INTRODUCTION
Panax ginseng C. A. Meyer (ginseng) is a herbal root with the ability to improve a weak constitution, prolong life, cure diseases and enhance body health (Gillis, Reference Gillis1997; Hu, Reference Hu1976). The cultivation of ginseng is complex due to its long cultivation period, requirement for deep shade and need for soils that are rich in nutrients, slightly acidic, deep and well drained. The Changbai Mountains area is considered to be a region that produces some of the finest cultivated ginseng in the world. This region has gained a reputation for the high-quality mass production of authentic ginseng. It is estimated that the Changbai Mountains account for 85% of the ginseng produced in China and 70% of the ginseng produced in the world (Sun and Zhou, Reference Sun and Zhou2008). Ginseng cultivation faces several problems, including the barrier of continuous cultivation, which has been frequently reported, and significant effort has been devoted to its elucidation (Zhang et al., Reference Zhang, Chen, Wang, Xu and Liu2008). A widespread problem is red skin disease, which has been postulated to result from physiological stress rather than pathological infection (Gao et al., Reference Gao, Zhang and Hai2011; Zhao and Li, Reference Zhao and Li1998; Zhao et al., Reference Zhao, Li, Guo, Liu and Liu2001). Therefore, soil conditions may be an important factor related to the incidence of red skin disease.
Albic luvisols, also termed ‘Baijiang soil’ in China, is an important soil type for ginseng cultivation and is widely distributed in Northeast China. A mixture of humus and albic horizon (generally 1:1) is combined to create raised bed soils (Jilin Province Soil and Fertilizer Department, 1990). It is reported that ginseng cultivation may acidify bed soils at some depths (Xie and Xu, Reference Xie and Xu1996; Zhao and Li, Reference Zhao and Li1998). Soil acidification can lead to the dissolution of active Al and increase Al phytotoxicity. Al toxicity is one of the most significant constraints limiting plant growth and crop production in acidic soils (pH < 5.0; Kochian et al., Reference Kochian, Pineros and Hoekenga2005). The primary symptom of Al toxicity is the rapid inhibition of root growth, resulting in a reduced and damaged root system that limits water and mineral nutrient uptake (Delhaize and Ryan, Reference Delhaize and Ryan1995). One of the Al resistance mechanisms in higher plants is the production of phenolic compounds in response to Al stress (Matsumoto et al., Reference Matsumoto, Hirasawa, Morimura and Takahashi1976; Ofei-Manu et al., Reference Ofei-Manu, Wagatsuma, Ishikawa and Tawaraya2001; Osawa et al., Reference Osawa, Endo, Hara, Matsushima and Tange2011). The accumulation and oxidation of phenolic compounds have been suggested to lead to the formation of brown to red pigments in plant tissues (Pourcel et al., Reference Pourcel, Routaboul, Cheynier, Lepiniec and Debeaujon2006). Our preliminary study showed that there was a high level of Al3+ present in the ginseng bed soils and that ginseng roots with red skin disease contained higher Al content (Li, Reference Li2009). In the present study, ginseng red skin disease was hypothesized to be related to Al toxicity or the Al resistance response in ginseng. Therefore, determining Al speciation in the mixed ginseng bed soils is necessary to assess the risk of their potential toxicity.
In this study, the bed soils and ginseng roots were sampled at a ginseng farm located in the Changbai Mountains in Northeast China. To systemically study the causes of red skin disease, we investigated soil characteristics and evaluated various Al pools in bed soils by selective extraction.
MATERIALS AND METHODS
Description of the sampling area and the cultivating method of ginseng
The study areas were the ginseng cultivation fields (41°32′N, 128°09′E) in the Changbai Mountains in MaLuGou country, Changbai autonomous county, Jilin Province, China. Prior to ginseng cultivation, the location contained a mixed hardwood forest. Large areas albic luvisols rich in volcanic ash was derived from loess under the native forest. Local farmers deforested the area by cutting and burning the trees and then used mixed humus and albic horizons (1:1) to create raised ginseng bed soils. An artificial plastic shade was utilized above the bed soils for ginseng cultivation. This area is characterized by a mountain climate with a dry and windy spring, a rainy summer, a cool and foggy autumn, and a cold and long winter. The mean annual temperature varies between 3.3 and 7.3 °C, with the mean temperature in July ranging from 8.7 to 19.3 °C and the mean temperature in January ranging from −23.3 to −16.1 °C. The annual solar radiation is 124.01 MJ m−2, and the mean annual precipitation is over 1400 mm, which is the heaviest precipitation in Northeast China (Yang et al., Reference Yang, Kim, Yun, Lee, Kwon and Kang1997; Yang and Xu, Reference Yang and Xu2003).
