Introduction
Mosses, including species in the Bryum, Amblystegium, and Brachythecium genera, are common weeds in golf courses, particularly on putting greens (Burnell et al. Reference Burnell, Yelverton, Neal, Gannon and McElroy2004; Cook et al. Reference Cook, McDonald and Merrifield2002; Happ Reference Happ1998; Kennelly et al. Reference Kennelly, Todd, Settle and Fry2010). Silvergreen bryum moss [Bryum argenteum Hedw.] is the most pervasive moss species on greens in the United States (Kennelly et al. Reference Kennelly, Todd, Settle and Fry2010). Beginning in the 1980s, B. argenteum was recognized in the United States as an undesirable colonizer of golf course tees and greens (Taylor and Danneberger Reference Taylor and Danneberger1996), where it adversely affected golf ball roll dynamics and putting green aesthetics (Figure 1A). Research has focused on controlling these moss populations (Cook et al. Reference Cook, McDonald and Merrifield2002; Kennelly et al. Reference Kennelly, Todd, Settle and Fry2010; Post et al. Reference Post, McCall and Askew2016). Several chemical and cultural control strategies have attempted to eradicate this species in putting greens, but with limited success (Raudenbush Reference Raudenbush2015). Putting greens present an altered microhabitat that is ideal for B. argenteum: this species is a specialist requiring disturbance to survive, and this disturbance is provided through daily mowing, frequent aeration, foot traffic (golfers), and frequent irrigation to a sand-based root zone. Additionally, B. argenteum is desiccation tolerant (Proctor et al. Reference Proctor, Oliver, Wood, Alpert, Stark, Cleavitt and Mishler2007), which allows it to survive prolonged periods without water—an important consideration given the increased restrictions on water usage on golf courses. Bryum argenteum is capable of acclimating to high or low light conditions within the same colony (Green et al. Reference Green, Schroeter and Seppelt2000). It is also capable of producing prodigious amounts of specialized asexual propagules that float (bulbils; Stark et al. Reference Stark, McLetchie and Eppley2010), and fragmentation of its shoots only serves to propagate mosses, because the entire plant body is totipotent (capable of regenerating new plants; Glime Reference Glime2017). Thus it is no surprise that this moss has proven to be a formidable weed species for golf course superintendents.
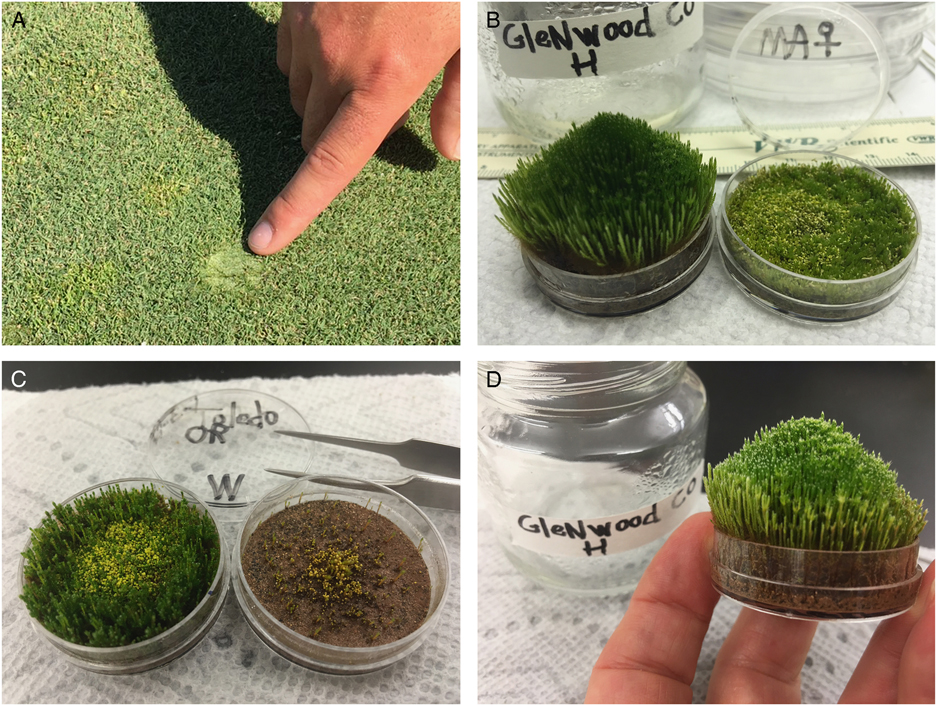
Figure 1 (A) Example of a patch of Bryum argenteum on a putting green. (B) Comparison of a green genotype vs. a native genotype of B. argenteum after 150 d in nutrient-rich culture. (C) Comparison of nutrient-enriched vs. nutrient-deprived cultures of the same genotype. (D) Close-up of the Glenwood, CO, green genotype after 150 d in culture.
