Introduction
Cloning of mammalian embryos using oocyte and somatic cells has been examined for over a decade since the first successful production of the cloned animal ‘Dolly’ (Wilmut et al., Reference Wilmut, Schnieke, McWhir, Kind and Campbell1997); it has been discovered that several mammalian oocyte can reprogramme somatic cell nuclei and can support development of the cloned embryos (Kato et al, Reference Kato, Tani, Sotomaru, Kurokawa, Kato, Doguchi, Yasue and Tsunoda1998, bovine; Wakayama et al., Reference Wakayama, Perry, Zuccotti, Johnson and Yanagimachi1998, mouse; Baguisi et al, Reference Baguisi, Behboodi, Melican, Pollock, Destrempes, Cammuso, Williams, Nims, Porter, Midura, Palacios and Ayres1999, goat; Polejaeva et al., Reference Polejaeva, Chen, Vaught, Page, Mullins, Suyapa, Dai, Boone, Walker, Ayares, Colman and Campbell2000, pig; Chesné et al., Reference Chesné, Adenot, Viglietta, Baratte, Boulanger and Renard2002 rabbit; Shin et al., Reference Shin, Kraemer, Pryor, Liu, Rugila, Howe, Buck, Murphy, Lyons and Westhusin2002, cat; Zhou et al, Reference Zhou, Renard, Le, Brochard, Beaujean, Cherifi, Fraichard and Cozzi2003, rat; Galli et al, Reference Galli, Lagutina, Crotti, Colleoni, Turini, Ponderato, Duchi and Lazzari2003, horse; Woods et al., Reference Woods, White, Vanderwall, Li, Aston, Bunch, Meerdo and Pate2003, mule; Lee et al., Reference Lee, Kim, Jang, Oh, Yuda, Kim, Shamim, Kim, Kang, Schatten and Hwang2005, dog; Li et al., Reference Li, Sun, Chen, Liu, Wisely, Zhou, Renard, Leno and Engelhardt2006, ferret; Shi et al., Reference Shi, Lu, Wei, Cui, Yang, Wei and Liu2007, buffalo; Wani et al., Reference Wani, Wernery, Hassan, Wernery and Skidmore2010, camel). The somatic cell nuclear transfer (SCNT) technique has been proposed to help preserve endangered animals. Usually, the SCNT technique requires recipient oocyte cytoplasm to transplant the nucleus for nuclear reprogramming and for the production of cloned offspring. However, collection of oocytes from endangered species is almost impossible because of the small population numbers of these species. Recently, several groups have reported the technique of interspecies nuclear transfer (ISCNT) in which donor nuclei were transplanted into oocyte cytoplasm that had been obtained from different animal species (Lanza et al., Reference Lanza, Cibelli, Diaz, Moraes, Farin, Hammer, West and Damiani2000; Loi et al., Reference Loi, Ptak, Fulka, Cappai and Clinton2001; Gómez et al., Reference Gómez, Pope, Giraldo, Lyons, Harris, King, Cole, Robert, Godke and Dresser2004, Reference Gómez, Pope, Kutner, Ricks, Lyons, Ruhe, Dumas, Lyons, López, Dresser and Reiser2008, Reference Gómez, Pope, Ricks, Lyons, Dumas and Dresser2009; Kim et al., Reference Kim, Jang, Oh, Yuda, Kim, Hwang, Hossein, Kim, Shin, Kang and Lee2007; Oh et al., Reference Oh, Kim, Jang, Kim, Hong, Park, Park, Park, Sohn, Kim, Shin and Lee2008; Folch et al., Reference Folch, Cocero, Chesné, Alabart, Domínguez, Cognié, Roche, Fernández-Árias, Martí, Sánchez, Echegoyen, Beckers, Bonastre and Vignon2009; Yang et al., Reference Yang, Li, Pang, Yang, Qin, Chen, Zhang, Huang, Zheng, Huang and Liang2010). To date, the successes of cloned offspring by the ISCNT have been reported in animals such as gaur (gaur–bovine, intra-genus, Lanza et al., Reference Lanza, Cibelli, Diaz, Moraes, Farin, Hammer, West and Damiani2000), mouflon (mouflon–sheep, inter-subspecies, Loi et al., Reference Loi, Ptak, Fulka, Cappai and Clinton2001), African wild cat (African wild cat–domestic cat, inter-subspecies, Gómez et al., Reference Gómez, Pope, Giraldo, Lyons, Harris, King, Cole, Robert, Godke and Dresser2004), grey wolf (grey wolf–dog, inter-subspecies, Kim et al., Reference Kim, Jang, Oh, Yuda, Kim, Hwang, Hossein, Kim, Shin, Kang and Lee2007; Oh et al., Reference Oh, Kim, Jang, Kim, Hong, Park, Park, Park, Sohn, Kim, Shin and Lee2008), sand cat (sand cat–domestic cat, inter-subspecies, Gómez et al., Reference Gómez, Pope, Kutner, Ricks, Lyons, Ruhe, Dumas, Lyons, López, Dresser and Reiser2008; 2009), bucardo (buccard–ibex, inter-subspecies, Folch et al., Reference Folch, Cocero, Chesné, Alabart, Domínguez, Cognié, Roche, Fernández-Árias, Martí, Sánchez, Echegoyen, Beckers, Bonastre and Vignon2009) and river buffalo (river buffalo–swamp buffalo, inter-subspecies, Yang et al., Reference Yang, Li, Pang, Yang, Qin, Chen, Zhang, Huang, Zheng, Huang and Liang2010). These studies revealed that the ISCNT is an effective approach for the reproduction of endangered species.