Sampling procedures
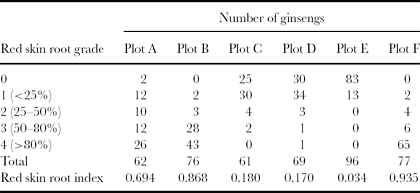
The study was carried out in September 2009 during the ginseng harvest period. We selected six plots (A–F) containing 6-year-old ginseng plants (each plot was 1 m2), which belonged to different farmers. From each plot, all of the ginseng roots were harvested, numbered and stored at 4 °C for transportation. In the laboratory, each ginseng root was classified using five grades corresponding to the percentage of the area of red skin (Li et al., Reference Li, Tian, Sun, Guo and Liu1999). The grades ranged from 0 to 4, representing healthy ginseng without red skin and a red skin area less than 25%, 25–50%, 50–80% and more than 80% of the root epidermal (Table 1). Ginseng roots representative of the different grades were photographed using a Nikon camera. The red skin disease indices in each plot were computed as ∑(number × red skin grade)/(total number × the highest red skin grade) (Li et al., Reference Li, Tian, Sun, Guo and Liu1999). The soils were sampled from each plot at three depths at intervals of 0–5 cm, 5–10 cm and 10–15 cm, representing soils from the upper root zone, the root zone and below the root zone, respectively. Each soil sample consisted of a mixture of five subsoils from the same plot. A portion of the soil samples was stored at 4 °C for nitrate determination, and the remainder were air dried and passed through a 2-mm sieve for laboratory analysis.
Table 1. Red skin disease indices in the six plots.

Analysis of ginseng root Al content
Ginseng roots with different red skin grades were rinsed five times with distilled water to remove attached soil. Fibrous roots were cut, and the ginseng head, epidermis and periderm (2–3 layers cells below epidermal) were separated from the roots using a bamboo knife. All of the samples were dried, crushed and digested in an acid mixture (HNO3: HClO4 4:1). The amount of Al present in the digest solution was quantified using an atomic absorption spectrophotometer (AAS) with a graphite furnace atomizer (Perkin-Elmer AAnalyst 700).
Soil characteristics analysis
Soil moisture was measured by gravimetry after the soil was dried at 105 °C. The bulk density of soil samples was determined from three replicates of undisturbed 28.6 cm3 soil cylinders and computed according to soil moisture. The pH in water and in a 1 M KCl solution (w:v, 1:2.5) was measured using a pH meter (PHS-3C, Leichi, Shanghai, China). The organic matter concentration, as total organic carbon, was determined using the dry combustion method. The nitrate concentration in soil was extracted using a 1 M KCl solution and analysed by dual-wavelength ultraviolet spectrophotometry according to Norman et al. (Reference Norman, Edberg and Stucki1985).
Exchangeable cations were extracted with 1 M NH4Cl (soil: extractant, 1:50) and determined by AAS (Ca, Mg and Al) and using a flame photometer (Na and K). The effective cation exchange capacity (ECEC) was calculated as the sum of Ca, Mg, Na, K and Al (Kamprath, Reference Kamprath1970). Al saturation (Al: ECEC) and the molar ratio of the base cations (BC, Ca+Mg+K+Na) to Al were also calculated.
Al fractionation
Different forms of Al were extracted from the soil solid phase using several extraction solutions. Al extracted using 0.5 M NaOH (soil:extractant, 1:100, with shaking for 16 h) (Aln) estimates the total free Al (Borggaard, Reference Borggaard1985). Al extracted using 0.2 M ammonium oxalate-oxalic acid at pH 3 (soil:extractant, 1:50, with shaking for 4 h in the dark) (Alo) estimates total non-crystalline Al (Buurman et al., Reference Buurman, Van Lagen and Velthorst1996; García-Rodeja et al., Reference García-Rodeja, Nóvoa, Pontevedra, Martínez-Cortizas and Buurman2004). Al extracted using 0.1 M Na-pyrophosphate (pH 10) (soil:extractant, 1:100, with shaking for 16 h) (Alp) estimates the total organically bound Al (Buurman et al., Reference Buurman, Van Lagen and Velthorst1996). Extraction using 0.5 M CuCl2 (pH 2.8) (soil: extractant, 1:10, with shaking for 2 h) (AlCu) estimates aluminium forming low- and medium-stability complexes with organic matter (Juo and Kamprath, Reference Juo and Kamprath1979). Al in extracts was measured in duplicate by AAS with a graphite furnace atomizer.