An infestation of B. argenteum typically begins as a small, circular colony (<5 cm in diameter). If this colony is not mechanically removed with a knife or cup cutter, it will persist in the putting green (Raudenbush Reference Raudenbush2015) either (1) as new colonies establishing close to the original colony, forming pure patches; or (2) interwoven with the grass canopy, that is, not exhibiting discrete patch-forming behavior (Z Raudenbush, personal observation). Given that growth rates for mosses are more than an order of magnitude less than growth rates for grasses (Martin and Adamson Reference Martin and Adamson2001), it is surprising that B. argenteum can compete for space and spread into uniform patches among the grasses. Most golf course superintendents use chemical control strategies to keep B. argenteum populations in check. A product containing the active ingredient carfentrazone-ethyl, which inhibits chlorophyll synthesis and ultimately results in lipid peroxidation (Senseman Reference Senseman2007), is usually applied. Unfortunately, this contact herbicide merely injures the shoot tissue, and new shoot growth is typically observed 2 to 3 wk after application (Raudenbush Reference Raudenbush2015; Raudenbush et al. Reference Raudenbush, Keeley and Stark2015). However, carfentrazone-ethyl has been shown to reduce the development of protonemata from spores and bulbils of B. argenteum by >80% (Post et al. Reference Post, McCall and Askew2016), indicating a degree of effectiveness in preventing colonization of putting greens by mosses. Additionally, many superintendents have observed reductions in populations after foliar applications of zinc-containing products, ferrous sulfate, and copper sulfate, with the literature revealing mixed results. Overall, most researchers reported approximately 30% to 50% B. argenteum control using three to four repeated applications of the aforementioned products (Raudenbush Reference Raudenbush2015).
Protocols for preventing colonization and controlling species populations benefit from understanding the life history of the species, that is, its growth, regeneration, and reproductive strategies and/or characteristics central to its survival and continuation, which may allow the targeting of such key life-history events. Eradication protocols can be informed by identifying differences between the life histories of successful invaders and noninvaders of putting greens. Several studies have documented life-history differences that are due to genetic differences within bryophyte species (ecotypes). Evidence of ecotypic variation in B. argenteum’s life-history traits indicates that the most vigorous genotypes (genetic strains) tend to originate from the harshest environments (Antarctica, metal mine tailings, and deserts; Horsley et al. Reference Horsley, Stark and McLetchie2011; Longton Reference Longton1981; Longton and MacIver Reference Longton and MacIver1977; Shaw and Albright Reference Shaw and Albright1990; Shaw et al. Reference Shaw, Beer and Lutz1989). Life history and physiological ecotypic divergence in other bryophyte species were shown to occur in ceratodon moss [Ceratodon purpureus (Hedw.) Brid.] (Shaw and Beer Reference Shaw and Beer1999) and Marchantia inflexa Nees & Mont. (Brzyski et al. Reference Brzyski, Taylor and McLetchie2014; Marks et al. Reference Marks, Burton and McLetchie2016). The success of invasive plant species often derives from a faster growth rate giving the invasive a competitive advantage over native species in the same habitat, possibly allowing the invasive to exploit vacant niches in an established community (Reichmann et al. Reference Reichmann, Schwinning, Polley and Fay2016). Although alien and native species may exhibit wide variation in life-history traits, few studies have assessed genotypic variation in life-history traits between invasive strains and noninvasive strains of the same species (Brzyski et al. Reference Brzyski, Taylor and McLetchie2014). One goal of this study was to test for the presence of life-history differences between putting green and native individuals of B. argenteum using a common garden approach.