Several non-human primates are also classified as highly endangered species. Additionally, all non-human primates are registered under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) List. The SCNT could be applied to reproduction of endangered primates. Cynomolgus monkey is an experimental animal that is used widely in many fields such as medicine, pharmacokinetics and developmental biology. Experience in use of the ISCNT in cynomolgus monkey is expected to help in the cloning of endangered primates.
The characteristics of the culture medium used in the in vitro culture of mammalian embryos has certain effects on the success of embryo development. In mice, Gardner & Lane (1996) reported that the use of medium that contained non-essential amino acid, glutamine and EDTA alleviated the 2-cell block and accelerated subsequent embryo development. By contrast, removal of glucose from the culture medium resulted in a significant decrease in implantations. In bovine, Iwata et al. (Reference Iwata, Akamatsu, Minami and Yamada1998) reported that a low concentration (1.5 mM) of glucose in culture medium is beneficial; however, high concentration (4.5 mM) of glucose in culture medium reduced blastocyst development. Thus, selection of suitable culture medium for each species must be considered to achieve success of embryo development. To date, it is not known which embryo culture medium is suitable for the nuclear donor or the oocyte recipient for ISCNT embryos.
In the present study, we performed the ISCNT between monkey somatic nuclei and rabbit enucleated metaphase II (MII) oocytes (monkey–rabbit embryos). In the first experiment, to determine the most suitable culture medium for monkey–rabbit ISCNT embryos, we cultured the ISCNT embryos in two different types of culture medium, which were used for rabbit embryos (Zheng et al., Reference Zheng, Jiang, Zhang, Sun and Chen2004) and were optimized and used for cynomolgus monkey embryos (Schramm & Bavister, Reference Schramm and Bavister1996; Narita et al., Reference Narita, Tsuchiya, Takada and Torii2007). In the second experiment, we investigated the developmental characters such as the blastocyst cell numbers and the fate of mitochondria of the monkey–rabbit ISCNT embryos.
Materials and methods
Animals
All rabbits (New Zealand White, NZW) were assigned from Kitayama Labes (Nagano, Japan) and maintained in light-controlled and air-conditioned rooms. All cynomolgus monkeys were bred at Eve BioScience (Wakayama, Japan). All animal procedures conformed to the Guidelines of Kinki University for the Care and Use of Laboratory Animals.
Embryos culture medium
Two types of medium were used for embryo culture. TCM199 medium (Nissui Pharmaceutical) that contained 0.3% (w/v) BSA (M199), which was usually used for rabbit embryos, and CMRL-1066 medium (Invitrogen) that contained pyruvic acid (Sigma), lactic acid (Sigma), l-glutamine (Sigma) and 20% fetal bovine serum (FBS, HyClone) (mCMRL-1066), which was used for monkey embryos, were used for the culture of monkey–rabbit embryos, rabbit–rabbit embryos or rabbit embryos.
Establish and culture of donor cells
Ear skin tissue from NZW rabbit and cynomolgus monkeys was shaved and was then washed in 7.5% (w/v) povidone-iodine for 5 min and 70% (v/v) ethanol for 2 min. Cleaned tissues were sliced into small pieces and cultured on cell culture dishes in Dulbecco's modified Eagle medium (DMEM, Gibco) that contained 10% (v/v) FBS. For the 1–2-week culture, fibroblasts were grown out around tissue pieces. Established fibroblasts were detached for 1 min by Dulbecco's phosphate-buffered saline (D-PBS) that contained 0.25% (w/v) trypsin and 1 mM EDTA. Subsequently, cell suspensions were washed in DMEM that contained 10% FBS. The cells were centrifuged at 350 g for 10 min to obtain a cell pellet. The pellet was resuspended in DMEM that contained 10% FBS and placed in culture at 37°C in an atmosphere of 5% CO2 in air. Monolayers were grown to 80–90% confluency, primary culture cells were used for future experiments. Cells at passages 3–6 were used as donors. The cell cycle control was contact inhibition for 1–2 days prior to nuclear transfer. At the time of the experiments, the donor cells were digested with 0.25% (w/v) trypsin and 1 mM EDTA to produce single cells. The single donor cells were suspended in polyvinyl pyrrolidone (PVP, Sigma) and droplets were placed on the micromanipulation chamber.