RESULTS
Characteristics of red skin disease of ginseng and the disease indices in six plots
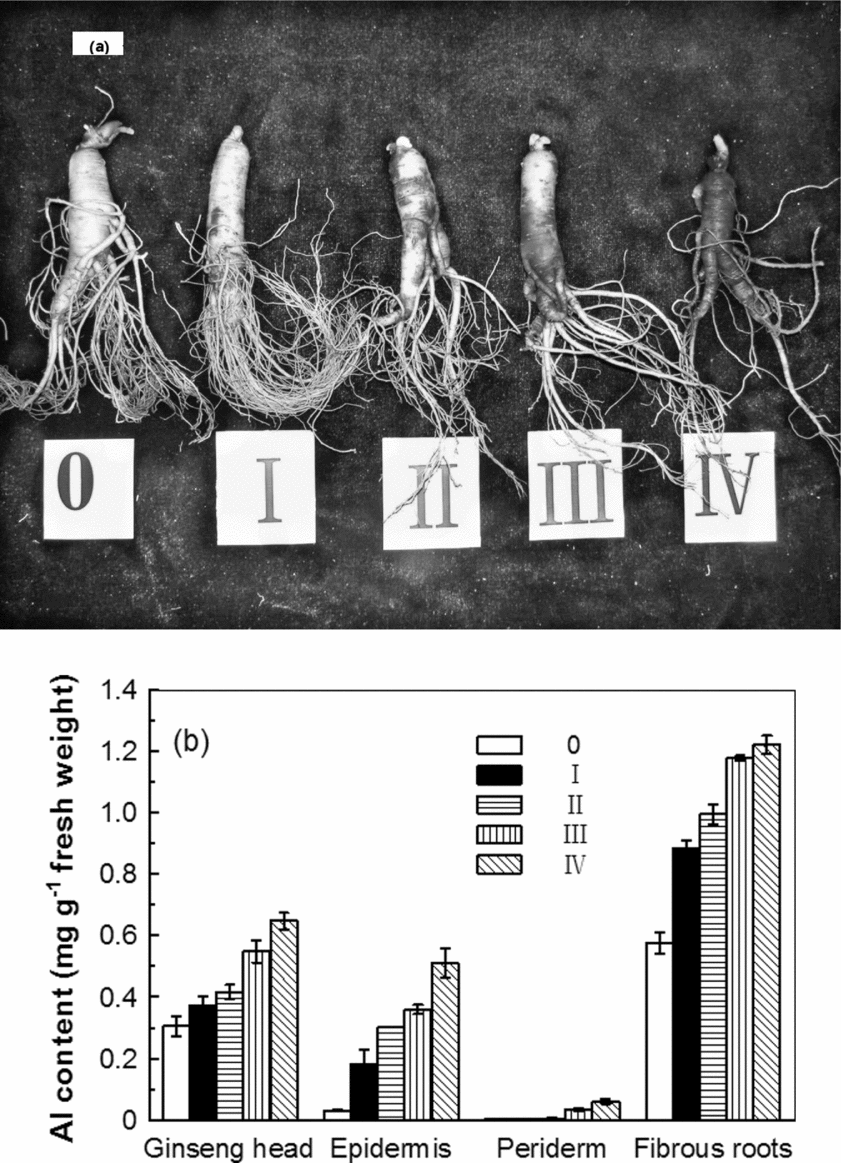
Figure 1a describes the typical five disease grades according to the red skin area. Ginseng with a higher red skin grade had fewer lateral roots and accumulated more Al in the head and root tissues, especially in the fibrous roots (Figures 1a and b). More Al accumulated in the epidermal tissues, and less accumulated in the periderm (Figure 1b). The red epidermis initially appeared in the main roots, followed by the lateral roots, until it finally covered the entire root. The red zone was limited to the epidermal and peridermal tissues. The red epidermal cells were easy to remove; however, there were no fungal spores or mycelium visible in or between cells observed by microscopy (data not shown).