Genetic differences between two population types can result from strong selection of offspring produced within each population type (local adaptation) or selection of specific genotypes that colonize the novel sites. In the latter case, there will be a larger variation in life-history traits in the native populations than in more recently colonized sites (Brzyski et al. Reference Brzyski, Taylor and McLetchie2014). Locally adapted genotypes are characterized by higher fitness in the localized habitat relative to nonlocal habitats, a trait well documented in plants and more likely under conditions of low gene flow (Sánchez et al. Reference Sánchez, Alonso-Valiente, Albert and Escudero2017). In mosses, only a few studies have documented local adaptation (e.g., Mikulášková et al. Reference Mikulášková, Hájek, Veleba, Johnson, Hájek and Shaw2015; Shaw et al. Reference Shaw, Beer and Lutz1989), and gene flow among populations is expected to be large due to high dispersal rates (Barbé et al. Reference Barbé, Fenton and Bergeron2016; Hájek et al. Reference Hájek, Roleček, Cottenie, Kintrová, Horsák, Poulíčková, Hájková, Fránková and Dítě2011; Sundberg Reference Sundberg2013). These characteristics suggest that golf course populations of mosses may not be locally adapted unless gene flow is restricted, perhaps by golf course management practices (i.e., moss sexual structures cut by mowing).
We hypothesized that there may be life-history and developmental differences in the timeline of colony establishment that enable plants of B. argenteum to survive and flourish in golf course putting greens compared with native habitats, and these differences may be related to inorganic nutrient status contributing to the ability of B. argenteum colonies to persist in a putting green habitat.
Materials and Methods
Species Description
Bryum argenteum is a polymorphic and cosmopolitan species that occurs on all continents in both natural and disturbed sites (Longton Reference Longton1981; Spence Reference Spence2014). As a bryophyte, this species germinates from a haploid spore, develops initially into a filamentous protonemal stage, and then into the dominant leafy gametophyte stage. Gametophytes can reproduce sexually to produce spores or asexually via fragmentation, gemmae, and/or bulbils. Bryum argenteum is common in urban areas along streets and in sidewalks, parks, and golf course putting greens (Raudenbush et al. Reference Raudenbush, Keeley and Stark2015). Plants take on a silvery appearance in the field, because the hyaline upper leaves have regions devoid of chlorophyll. Protonemata produce deciduous gemmae (protonemal gemmae), and shoots can produce deciduous shoot tips (bulbils). Both of the specialized asexual propagules can float, with gemmae produced by juvenile colonies and bulbils produced by mature colonies (Horsley et al. Reference Horsley, Stark and McLetchie2011). The species has separate male and female plants (haploid dioecy), with sex expression day-neutral, pH independent, and requiring a light intensity of at least 50 µmol m−2 s−1 (Chopra and Bhatla Reference Chopra and Bhatla1981). Female-biased sex ratios are common in B. argenteum, with male colonies tending to occur in altered urban areas and high-elevation native habitats, and females more abundant in arid land regions of North America (Stark et al. Reference Stark, McLetchie and Eppley2010).
Obtaining Collections
Putting green specimens were collected from golf courses in the United States and Canada by providing superintendents with sampling kits. Each kit contained a prepaid padded envelope, three small coin envelopes, a sampling survey form, and instructions for harvesting three separate specimens from putting greens. Superintendents were instructed to (1) harvest a 3- to 4-cm-diameter colony of pure moss using a knife or small soil probe; (2) immediately enclose the specimen in one of the provided coin envelopes; (3) complete the sampling survey form (contact information and site description); and (4) package the specimen(s) and survey form in the prepaid envelope and mail. Forty-five sampling kits were distributed to superintendents during regional and national presentations on moss control, and ~21 responses were received. Responding golf course superintendents mailed dry moss cores, in each case B. argenteum (identities confirmed by John Brinda of the Missouri Botanical Garden), from the following states: California (6), Ohio (3), South Dakota (2), Colorado (1), Illinois (1), Minnesota (1), Nevada (1), Oregon (1), and Alberta, Canada (1). For specimens not from putting greens, the coauthors made opportunistic collections from a variety of locations, including the states of Nevada (4), Oregon (3), California (2), Arizona (2), Georgia (1), Kentucky (1), Massachusetts (1), New Mexico (1), Pennsylvania (1), and Washington (1). The 34 accessions used in the present experiment are given in Table 1.
Table 1 Location of Bryum argenteum specimens cloned to pure, single-genotype cultures in the experiment.