Sperm collection
Rabbit semen was collected by ejaculation from adult male NZW rabbits. The semen was diluted with 2 ml of HTF medium that contained 0.3% (w/v) bovine serum albumin (BSA; Sigma), and centrifuged for 5 min at 200 g at room temperature, after which time the supernatant was removed. For swim-up, 1 ml of HTF medium was mounted on a centrifuged pellet for 30 min. Monkey semen was collected by ejaculation from adult male cynomolgus monkeys. The semen was incubated for 30 min to separate seminal plasma. The separated semen was diluted with 2 ml of BWW medium that contained 0.3% (w/v) BSA. The diluted semen was added to equal amounts of Tris–TES–egg yolk (TTE) buffer and cooled from room temperature to 4°C at a rate of 1°C every 10 min. The cooled semen was added to an equivalent amount of TTE buffer that contained 12% (v/v) glycerol. The semen was sucked up into a 1 ml freezing straw, frozen in the air layer above liquid nitrogen and stored in liquid nitrogen until use. The frozen semen was thawed just before intracytoplasmic sperm injection (ICSI). For thawing, frozen semen was heated to 37°C by immersion in water. Thawed semen was added to 1 ml BWW medium and centrifuged for 5 min at 200 g at room temperature, after which time the supernatant was removed. Sperm were washed by swim-up into 1 ml of BWW medium for 30 min. Washed rabbit and monkey sperm were used for ICSI.
Oocyte collection
For preparing oocytes, mature female NZW rabbits were superovulated by pregnant mare serum gonadotrophin (PMSG) and human chorionic gonadotrophin (hCG). Each rabbit was administered with 80 IU PMSG, and then 75 IU hCG on 3 days after the PMSG administration. At 14–15 h after hCG, cumulus–oocytes complexes (COCs) were collected by flushing the removed oviducts with MII medium. COCs were exposed to 0.1% (w/v) hyaluronidase in MII medium that contained 0.3% (w/v) BSA for 2–4 min. And then MII oocytes were denuded from COCs by gently pipetting. Rabbit oocytes were used for ICSI and for recipient oocytes of SCNT. Mature female cynomolgus monkeys were superovulated by recombinant human FSH (rhFSH) and hCG. Each monkey was administered 1.8 mg gonadotrophin-releasing hormone (GnRH) agonist when menstrual blood was observed. Two weeks after the GnRH agonist injection, the monkeys were administered with 37 IU rhFSH twice a day for 10 days. The monkeys were administered with 300 IU/kg hCG12 h after final rhFSH administration. At 40–42 h after hCG, cynomolgus monkey COCs were collected from ovarian follicles by aspiration using 2.5 ml syringe with TALP–HEPES medium that contained heparin on laparoscopy. COCs were exposed to 0.1% (w/v) hyaluronidase in TALP–HEPES medium for 2–4 min. And then, MII oocytes were denuded from COCs by pipetting. These oocytes were used for ICSI.
Intracytoplasmic sperm injection
ICSI was performed with a micromanipulator (Narishige). Briefly, three 10 μl droplets and one 20 μl droplet of HEPES-buffered medium (rabbit: M2 medium, monkey: TALP–HEPES medium) and three 5 ml droplets of HEPES-buffered medium that contained 10% (w/v) PVP were placed in micromanipulation chamber and covered with mineral oil. Sperm were placed in a 20 μl droplet of HEPES-buffered medium in the micromanipulation chamber. The micromanipulation chamber was mounted on an inverted microscope (Olympus, IX-70) equipped with Hoffman Modulation Contrast System and micromanipulators. An individual sperm was transferred to PVP droplet, immobilized by striking the tail, aspirated into an ICSI pipette (Humagen, MIC-35–30), and injected into the cytoplasm of an MII oocyte away from the polar body.
Somatic cell nuclear transfer
Enucleation and nuclear transfer were done with a micromanipulator. Briefly, six 10 μl droplets of M2 medium, three 10 μl droplets of M2 medium that contained 7.5 μg/ml cytochalasin B (Sigma) and 5 μg/ml Hoechst 33342 (Sigma), and nine 5 μl droplets of M2 medium that contained 10% PVP were placed in the micromanipulation chamber and covered with mineral oil. The micromanipulation chamber was mounted on an inverted microscope equipped with Hoffman optics and micromanipulators. Denuded oocytes were incubated for 10 min in M2 medium that contained 7.5 μg/ml cytochalasin B and 10 μg/ml Hoechst 33342 stain before enucleation. The MII spindle and a small amount of the cytoplasm were aspirated using a 10–15 μm glass pipette. The aspirated MII spindle was to ensure the enucleation success using ultraviolet (UV) light irradiation. Enucleated oocytes were incubated for 1 h in mCMRL-1066 before donor cell injection. Donor cells were selected according to small cell size and smooth plasma membrane. Donor cells were injected into enucleated oocytes using an 8–10 μm glass pipette. Reconstructed oocytes were incubated for 1.5 h in M199 before oocyte activation. Reconstructed oocytes were activated by electrical stimulation using an electro cell fusion machine (Shimadzu, SSH-10) and a 2 mm fusion chamber (Shimadzu, FRC-22S). Electrical stimulation condition was three sets of two DC pulses of 2.5 kv/cm for 30 μs spaced in 0.3 mM d(–)-mannitol solution that contained 0.1 mM CaCl2·2H2O and 0.1 mM MgSO4·7H2O. Activated oocytes were incubated for 2 h in M199 and 2 mM 6-dimathyl-aminopurine (6-DMAP, Sigma) and 7.5 μg/ml cytochalasin B.
