reference Kozlik-Feldmann, Lorber and Sievert17 The other Amplatzer family devices (AMVSD Occluder, ADOI, ADOII, AVPII; Abbott Medical, Plymouth, MN, United States of America) are now considered for off-label use and have shown results comparable to surgery. Reference Mijangos-Vázquez, El-Sisi and Sandoval Jones18–Reference El-Sisi, Sobhy and Jaccoub21 A new Amplatzer device (Amplatzer Membranous VSD Occluder 2, AGA Medical Corporation, St Jude, MN, USA) was designed to prevent conduction abnormalities and favourable results were reported in the initial experience, but no follow-up data are available. Reference Tzikas, Ibrahim and Velasco-Sanchez22 The devices of other manufactures are also being used worldwide most commonly from China and the rest of Asia. Reference Yang, Kong and Sheng15,Reference Yang, Yang and Wan23–Reference Zhou, Pan and Guan25
LifeTechTM Konar-MF VSD Occluder is a new device specifically designed for VSD closure, which is soft and flexible, can be delivered both ante and retrograde and offers a hybrid design between single and double-disc devices. Reference Schubert, Kelm and Koneti26–Reference Tanidir, Baspinar and Saygi28 The initial experience is encouraging and our experience adds to the currently limited literature available on the initial and early results including patients with mild right coronary cusp prolapse with no or trace to mild aortic regurgitation.
Our data are important for various reasons. First, it includes the short-term follow-up of over a year. There was no incidence of complete heart block, no late embolisation, and no new onset of aortic or tricuspid regurgitation. The device embolisation occurred in only one patient during the procedure needing a larger device. The design of the device is such that either end can be snared and the softness allows it to be easily pulled into the same sheath size, which is used to deliver the device.
Second, the incidence of prolapse of the right coronary cusp associated with perimembranous outlet ventricular septal defect is high in our population. Reference Kazmi, Sadiq and Hyder29 We decided to close these defects if patients had a perimembranous ventricular septal defect with mild right coronary cusp prolapse and aneurysm on the right ventricular end of the ventricular septal defect. In these cases, the conventional double-disc device may cause further complications in particular acute severe aortic regurgitation or progression of aortic regurgitation with time. Reference Chen, Li and Li30 The implantation of MFO Occluder was convenient in perimembranous ventricular septal defect with aneurysm because the retention disc can be put entirely within the aneurysm and the cylindrical portion of the device secures itself in an opening of the aneurysm on the right ventricular side with right ventricle disc ensuring complete closure. Therefore, the device stayed under the aortic valve cusp preventing any further progression of the prolapsing leaflet, hence preventing progression of aortic regurgitation. Proper patient selection is the most important factor for procedural success in these cases. As there is no aortic rim, the measurement of the actual ventricular septal defect size is a challenge. We measured the ventricular septal defect size from the crest of the interventricular septum to the aortic valve annulus. Hence for successful ventricular septal defect device closure in these patients, pre-intervention profiling of ventricular septal defect by transthoracic and transesophageal echocardiography (0–180° profiling) as well as intraoperative profiling during left ventricular angiography are crucial.
The procedural success (100%) and immediate closure rates (79.5%) were high in our study. These results were comparable to studies using the conventional double-disc devices such as Amplatzer Membranous VSD Occluder, Amplatzer Muscular VSD Occluder, Amplatzer Duct Occluder, Amplatzer Duct Occluder II, AVPII, or modified devices such as modified VSD Occluder (being used in China) for perimembranous ventricular septal defect closure. Reference Bergmann, Germann and Nordmeyer19–Reference Zhou, Pan and Guan25 The closure rate increased to 97.5% at a median follow-up of 13 (15–18) months.
Fourth, the design of this device allows its use in few patients, where the ventricular septal defect RV end diameter is bigger than the left ventricular end diameter (two patients in our series). The unique design of the MFO Occluder achieves a stable position inside the defect while occluding the right ventricle end completely. Such patients have historically needed surgery.
Finally, the complications associated with attempted device closure have been related to various factors like a large device profile, a large delivery system, high clamping force caused by double-disc design, and high radial stress due to the hard profile of the device. The Konar-MF VSD Occluder is a low profile, soft device, and needs a small delivery system with ease of implantation from either route. There is a significantly reduced probability of trauma, low clamp force, and radial stress to the ventricular septum, and therefore the likelihood of damage to adjacent structures like aortic and tricuspid valves as well as the bundle of His greatly decreases. The left ventricular disc in Konar-MF VSD Occluder, however, is symmetrical (ADOI design) and long-term data are required to evaluate this limitation as complete heart block has been reported with this device too. Reference Haddad, Daou and Saliba27 Similarly, the right ventricular disc is like that of ADOII which may potentially cause tricuspid regurgitation.
Study limitations
The study population was relatively older children and the age range was wide. Since this experience was in its initial stage, the operators’ choice of defect size was also small to moderate and moderate rather than large. The study is essentially a clinical follow-up of just 1 year, hence, the safety profile in patients with aortic valve prolapse is uncertain and needs long-term follow-up, as the aortic regurgitation may show up in later years. Long-term data would be required to assess other long-term complications like complete heart block.
Conclusions
Percutaneous closure of ventricular septal defect using the Konar-MF device is safe and effective. The short-term follow-up shows a high closure rate with few complications and no case of complete heart block. This device can also be safely used in patient with perimembranous ventricular septal defect, aneurysm formation, and a mild prolapse of the right coronary cusp. With increasing experience, the device may be used in younger patients with large ventricular septal defects.
Acknowledgement
We would like to acknowledge Dr Muhammad Akhter Sultan, fellow pediatric cardiology and Mr. Abdul Razzaq, Cath lab technician for helping in data collection for the study.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Drug Regulatory Authority of Pakistan, GL No. DRAP/PS-002/01) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review board of The Children’s Hospital and Institute of Child Health Lahore and institutional ethical committees of The Children’s Hospital and Institute of Child Health Multan and Ch. Pervez Elahi Institute of Cardiology Multan. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals (National Bioethics Committee of Pakistan Research Council, Rule 9(1) of Bio-Study Rules 2017) and has been approved by the institutional review board of The Children’s Hospital and Institute of Child Health Lahore and institutional ethical committees of The Children’s Hospital and Institute of Child Health Multan and Ch. Pervez Elahi Institute of Cardiology Multan.