Decontamination and Culturing
Dried shoot apices were clipped and placed into culture on hydrated sand media. Locally collected pH-neutral sandstone-derived sand was sieved (355 µm), dry-sterilized, placed into plastic Petri dishes (inner diameter=35 mm), and watered weekly with sterile distilled water. If contaminating algae, bacteria, or fungi were present as the culture developed, a single shoot apex was removed and subcultured on fresh medium. Subculturing was repeated until contaminants were absent from the culture. When pure (nonaxenic) cultures were mature (~3 mo, producing shoots throughout the Petri dish), a final subculture was made, and these shoots were grown to maturity and used in the present experiment. Some genotypes were subcultured over multiple generations. Each experimental culture was initiated by removing a single shoot, cutting it to a length of 2 mm with a straight edge, and planting it upright in the center of a 35-mm plastic Petri dish. Plants were grown in a plant growth chamber (Percival E30B, Boone, IA) fitted with both fluorescent and incandescent lights that created a photosynthetic photon flux density (PPFD) of ~220 µmol m−2 s−1 photosynthetically active radiation (range 170 to 270 µmol m−2 s−1), with photoperiod set at 12 h (20 C lighted, 8 C darkened).
Cultures were inspected daily until regeneration occurred by protonema extending from the original shoot axis. For other observations, cultures were examined daily through day 7, twice per week through day 21, then once a week thereafter through the conclusion of the experiment (day 150). When shoots began to brush up against the ceiling of the petri dish, the lid was removed and the petri dish was placed inside a glass jar (7 by 6 cm, height by diameter) with a translucent lid. Water or nutrient solution was applied as needed on a weekly basis to the edge of the sand media.
Experimental Design
To assess the effects of nutrient limitation, two nutrient treatments were applied to all 34 genotypes (17 from putting greens; 17 from native habitats, i.e., not from a putting green): (1) nutrient enriched, watered with a 30% strength of Hoagland’s solution (Hoagland and Arnon Reference Hoagland and Arnon1938), which supplies all of the required inorganic nutrients for plants and no carbon compounds; and (2) nutrient deprived, watered with sterilized (autoclaved) distilled water. Watering and nutrient application were by dropper bottles refrigerated to ~3 C when not in use. All 34 genotype cultures were assigned to each treatment.
Developmental Phenological Observations
To assess the effects of habitat (native vs. green) and nutrient limitation, the time (days) when the following developmental event occurred was recorded: shoot regeneration (from protonema), original shoot axis branching, first shoot induction, first bulbil production, first protonemal gemma formation, and first inflorescence production. A bulbil was recognized only after it had fully detached from the parent shoot or, in the case of protonemally produced bulbils, detached from the protonemal filaments (i.e., it was lying on the medium).
Life-History Trait Assessments
To assess the effects of habitat (native vs. green) and nutrient treatment (nutrient enriched vs. nutrient deprived), growth was assessed as (1) the maximum linear distance protonemal filaments extended from the original planted shoot apex (mm on day 21), (2) the number of protonemal shoots (shoots produced directly from protonemal mats) on day 21, (3) height (mm) of the tallest shoot after 5 mo in culture (measured from medium surface to shoot apex), and (4) aerial rhizoid cover on day 50. Asexual reproductive output was assessed as (5) cover of protonemal gemmae on day 50, and (6) the number of bulbils produced by day 50. Sexual reproductive effort was assessed by counting the number of inflorescences produced after 5 mo. All observations were made through a dissecting microscope at up to 60×, estimating cover by eye to the nearest 10% relative to the total cover of the culture (e.g., cover of protonemal gemmae was the percentage of the total protonemal area occupied by the gemmae; cover of aerial rhizoids was estimated in a similar manner) and estimating lengths of protonema and shoots using a millimeter ruler held over or nestled within the culture to the nearest 0.1 mm.