Embryo culture
After the activation, monkey–rabbit ISCNT embryos and rabbit–rabbit SCNT embryos were incubated in M199 or mCMRL-1066 until 120 h post activation (hpa). Rabbit ICSI embryos were cultured by mCMRL-1066 until 120 h post sperm injection (hpi). Monkey ICSI embryos were cultured in mCMRL-1066 until 168 hpi. All embryos were cultured in an atmosphere of 90% N2, 5% O2, and 5% CO2 with high humidity at 37.5°C.
Cell number counting
The developmental kinetics of rabbit ICSI embryos, rabbit–rabbit SCNT embryos and monkey–rabbit ISCNT embryos were observed every 24 h until 120 hpi or hpa. Monkey ICSI embryos were observed every 24 h until 168 hpi. Embryos reaching the blastocyst stage were stained by 10 μg/ml Hoechst 33342 stain, and were placed on glass slides. The stained blastocysts were photographed under UV light irradiation using a microscope. The pictures were used for counting the cell numbers.
Mitochondrial DNA analysis
A region of the mitochondrial DNA cytochrome b (cytb) gene was analyzed by PCR with two sets of primers. The sequences of cynomolgus monkey cytb primers were: forward 5′-CTCCTCAATTGCACATATCA-3′, and reverse 5′-AAGAAGGAGTATAATGCCGA-3′. The rabbit cytb primer sequences were: forward 5′-GCCATACACTACACCACAGA-3′, and reverse 5′-GAGGTATCGGATAAGTCAGC-3′. Amplification was performed at 94°C for 5 min, 94°C for 30 s, 55°C for 30 s and 72°C for 15 s for 25 cycles. The final PCR products were separated by agarose electrophoresis.
Statistical analysis
All data were analyzed with Fisher's exact test. The value of P < 0.05 was considered to be statistically significant.
Results
Influence of culture medium on monkey–rabbit ISCNT embryos and rabbit–rabbit SCNT embryos development
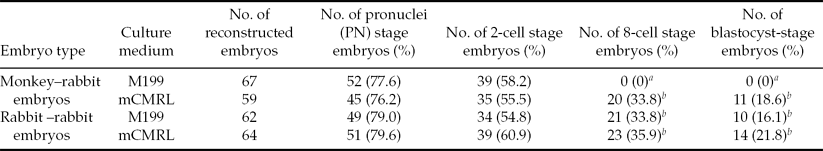
When the monkey–rabbit ISCNT embryos were cultured in mCMRL-1066, the monkey–rabbit ISCNT embryos developed to the blastocyst stage (17%; Table 1 and Fig. 1). In contrast, when the monkey–rabbit ISCNT embryos were cultured in M199, all embryos arrested by the 4-cell stage. However, rabbit–rabbit SCNT embryos developed to the blastocyst stage in both M199 (16%) and mCMRL-1066 media (22%).
Table 1 In vitro development of monkey–rabbit interspecies somatic cell nuclear transfer (ISCNT) embryos and rabbit–rabbit somatic cell nuclear transfer (SCNT) embryos cultured in two different media

a ,b Values within same column with different superscripts differ significantly (P < 0.05).