Figure 1. Characterization of ginseng red skin disease. (a) Ginseng roots were classified according to the percentage of the area of their red skin. (b) The Al content of the different ginseng root tissues classified with different red skin disease grades.
Table 1 presents the ginseng numbers in each red skin disease grade and the calculated red skin disease indices in each plot. Plot E, with the lowest index of 0.034, contained the most healthy ginseng. Plot F, with the highest index of 0.935, contained the highest number of roots exhibiting grade 4 red skin disease.
Soil physico-chemical properties of different plots
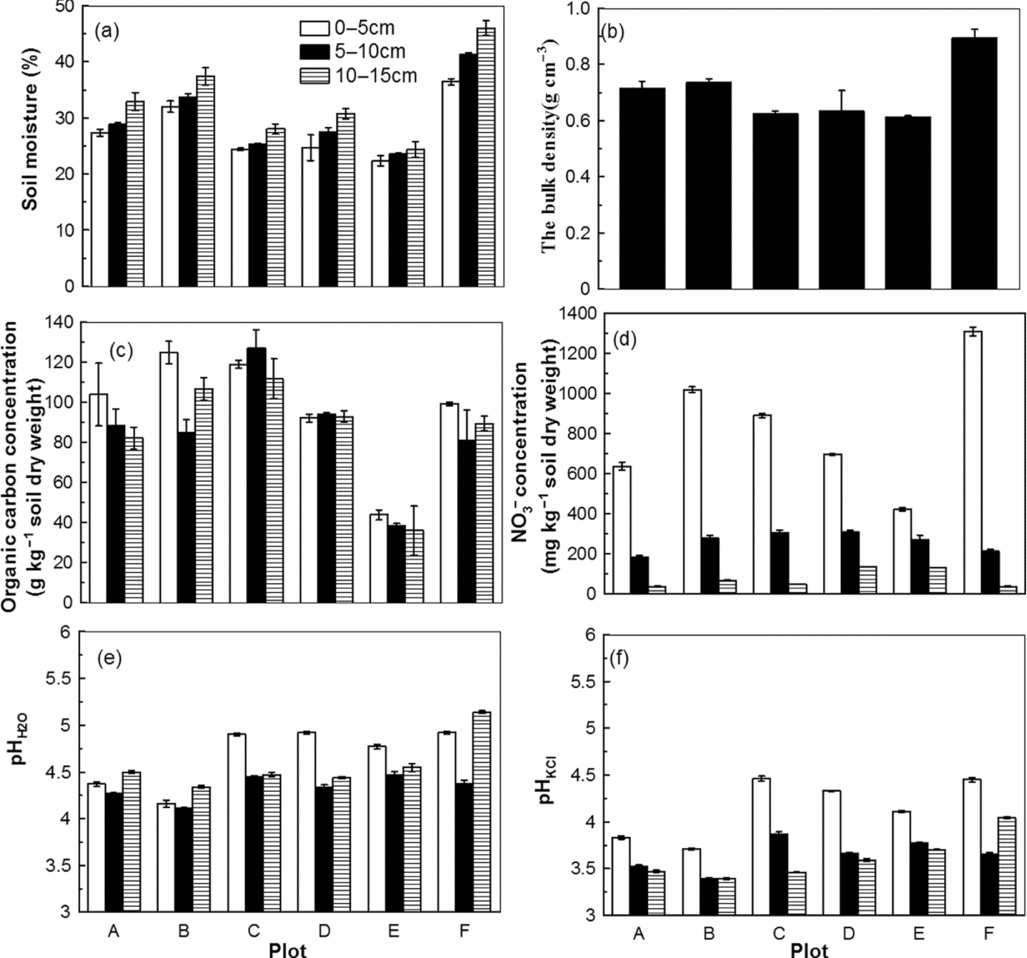
For all plots, the soil moisture increased with soil depth (0–5 cm, 5–10 cm, 10–15 cm). Plots A, B and F, which had higher red skin disease indices, contained higher soil moisture at all the depths, compared with plots C, D and E (Figure 2a). The soil moisture of plot F was nearly twofold that of plot E.