Chlorophyll Fluorescence and Chlorophyll Content
To assess the effects of habitat (native vs. green) and nutrient treatment (nutrient enriched vs. nutrient deprived), chlorophyll fluorescence and content were determined among treatments. At the conclusion of the experiment (150 d), ~12 vegetative shoots (without inflorescences at or near the shoot apex) were removed from the culture at a point approximately halfway from the dish center to the dish edge, placed in sterile water droplets, trimmed to ~2 mm in length (some nutrient-deprived shoots did not reach 2 mm and were used in their entirety), and placed into a fluorescence clip on a chemical wipe dipped in water contained in a larger glass Petri dish; the dish was lidded, allowing the shoots to remain hydrated at high relative humidity. It was not possible to exclude all bulbils from the shoots in cultures that produced high numbers (hundreds) of bulbils. Inflorescences along the lower regions of shoots were manually removed with forceps. Clips were dark adapted for 30 min, after which a modulated chlorophyll fluorometer (FMS2, Hansatech, King’s Lynn, UK) incorporating the saturation pulse method (Bilger et al. Reference Bilger, Schreiber and Bock1995) was used to determine the maximum photochemical efficiency of dark-adapted photosystem II (PSII; F v/F m) in clips sealed from incident light. Subsequently, the effective quantum yield of PSII photochemistry (φPSII) was determined from the following light program on the same shoots: emission of an actinic light (300 µmol m−2 s−1 PPFD) with 30-s equilibration periods before delivery of saturating light pulses (initial 8,000 µmol m−2 s−1 PPFD) at increasing light intensities (Genty et al. Reference Genty, Briantais and Baker1989). F v/F m is a measure of the general condition of the photocenters, and φPSII represents the fraction of excitation energy flowing through PSII and indicates active photosynthesis (Green and Proctor Reference Green and Proctor2016). Additionally, the value F m (maximal fluorescence) was used to provide a rough comparative measure of the amount of photocenter activity, and thus of the total potential photosynthetic activity and an indication of cell damage incurred by the shoots (Proctor Reference Proctor2003). The program used also produced nonphotosynthetic quenching estimates (NPQ, qNP, qP). Chlorophyll content was assessed on a different set of 12 shoots from each culture selected in the manner described earlier, with the shoots aligned by their apices and juxtaposed in a single row on a hydrated chemical wipe. A series of five measurements was implemented using a chlorophyll-content meter (Opti-Sciences CCM-300, Hudson, New Hampshire), yielding a mean chlorophyll concentration (mg m−2).
Statistics
For phenological/developmental traits, ANOVA was used to test whether habitat (native vs. green) and nutrient treatments (nutrient enriched vs. nutrient deprived) affected the response variables of shoot regeneration from protonema, branching of the original shoot axis, shoot induction, bulbil induction, and sex organ induction time. Across all ANOVAs for phenological/developmental traits, the interaction between habitat and nutrient treatment was not significant, and the interaction terms were dropped from the models. For allocation traits, ANOVA was used to test whether habitat and nutrient treatment affected the response variables of growth traits (protonema length at 3 wk, height of tallest shoot, number of shoots, and rhizoid cover), asexual reproduction traits (cover of protonemal gemmae and number of bulbils produced by day 50), and a sexual trait (number of inflorescences produced after 5 mo). Interaction terms were dropped when not significant. Response variables of size were log transformed, and percent data were arcsine transformed to improve normality. For allocation traits, ANOVA was used to test whether habitat and nutrient treatment affected the response variables of F v /F m, F m, ɸPSII, qP, qNP, and NPQ. Interaction terms were dropped when not significant. All statistical analyses were conducted in SAS v. 9.4 (SAS Institute, Cary, NC).
Results and Discussion
Developmental Phenology
For developmental phenological traits, the interaction between habitat and nutrient treatment was not significant, and the interaction terms were dropped from the models. Shoot regeneration from protonema originating from the original shoot axis occurred about 1 d earlier (day 5 vs. day 6) in green genotypes than in native genotypes (F(1/65)=8.27, P=0.005; Figure 2A). The time to shoot regeneration was not affected by nutrient treatment (F(1/65)=0.18, P=0.669). Branching of the original shoot axis occurred from 8 to 11 d after planting and was not related to habitat (F(1/65)=0.22, P=0.644) or nutrient treatment (F(1/65)=2.98, P=0.0892). Shoot induction occurred earlier in green genotypes (day 17 to 18 vs. day 21; F(1/65)=17.76, P=0.0001; Figure 2B) but did not differ between the nutrient treatments (F(1/65)=1.05, P=0.3083). Bulbil induction did not differ between habitats or between nutrient treatments (F(1/ 49) =1.64, P=0.206, and F(1/49)=0.00, P=0.98, respectively). Gemma induction occurred during the 3rd or 4th week of growth and was earlier in native habitats relative to green habitats (35±2 vs. 29 ±1 d, respectively; F(1/47)=9.90, P=0.0029) and did not differ between the nutrient treatments (F(1/47)=0.00, P=0.963). Within the nutrient-enriched treatment, 8 of the green genotypes produced gemmae over the course of the experiment, compared with 16 of the native genotypes. Time to sex induction did not differ by habitat (F(1/41)=3.16, P=0.0831) but was earlier in the low-nutrient treatment relative to the enriched-nutrient treatment (75±6 vs. 98±6 d, respectively; F(1/41)=3.16, P=0.02).