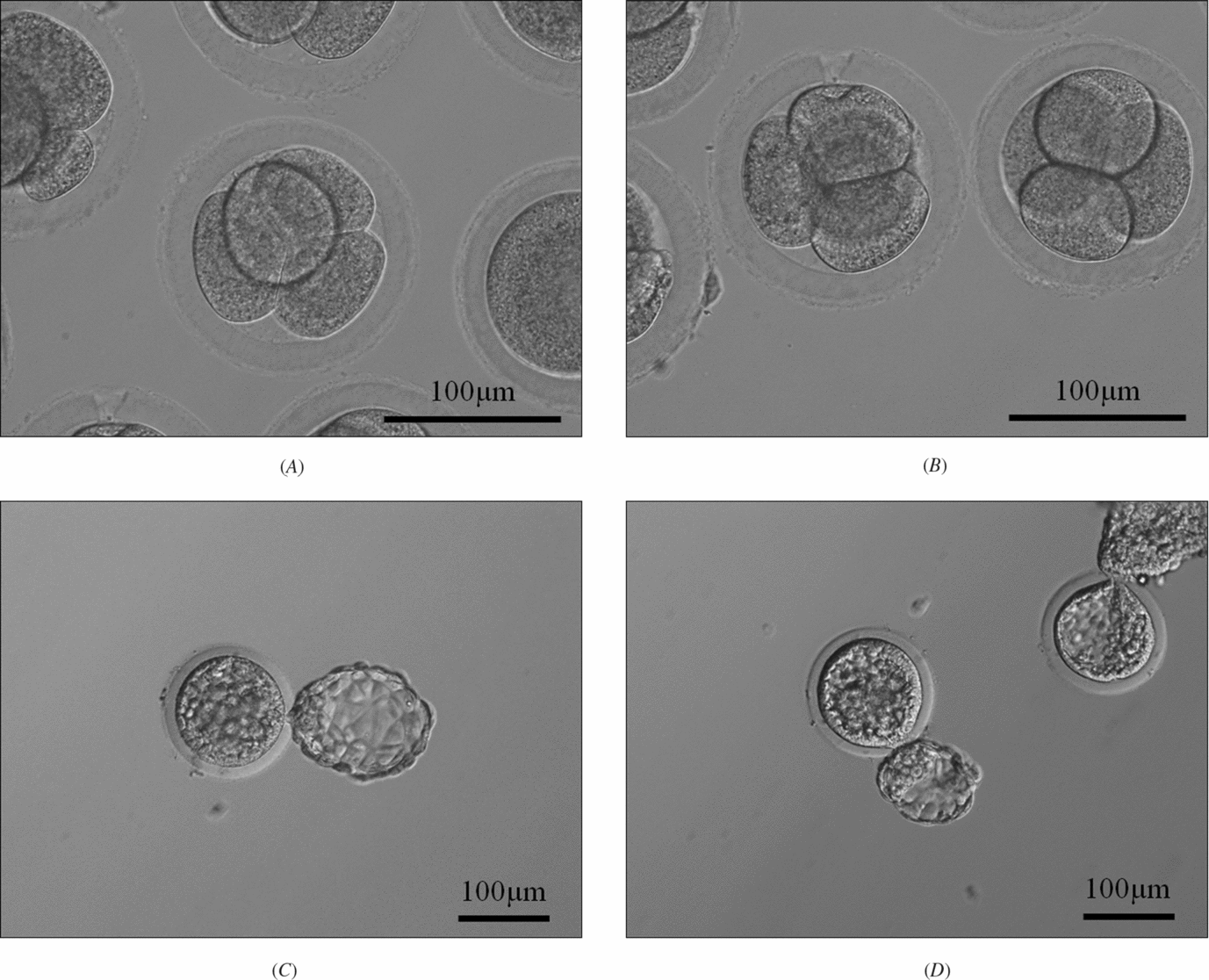
Figure 1 Rabbit–rabbit somatic cell nuclear transfer (SCNT) embryos and the monkey–rabbit interspecies somatic cell nuclear transfer (ISCNT) embryos. (A) Rabbit–rabbit SCNT embryos at the 4-cell stage at 24 h post activation (×200). (B) Monkey–rabbit ISCNT embryos at the 4-cell stage at 24 h post activation (×200). (C) Rabbit–rabbit SCNT embryos at the blastocyst stage at 120 h post activation (×100). (D) Monkey–rabbit ISCNT embryos at the blastocyst stage at 120 h post activation (×100).
The cell number of blastocyst stage
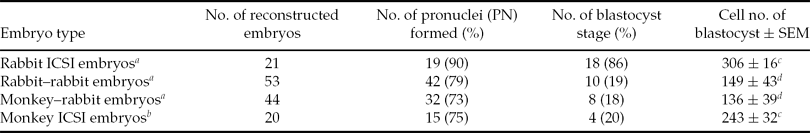
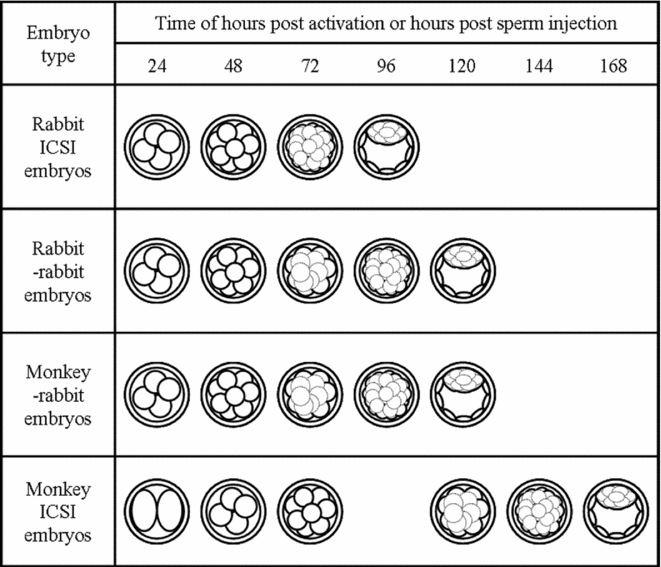
Rabbit ICSI embryos developed to the 4-cell stage at 24 hpi, to the 8-cell stage at 48 hpi, to the late morula stage at 72 hpi, and to the blastocyst stage at 96 hpi. Rabbit–rabbit SCNT embryos and monkey–rabbit ISCNT embryos developed to the 4-cell stage at 24 hpa, to the 8-cell stage at 48 hpa, to early morula stage at 72 hpa, to the late morula stage at 96 hpa, and to the blastocyst stage at 120 hpa. Monkey ICSI embryos developed to the 2-cell stage at 24 hpi, to the 4-cell stage at 48 hpi, to the 8-cell stage at 72 hpi, to the early morula stage at 120 hpi, to the late morula stage at 144 hpi, and to the blastocyst stage at 168 hpi. The developmental stages of monkey–rabbit embryos at each observation time were similar to those of rabbit–rabbit embryos (Fig. 2). The cleavage timing of the monkey–rabbit ISCNT embryos was later than that of the rabbit ICSI embryos by about 24 h, earlier than that of monkey ICSI embryos about 48 h and almost the same as that of rabbit–rabbit SCNT embryos. As for the average number of cells in the blastocyst stage, rabbit ICSI embryos had 306 cells, rabbit–rabbit SCNT embryos had 149 cells, monkey–rabbit ISCNT embryos had 136 cells and monkey ICSI embryos had 243 cells. The number of cells in the monkey–rabbit ISCNT blastocysts was lower than that of rabbit ICSI embryos, and was similar to rabbit–rabbit SCNT embryos (Table 2).
Table 2 The cell number of rabbit intracytoplasmic sperm injection (ICSI) embryos, rabbit–rabbit somatic cell nuclear transfer (SCNT) embryos, monkey–rabbit interspecies somatic cell nuclear transfer (ISCNT) embryos and monkey ICSI embryos at the blastocyst stage after culture in mCMRL-1066 medium

a Cell number of rabbit ICSI blastocysts, rabbit–rabbit blastocysts and monkey–rabbit blastocyst was observed at 120 h post sperm injection or 120 h post sperm injection.