Figure 2. The soil moisture (a), bulk density (b), organic carbon concentration (c), NO3− concentration (d), pHH2O (e) and pHKCl (f) of the six plots.
Bulk density is an index of soil compaction. The soil bulk density of all the plots was less than 1 g cm−3; this was less than the average for cultivation soil, which generally has a bulk density of 1–1.3 g cm−3. Plots A, B and F, with higher red skin disease indices, also exhibited higher soil bulk densities; plot F had the greatest bulk density with 0.9 g cm−3 (Figure 2b).
The organic carbon concentration fluctuated among the different plots and depths. Plot E, which exhibited the lowest disease index, contained the lowest total organic carbon concentration at all three depths (Figure 2c).
The nitrate concentrations were markedly different among the three depths of the different plots and were significantly higher at the surface depth (0–5 cm) in each plot (Figure 2d). Plot F, with the highest red skin disease index, accumulated the highest nitrate concentration at the surface depth.
The ginseng bed soils were acidic with pHKCl (Figure 2f) ranging from 3.4 to 4.5, and the pHH2O of all plots was consistently below 5.0 (Figure 2e). Soil samples from the 5–10 cm depth (root zones) had lower pHH2O values compared with those measured at the higher and lower soil depths.
Variation in major cations and Al risk assessment
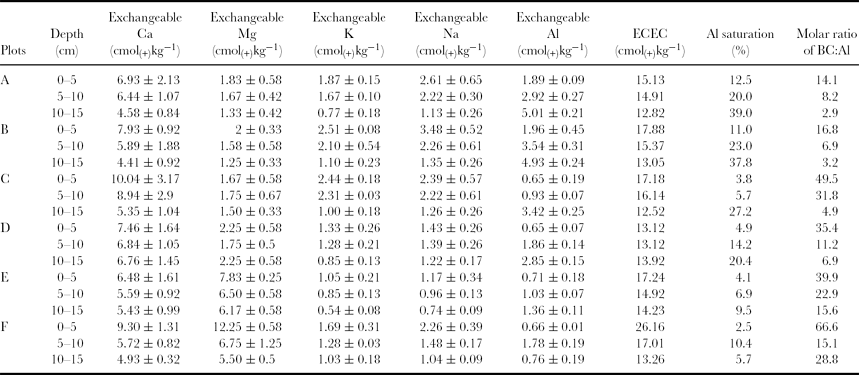
Major exchangeable cations (Ca, Mg, K, Na and Al) were measured, and the ECEC, Al saturation and molar ratio of BC to Al were calculated to evaluate Al toxicity (Table 2). Exchangeable Fe and Mn were also tested, but they were present in negligible amounts (data not shown). All the six plots and depths contained exchangeable Al that ranged from concentrations of 50–450 mg kg−1, with the concentrations increasing with depth. The variation in exchangeable Al was also negatively correlated to the pHs. Plots C, D and E, which exhibited lower red skin disease indices, contained lower concentrations of exchangeable Al in their root zones (5–10 cm; Table 2).
Table 2. The exchangeable cations, aluminium saturation and molar ratio of BC to Al of the soils in the six plots.

In contrast with exchangeable Al, the cations Ca, Mg, K and Na were present in higher concentrations in the surface soil. The ECEC ranged from 12 to 26 cmol(+)kg−1 and was also higher in the surface soil (Table 2).
Al saturation in soils is widely used for the risk assessment of Al toxicity. Plots A and B contained higher levels of Al saturation, while plot E contained the lowest level, and Al saturation at the depth of 10–15 cm was higher than at the 5–10 cm depth (Table 2).
The values of the molar ratio of BC to Al varied in different plots, and were negatively correlated with the Al saturation. Plots A and B, with higher red skin disease indices, had lower molar ratios of BC to Al compared with plots C, D and E, and the values in their root zones were below 10 (Table 2).