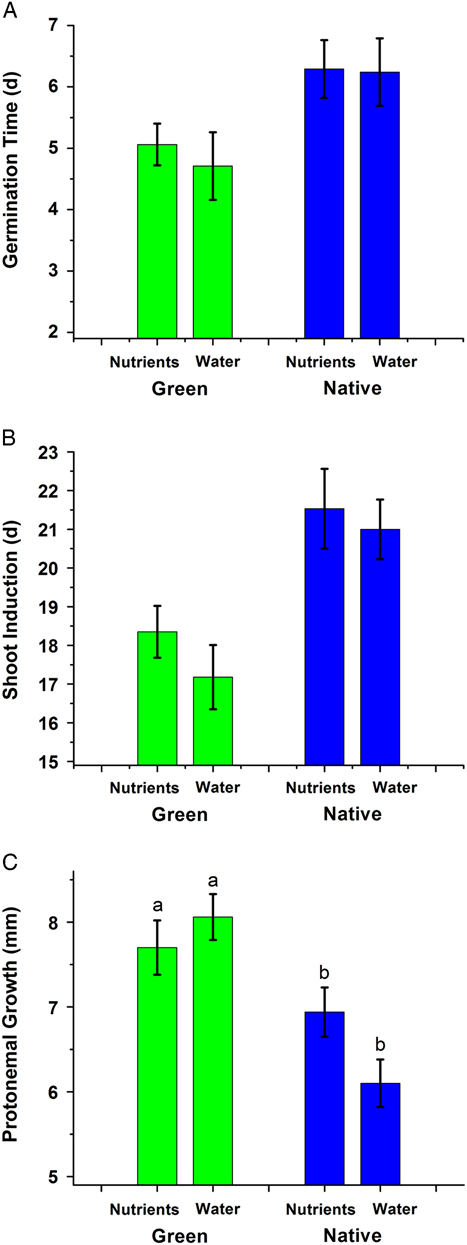
Figure 2 (A) Time to germination of detached shoot apices of Bryum argenteum placed into culture, as judged by the appearance of protonemal filaments. (B) Time to first shoot induction of B. argenteum placed into culture, as judged by the appearance of shoot primordia arising from protonemal filaments. (C) Length of protonemal extension from the original shoot apex on day 21 of B. argenteum placed into culture, as judged by the linear distance of protonemal filaments from the center of the culture dish. Mean±1 SE; in C, difference lowercase letters indicate P<0.05; within native plants the nutrient different was marginally significant (P=0.064). Green, genotypes derived from North American golf course putting greens; Native, genotypes derived from habitats other than putting greens; Nutrients, supplied with a 30% Hoagland’s solution; Water, supplied with only sterile distilled water.
Allocation to Life-History Traits
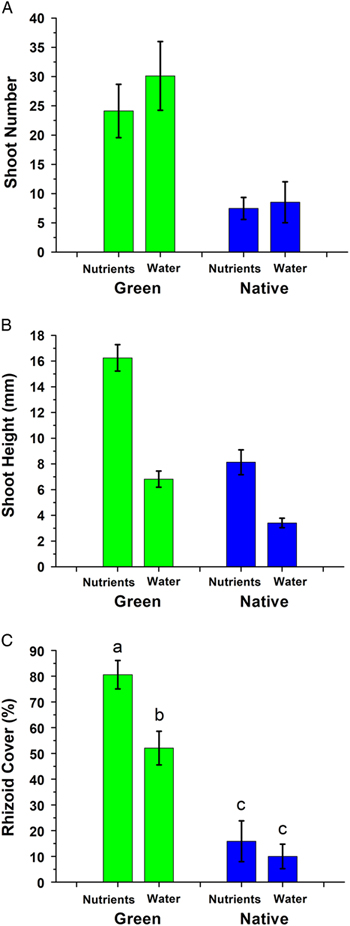
Growth was assessed through the rate of protonemal extension, the number of protonemal shoots, shoot height, and aerial rhizoid cover. Regarding protonemal extension by day 21 of the experiment, a significant interaction (F(1/64)=4.58, P=0.036) was due to the green genotypes in the low-nutrient treatment having slightly longer protonema than those from enriched-nutrient treatments, while the reverse occurred for native genotypes. Protonema extended farther in green genotypes than in native genotypes (F(1/64)=22.24, P<0.0001; Figure 2C). These protonemata from green genotypes produced more than three times the number of shoots over the first 21 d of the experiment (F(1/65)=20.79, P<0.0001; Figure 3A). There was no nutrient treatment effect on shoot production (F(1/65)=0.71, P=0.403). At the conclusion of the experiment (5 mo), the shoots from the green genotypes were more than two times the length of the shoots in the native genotypes (F(1/64)=61.02, P<0.0001; Figure 3B). Similarly, high nutrients resulted in shoots more than two times taller than those of low-nutrient conditions (F(1/64)=84.97, P<0.0001; Figure 1B and D). Regarding rhizoid cover, a significant interaction (F(1/64)=4.38, P<0.04) was due to the greater difference between the nutrient treatments in the green genotypes versus the native genotypes. Rhizoid cover was greater among the green genotypes than the native genotypes, averaging ~80% in the nutrient-enriched treatment when assessed on day 50 of the experiment, compared with ~15% rhizoid cover in native genotypes (F(1/64)=60.55, P<0.0001), and greater in the nutrient-enriched treatment than in the low-nutrient treatment (F(1/64)=60.55, P<0.0001; Figure 3C). Asexual reproduction was assessed through gemma cover and the number of bulbils. Green genotypes produced less protonemal gemma