b Cell number of monkey ICSI blastocysts was observed at 168 h post sperm injection.
c ,d Values within same column with different superscripts differ significantly (p < 0.05).
SEM, standard error of the mean.

Figure 2 Cleavage timing of rabbit intracytoplasmic sperm injection (ICSI) embryos, rabbit–rabbit somatic cell nuclear transfer (SCNT) embryos, monkey–rabbit interspecies somatic cell nuclear transfer (ISCNT) embryos and monkey ICSI embryos. All embryos were cultured in mCMRL-1066 medium and observed every 24 h.
Mitochondrial fate of monkey–rabbit ISCNT embryos
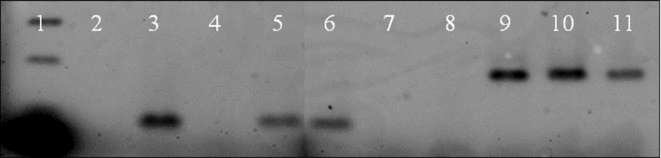
The cynomolgus monkey mitochondrial DNA could be detected in all monkey–rabbit embryos right after donor-cell injection and at the blastocyst stage (Fig. 3). Rabbit mitochondrial DNA could be detected in all monkey–rabbit ISCNT embryos both at the donor cell-injected oocyte and at the blastocyst stages. This result suggested that monkey–rabbit embryos were heteroplasmic.

Figure 3 Mitochondrial analysis by polymerase chain reaction (PCR) using rabbit cytochrome b (cytob) gene-specific primer or cynomolgus monkey cytob gene-specific primer. Lane 1: 100-bp ladder marker. Lanes 2–6: using rabbit cytob primer (PCR product length is 95 bp). Lanes 7–11: using monkey cytob primer (PCR product length is 177 bp). Lanes 2 and 7: negative control (distilled water). Lanes 3 and 8: rabbit DNA sample from fibroblast. Lanes 4 and 9: monkey DNA sample from fibroblast. Lanes 4 and 10: monkey–rabbit ISCNT embryos just after nuclear injection. Lanes 5 and 11: monkey–rabbit ISCNT embryos at blastocyst stage.
Discussion
Interspecies somatic cell nuclear transfer (ISCNT) is a technique to reprogramme the animal somatic cell nucleus using the oocyte cytoplasm of other species. In this study, we succeeded in producing monkey–rabbit ISCNT blastocysts. In previous reports, the development to blastocyst is one of indicators of reprogramming (Thongphakdee et al., Reference Thongphakdee, Numchaisrika, Omsongkram, Chatdarong, Kamolnorranath, Dumnui and Techakumphu2006; Zhao et al., Reference Zhao, Ouyang, Nan, Lei, Song, Sun and Chen2006, Reference Zhao, LI, Cao, Jiang, Ouyang, Nan, Lei, Song, Sun and Chen2007). Our result suggested that rabbit oocytes can reprogramme cynomolgus monkey fibroblast nuclei by these criteria.