Al fractionation in the solid phase of soils in different plots
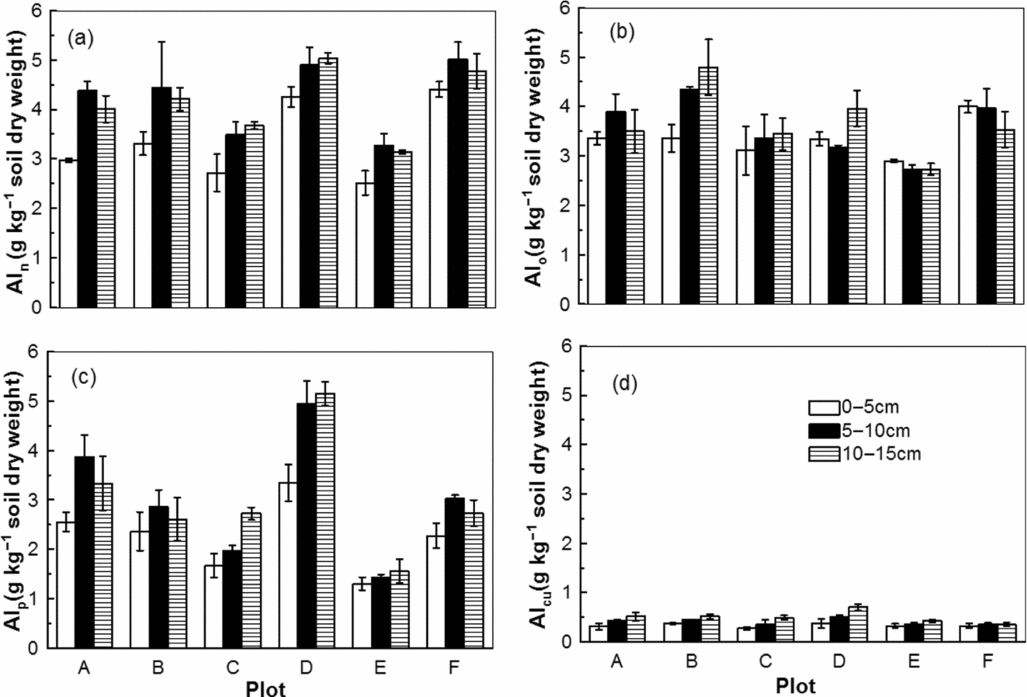
There were clear differences among the concentrations of Al species in the six plots (Figure 3). The concentration of Aln, an estimate of total ‘free’ Al, ranged from 2.5 to 5.0 g kg−1 among the six plots. Plots D and E, with lower red skin disease indices, contained lower concentrations of Aln (Figure 3a). The concentration of Alo, an estimate of total non-crystalline Al, ranged from 2.7 to 4.8 g kg−1. Plots C, D and E contained lower Alo concentrations than plots A, B and F, which was consistent with their corresponding red skin disease indices (Figure 3b). Alp values, an estimate of the total organically bound Al, were generally lower than those of Alo, except in plot D (Figure 3c). AlCu, which is used to estimate Al forming low- and medium-stability complexes with organic matter, was present with no significant differences among all depths of the six plots (Figure 3d).

Figure 3. Al fractionation in the soil solid phase of the six plots. The fractions were extracted with 0.5 M NaOH for Aln (a), 0.2 M ammonium oxalate-oxalic acid at pH 3 for Alo (b), 0.1 M Na-pyrophosphate (pH 10) for Alp (c) and 0.5 M CuCl2 (pH 2.8) for AlCu (d).
DISCUSSION
Red skin disease is commonly found in the bed soils that originate from albic luvisol in the Changbai Mountains region. Ginseng roots suffering from red skin disease present symptoms similar to Al toxicity, such as the inhibition of root growth, Al accumulation and a peeling epidermis (Figure 1a; Delhaize and Ryan, Reference Delhaize and Ryan1995; Li, Reference Li2009; Ma, Reference Ma2005). As the red skin disease grade increased, the inhibition of root growth became increasingly apparent. A higher concentration of Al accumulated in the fibrous roots; these roots contain the largest root surface area, thereby facilitating Al uptake (Figures 1a and b). In this study, we did not find fungal spores or mycelium in or between cells in the diseased ginseng roots (data not shown). This is consistent with the view of several Chinese researchers that the red skin in the ginseng roots is a physiological disorder rather than a symptom of infection (Gao et al., Reference Gao, Zhang and Hai2011; Li et al., Reference Li, Tian, Sun, Guo and Liu1999; Zhao and Li, Reference Zhao and Li1998; Zhao et al., Reference Zhao, Li, Guo, Liu and Liu2001) and that it is unrelated to rusty root in Panax quinquefolius L (Campeau and Proctor, Reference Campeau and Proctor2003). Al toxicity and accumulation in plant tissues are closely related to the availability of Al in soils (Dong et al., Reference Dong, Ramsey and Thornton1995; Hoyt and Nyborg, Reference Hoyt and Nyborg1971, Reference Hoyt and Nyborg1972). For this reason, the soil characteristics in the six plots were examined to determine their relation to the incidence of ginseng red skin disease.