cover than native genotypes (F(1/65)=32.94, P<0.0001; Figure 4A). There was no difference in gemma cover between the nutrient treatments (F(1/64)=0.64, P=0.434). Regarding bulbil production, a significant interaction of habitat and nutrient level (F(1/64)=6.25, P<0.015) was due to the greater difference between the nutrient treatments in the native genotypes compared with the green genotypes (Figure 4B). Green genotypes had fewer bulbils as assessed on day 50 than native genotypes (F(1/64) =12.96, P<0.0006; Figure 4B). Genotypes in nutrient-enriched treatments had more bulbils than genotypes in low-nutrient conditions (F(1/64)=7.41, P<0.0083). Sexual reproduction, assessed through the number of inflorescences produced over the course of the 5-mo experiment, did not differ between habitats (52 ±14 for green vs. 45 ±13 inflorescences for native; F(1/65)=0.18, P<0.672). Genotypes cultured in enriched nutrients had more inflorescences than genotypes cultured in low nutrients (F(1/64)=10.0, P=0.0024). Among the green genotypes, 14 of 17 genotypes cultivated for 6 mo (1 mo beyond the experimental period) produced perichaetia (female), with 3 nonexpressing genotypes and no male expression.

Figure 3 Number of shoots produced by protonema on day 21 of Bryum argenteum placed into culture, as judged by visual shoot counts at 60×magnification (A); height of the tallest shoot at the conclusion of the experiment (150 d) of B. argenteum placed into culture, as the linear distance from the medium surface to the shoot tip (B); and percent cover occupied by aerial rhizoids on day 50 of B. argenteum placed into culture, as the estimated percent visual cover of protonemata occupied by rhizoids (C). Mean±1 SE; in C, different lowercase letters indicate P<0.05. Green, genotypes derived from North American golf course putting greens; Native, genotypes derived from habitats other than putting greens; Nutrients, supplied with a 30% Hoagland’s solution; Water, supplied with only sterile distilled water.

Figure 4 Percent cover occupied by protonemal gemmae on day 50 of Bryum argenteum placed into culture, as the estimated percent visual cover of protonemata occupied by gemmae (A); number of bulbils produced by day 50 of B. argenteum placed into culture, as the estimated by visual counts of detached bulbils on the medium surface (B); and chlorophyll content at the conclusion of the experiment (150 d) of B. argenteum placed into culture, as determined on 12 representative shoots using a chlorophyll content meter (C). Mean±1 SE; in B, different lowercase letters indicate P<0.05. Green, genotypes derived from North American golf course putting greens; Native, genotypes derived from habitats other than putting greens; Nutrients, supplied with a 30% Hoagland’s solution; Water, supplied with only sterile distilled water.