The composition of in vitro culture medium is a very important factor in the early development of mammalian embryos. Nevertheless, to date, it had not been known which embryo culture medium was suitable for the nuclear donor or the oocyte recipient for ISCNT embryos. In a previous study, Dominko et al. (Reference Dominko, Mitalipova, Haley, Beyhan, Memili, McKusick and First1999) reported the in vitro development of interspecies SCNT embryos reconstructed with enucleated bovine oocytes and somatic cells from various mammalian. They succeeded in producing ISCNT embryos using sheep, pig and cynomolgus monkey donor cells that developed to the blastocyst stage, when embryos were cultured in CR1AA medium that was suitable for the culture of bovine embryos. For the ISCNT using rabbit oocytes, Chen et al. (Reference Chen, Wen, Zhang, Sun, Han, Liu, Shi, Li, Xiangyu, Lian, Kou, Wu, Chen, Wang and Zhang2002) reported that panda–rabbit embryos could develop to blastocysts when grown in M199 medium. In contrast, Wen et al. (Reference Wen, Yang, Cheng, Li, Liu, Sun, Zhang, Lei, Wu, Kou and Chen2003) reported that cat–rabbit embryos could develop in both M199 and SOF media, however, the cat–rabbit embryos cultured in SOF medium had a significantly higher rate of blastocyst development compared with the embryos cultured in M199 medium. Furthermore, Zhao et al. (Reference Zhao, Ouyang, Nan, Lei, Song, Sun and Chen2006) reported that camel–rabbit embryos could develop in M199 and SOF media, however, the embryos could not develop in mCR1aa medium. In this study, we cultured monkey–rabbit ISCNT embryos in two different types of culture medium, which were used for rabbit embryos (Zheng et al., Reference Zheng, Jiang, Zhang, Sun and Chen2004) and were used for cynomolgus monkey embryos (Schramm & Bavister, Reference Schramm and Bavister1996; Narita et al., Reference Narita, Tsuchiya, Takada and Torii2007). We succeeded in producing monkey–rabbit ISCNT blastocysts using mCMRL-1066 medium, which is suitable for culturing cynomolgus monkey embryos; the monkey–rabbit ISCNT embryos were arrested by the 4-cell stage when cultured in M199. Modified-CMRL-1066 medium contains lactic acid, pyruvic acid, FBS and coenzymes (FAD, NAD, NADP and coenzyme A). In contrast, M199 medium does not contain these components. These differences of medium components might influence the development of monkey–rabbit ISCNT embryos. We suggest that ISCNT embryos might prefer embryo culture medium of nuclear donor species for preimplantation development.
In the ISCNT, the selection of animal species for recipient oocytes is a very important factor affecting the outcome. Several researchers have confirmed that the cytoplasm of bovine and rabbit oocytes can reprogramme some kinds of somatic cells (Dominko et al., Reference Dominko, Mitalipova, Haley, Beyhan, Memili, McKusick and First1999, oocyte – bovine, donor cell – sheep, porcine, cynomolgus monkey and rat; Chen et al., 2002, oocyte – rabbit, donor cell – giant panda; Yang et al., 2003, oocyte – rabbit, donor cell – rhesus monkey; Jiang et al., Reference Jiang, Yang, Zhang, Zheng, Liu, Sun and Chen2004, oocyte – rabbit, donor – giant panda; Jiang et al., Reference Jiang, Chen, Nan, Ouyang, Sun and Chen2005, oocyte – rabbit, donor – ibex; Wen et al., Reference Wen, Bi, Xu, Yang, Zhu, Sun and Chen2005, oocyte – rabbit, donor cell – cat; Thongphakdee et al., 2006, oocyte – rabbit, donor cell – marbled cat; Zhao et al., Reference Zhao, LI, Cao, Jiang, Ouyang, Nan, Lei, Song, Sun and Chen2007, oocyte – rabbit, donor cell – camel and Tibetan antelope; Tao et al., Reference Tao, Liu, Zhang, Zhang, Fang, Han, Zhang, Liu, Ding and Zhang2009, oocyte – rabbit, donor cell – red panda). Especially, rabbit oocytes can reprogramme not only mammalian cells but also somatic cells from avian species (Liu et al., Reference Liu, Zhou, Chen, Zhang, Wen, Kou, Li, Sun and Chen2004, oocyte – rabbit, donor cell – chicken). These studies demonstrated that rabbit oocyte cytoplasm has high reprogramming potency for nuclei of other species. In this study, monkey–rabbit embryos and rabbit–rabbit embryos developed to the blastocyst stage at 120 hpa. In contrast, monkey ICSI embryos developed to the blastocyst stage later at 168 hpa. Cleavage timing of monkey–rabbit ISCNT embryos was almost the same as for the rabbit–rabbit SCNT embryos. The blastocyst cell numbers for monkey–rabbit ISCNT embryos was also almost