The ginseng bed soil, which consisted of a mixture of humus and albic soil horizons, had a low bulk density value of less than 1 g cm−3 (Figure 2a). Plots A, B and F, which contained the ginseng with higher red skin indices, had higher bulk densities and moisture contents (Figures 2a and b). This indicates that wet (damp) and compact soil conditions, with poor soil permeability and ventilation, promote the incidence and development of red skin disease. Higher nitrate concentrations were present at the soil surface (Figure 2d). This may be due to the artificial plastic shade for ginseng growth changing the water movement in the ginseng bed soils, resulting in the dominant upward capillary movement of water and causing nitrate accumulation at the surface. In addition, nitrate concentrations at the surface depths were in agreement with the red skin disease indices of the different plots (Figure 2d and Table 1).
The artificial ginseng bed soils that originated from albic luvisols were acidic (Figures 2e and f). In addition, the pHH2O and pHKCl values in the root zone (5–10 cm) were always lower compared with those above (0–5 cm) and below the root zone (10–15 cm; Figures 2e and f), suggesting that ginseng growth may acidify the root rhizosphere. Several root-induced processes may modify the pH in the rhizosphere, including charge balances associated with nutritional ionic absorption by roots, cation uptake by roots that leads to the extrusion of H+ and anion uptake that releases OH− (Haynes, Reference Haynes1990).
Al toxicity is one of the most important factors limiting plant growth in acidic soils (pH < 5.0). Many plant species, especially crops, are sensitive to micromolar concentrations of Al (Kochian et al., Reference Kochian, Pineros and Hoekenga2005). Exchangeable Al, ECEC, Al saturation and the molar ratio of BC to Al were used to evaluate Al toxicity (Table 2). Al extracted with 1 M NH4Cl is considered to be readily exchangeable (Peech et al., Reference Peech, Alexander and Dean1947) and is the standard method for determining salt exchangeable forms of Al in modern soil classification (FAO, 1998). The ginseng bed soils contained high concentrations of exchangeable Al that increased with soil depth and were negatively correlated with soil pH (Figures 2d and e; Table 2). With the decrease in pH as soil depth increased, the exchangeable Al increased. Plots A, B, and F, with higher ginseng red skin disease indices, had higher exchangeable Al concentrations in their root zones compared with the corresponding depths in plots C and E. Nitrate is known to play a major role in controlling the dissolution of Al3+ into the soil solution (Umemura et al., Reference Umemura, Usami, Aizawa, Tsunoda and Satake2003). The high concentration of exchangeable Al in the ginseng bed soil may be related to the higher nitrate accumulation (Figure 2d, Table 1).
The ECEC ranged from 12 to 26 cmol(+)kg−1 and was generally higher at the surface depth (Table 2), which may be related to the ginseng planting method. The plastic shade used for ginseng cultivation affected the water movement and led to the surface accumulation of certain dissolved cations. The Al saturation in soils is widely used for the risk assessment of Al toxicity in forests. In the present study, the Al saturation at the depth of 10–15 cm was generally higher than that at the 5–10 cm depth. Plots A and B, with higher red skin disease indices, had Al saturations higher than 20% (Table 2), which is considered to be the maximum amount acceptable for the development of Al-sensitive species (Mombiela and Mateo, Reference Mombiela and Mateo1984). The molar ratio of BC to Al is considered to be an indicator of the level of soil acidification and tree damage (Cronan and Grigal, Reference Cronan and Grigal1995; Posch et al., Reference Posch, de Smet, Hettelingh and Downing1995). In Europe, a value of 1 is often considered to be the critical level for a BC to Al ratio for coniferous forests. Sato and Wakamatsu (Reference Sato and Wakamatsu2001) have investigated soil solution chemistry at three forest areas containing granite bedrock in Japan, and they demonstrated that the values of BC to Al almost always exceed 10 in Japanese forest soils, even though the forest was exposed to considerable Al stress. Umemura et al. (Reference Umemura, Usami, Aizawa, Tsunoda and Satake2003) suggested that a BC to Al value of 2.5 is by no means sufficient for Al toxicity in Japanese cedar. In the present study, the BC to Al values varied in different plots and were negatively correlated with the Al saturation (Table 2). The root zones of plots C, D and E, with lower red skin disease indices, had BC to Al values greater than 10. In contrast, plots A and B, with higher disease indices, had values of less than 10. Ginseng, as a herbal plant, may be more sensitive to Al than tree species. Therefore, the Al saturations and the molar ratio of BC to Al in the root zones of plots A and B may be over the threshold toxicity for ginseng. Al toxicity may be an important factor related to red skin disease. Only plot F, with the highest red skin disease index, had a relatively lower Al saturation and higher BC to Al value (Table 2). However, plot F contained the highest bulk density, moisture content and nitrate concentration (Figure 2). Multiple factors, including Al, may promote the incidence of red skin disease.
Al fractionation in the soil solid phase is extremely complex and still not fully understood. Extraction of dry soil samples is suitable for the assessment of the potential risk of Al released from the soil solid phase to the soil solution (Drabek et al., Reference Drabek, Boruvka, Mladkova and Kocarek2003). Although this does not necessarily quantify the exact amount of Al available to plants, it provides a good approximation of the amount of Al that can potentially be released. Aln is an estimate of the total ‘free’ Al, because it can represent Al in gibbsite and 1:1 phyllosilicates with weak crystallinity (García-Rodeja et al., Reference García-Rodeja, Nóvoa, Pontevedra, Martínez-Cortizas and Buurman2004). Plots A, B, D and F had relatively higher Aln compared with plots C and E (Figure 3a); therefore, plots with a higher red skin disease index had a high level of total free Al in gibbsite and 1:1 phyllosilicate. Plot D, with a lower disease index, contained a higher amount of Aln. The higher proportion of Alp within plot D may reduce the Al toxicity (Figure 3c). Alo commonly represents dissolved short-range order Al hydroxides and oxyhydroxides, Al bound to organic matter and Al present in allophane and imogolite (Theng et al., Reference Theng, Russell, Churchman and Parfitt1982). Alo can reflect the reactive Al pool and is also termed the total non-crystalline Al (Álvarez et al., Reference Álvarez, Monterrose and Fernandez Marcos2002). The higher Alo concentrations in plots A, B, and F, especially in their root zones, are consistent with their red skin indices (Figure 3b). The negligible difference between Aln and Alo (Aln–Alo) suggests that the total free Al pool was dominated by reactive Al in the ginseng bed soils. In addition, the exchangeable Al concentrations correlated well with those of Alo in all the plots. The former may be dependent on the latter. Alp is used as a proxy for Al in organic complexes (Buurman et al., Reference Buurman, Van Lagen and Velthorst1996). It was positively correlated with the soil organic matter at all soil depths in all the plots, with the exception of plot D (Figure 3c). The largest concentration of Alp was found in plot D despite the low organic carbon concentration (Figures 2c, 3c). The molar ratio of C to Alp strongly depends on the degree of humification and the soil organic matter chemistry, which can provide a measure of the degree of the Al saturation of the humus (García-Rodeja et al., Reference García-Rodeja, Nóvoa, Pontevedra, Martínez-Cortizas and Buurman2004. AlCu represents potentially reactive non-exchangeable Al, which estimates the Al forming low-stability and medium-stability complexes with organic matter (Álvarez et al., Reference Álvarez, Monterrose and Fernandez Marcos2002; Juo and Kamprath, Reference Juo and Kamprath1979). There was no difference among different plots in AlCu concentrations (Figure 3d).
Above all, red skin disease of ginseng is a physiological disorder that is closely related to the physico-chemical characteristics of soil. The plots with higher red skin indices had a higher moisture content, bulk density and nitrate concentration in bed soils. Exchangeable Al concentrations, as well as Aln and Alo, represented pools of exchangeable Al and correlated well with the red skin disease indices in the six plots. The Al saturation and BC to Al values in the two plots with the highest disease indices appear to be over the threshold of toxicity for Al sensitive species. Therefore, compact soils with higher moisture, nitrate concentrations and active Al species are suggested to contribute to the incidence of ginseng red skin disease.
Acknowledgements
Financial support for this research was provided by the National Natural Science Foundation of China (No. 40903029) and the International Foundation for Science (C4711-1).