the same as for the rabbit–rabbit SCNT embryos. In contrast, at 120 hpi, monkey ICSI embryos developed to the morula stage. Monkey ICSI embryos developed to the blastocyst stage at 168 hpi. In previous studies, the cleavage timing of ISCNT embryos differed between reports. Examples of dependency on nuclei (Dominko et al, Reference Dominko, Mitalipova, Haley, Beyhan, Memili, McKusick and First1999, oocyte – bovine, donor cell – sheep, porcine, cynomolgus monkey and rat), on oocyte cytoplasm (Yang et al., Reference Yang, Han, Wen, Sun, Zhang, Zhang, Wu, Kou and Chen2003, oocyte – rabbit, donor cell – rhesus monkey; Liu et al., 2004, oocyte – rabbit, donor cell – chicken), of the differences between types of oocytes or nuclei (Chen et al., Reference Chen, Wen, Zhang, Sun, Han, Liu, Shi, Li, Xiangyu, Lian, Kou, Wu, Chen, Wang and Zhang2002, oocyte – rabbit, donor cell – giant panda; Wen et al., Reference Wen, Bi, Xu, Yang, Zhu, Sun and Chen2005, oocyte – rabbit, donor cell – cat) were reported. Most reports using rabbit oocyte have demonstrated dependency on the oocyte type (Yang et al., Reference Yang, Han, Wen, Sun, Zhang, Zhang, Wu, Kou and Chen2003, oocyte – rabbit, donor cell – rhesus monkey; Liu et al., 2004, oocyte – rabbit, donor cell – chicken) or shift type (Chen et al., Reference Chen, Wen, Zhang, Sun, Han, Liu, Shi, Li, Xiangyu, Lian, Kou, Wu, Chen, Wang and Zhang2002, oocyte – rabbit, donor cell – giant panda; Wen et al., Reference Wen, Bi, Xu, Yang, Zhu, Sun and Chen2005, oocyte – rabbit, donor cell – cat). In the case of ISCNT embryos, the timing of the cleavage might be influenced by the combination of choice of oocyte species and donor cell species.
For PCR analysis, both monkey and rabbit mitochondrial DNAs were detected from monkey–rabbit ISCNT embryos. These results showed that monkey–rabbit ISCNT embryos were heteroplasmic from between just after injection to blastocyst stage, findings that corresponded with a previous report (Yang et al., Reference Yang, Han, Wen, Sun, Zhang, Zhang, Wu, Kou and Chen2003, oocyte – rabbit, donor cell – rhesus monkey). In the case of fertilized mammalian embryos, paternal sperm mitochondria are usually eliminated after ubiquitination at the early developmental stage (Sutovsky et al., Reference Sutovsky, Moreno, Santos, Dominko, Simerly and Schatten2000). Accordingly, fertilized embryos have only maternal mitochondria. For SCNT in sheep, donor cell mitochondria were not detected from nuclear transfer-derived sheep (Evans et al., Reference Evans, Gurer, Loike, Wilmut, Schnieke and Schon1999). We speculate that mammalian oocytes might have an elimination pathway for extraneous mitochondria. For other ISCNT reports and in our study, donor cell mitochondria stayed in the embryos until the blastocyst stage (Yang et al., Reference Yang, Han, Wen, Sun, Zhang, Zhang, Wu, Kou and Chen2003 oocyte – rabbit, donor cell – rhesus monkey; Liu et al., 2004, oocyte – rabbit, donor cell – chicken; Yang et al., Reference Yang, Kou, Wang, Jiang, Mao, Sun, Sheng and Chen2004, oocyte – rabbit, donor cell – rhesus monkey; Hua et al., Reference Hua, Zhang, Song, Song, Zhang, Zhang, Zhang, Cao and Ma2008, oocyte – bovine, donor cell – sheep; Ma et al., Reference Ma, Yang, Hua, Cao, Li and Zhang2008, oocyte – sheep, donor cell – goat). Therefore, for ISCNT embryos, the elimination pathway for extraneous mitochondria might be defective.
In conclusion, our study demonstrated that: (1) rabbit oocytes can reprogram the cynomolgus monkey somatic cell nucleus, and can support preimplantation development; (2) monkey–rabbit ISCNT embryos develop well in monkey culture medium at early embryonic developmental stages; (3) the cell number of monkey–rabbit ISCNT embryos is similar to that of rabbit–rabbit SCNT embryos; and (4) mitochondria from monkey–rabbit ISCNT embryos are heteroplasmic from between just after injection as far as the blastocyst stage, that has roots in both rabbit oocytes and monkey somatic cells. Our study also suggested that selection of a suitable culture medium for the nuclear donor species is a very important factor for the success of in vitro development of ISCNT embryos.
Acknowledgements
We thank Dr G. Schatten, Dr T. Teramura, Ms N. Backes Kamimura and Ms J. Walhelm Kimura for manuscript support. This work was supported by Grant-in-Aid for the Century COE Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grant-in-Aid for Scientific Research (C).