Chlorophyll Fluorescence and Chlorophyll Content
The significant interaction effect for F v/F m between habitat and nutrients (F(1/50)=7.50, P=0.0085) was due to the difference in nutrient treatments being greater in the green genotypes than in the native genotypes. NPQ tended to be greater for green genotypes (F(1/50)=3.58, P=0.0641) and was greater when nutrients were withheld from cultures relative to nutrient-enriched cultures (F(1/50)=34.61, P<0.0001). The significant interaction effect (F(1/50)=6.79, P=0.0120) was due to the nutrient treatments having greater effect on the green genotypes compared with the native genotypes. F v/F m did not differ between habitats (green vs. native, F(1/51)=2.09, P=0.1542), but was greater when nutrients were withheld from cultures relative to nutrient-enriched cultures (F(1/51)=144.27, P<0.0001). A similar pattern was found for ɸPSII (F(1/51)=0.1, P=0.759, and F(1/51)=26.19, P<0.0001, habitat and nutrient treatments, respectively) and qP (F(1/51) =0.56, P=0.4596, and F(1/51)=43.97, P<0.0001, habitat and nutrient treatments, respectively). For qNP, habitats did not differ (F(1/150)=1.98, P=0.1660), but qNP was greater when nutrients were withheld from cultures relative to nutrient-enriched cultures (F(1/50)=29.82, P<0.0001). F m did not differ between habitats (green vs. native, F(1/51)=1.05, P<0.3110), but was lower when nutrients were withheld from cultures relative to nutrient-enriched cultures (F(1/51)=274.44, P<0.0001). Nutrients had a significant effect on chlorophyll content, with plants treated with nutrients exhibiting higher chlorophyll content (F(1/51)=175.66, P<0.001; Figures 1C, 4C). Chlorophyll content was unaffected by habitat (F(1/51)=3.37, P=0.0724).
In summary, plants of the moss Bryum argenteum collected from putting greens demonstrated divergent traits from those found in native habitats. Plants from putting greens reached developmental time points more rapidly and produced fewer specialized asexual structures (bulbils, gemmae) compared with plants from native habitats. Further, plants from the greens regenerated protonemata faster from detached shoot apices, and these protonemata extended laterally more rapidly from the original shoot apex than those of plants collected from native habitats. Shoot induction from the protonemal mat was quicker in plants from greens, and these colonies produced three times the number of shoots after 21 d compared with plants from native habitats. The tallest shoot of colonies originating from greens was twice the height of the tallest shoot from native habitats at the conclusion of the experiment (150 d). The cultures from greens produced much more extensive aerial rhizoid cover than cultures from native habitats, which could represent a trait favored in putting greens that improves competition with grasses. Unexpectedly, for germination time, protonemal extension rate, rhizoid cover, and shoot number, the green genotypes in cultures in which nutrients were withheld exhibited more aggressive growth than native genotypes in cultures in which where nutrients were allowed, suggesting adaptation to the putting green environment.
Chlorophyll fluorescence and content parameters did not exhibit differences between habitats, but in nutrient-deprived cultures, chlorophyll content and F m were much reduced, indicative of developmental damage to photosystems that was reflected in the general low regeneration vigor of nutrient-deprived cultures. Unexpectedly, the fluorescence parameters F v/F m and ɸPSII were both greater in nutrient-deprived cultures, a result that suggests more studies are needed on the effects of nutrients and photosynthetic stress in this species.
Results presented here suggest that green genotypes have been selected to be specialized on the putting greens, either because only specific genotypes were able to colonize these greens initially or selection acted on genotypes over time to produce better-adapted “putting green” genotypes (local adaptation). Future studies should test the contribution of these two selection patterns on the divergence in life history and development detected in this study. Faster development and early growth are characteristic of invasives and weedy plants (Baker Reference Baker1974; van Kleunen et al. Reference Van Kleunen, Weber and Fischer2010). Plant traits shown to correlate with faster growth in early life-history stages include greater total leaf area and specific leaf area in invasives compared with non-conspecific natives, with invasives having high photosynthetic efficiency, particularly in invaded environments (Leishman et al. Reference Leishman, Haslehurst, Ares and Baruch2007; Pattison et al. Reference Pattison, Goldstein and Ares1998; van Kleunen et al. Reference Van Kleunen, Weber and Fischer2010). Invasives may also have greater nitrogen-use efficiencies than native plants (Baruch and Goldstein Reference Baruch and Goldstein1999; Feng et al. Reference Feng, Fu and Zheng2008; Liu and van Kleunen Reference Liu and van Kleunen2017) and thus may more readily take advantage of nutrient pulses for growth (Liu et al. Reference Liu, Kong, Lu, Huang, Wang, Wang, Qu and Feng2017). The specialized green genotypes also differ markedly in their reproductive strategy, with little allocation to specialized asexual reproduction compared with native genotypes. Thus, green genotypes may have a “K”-type strategy (more allocation to biomass related to staying in place), whereas the native genotypes exhibit an “r”-type strategy (more allocation to offspring and dispersing; Grime Reference Grime1977; MacArthur and Wilson Reference MacArthur and Wilson1967; Planka Reference Planka1970). Putting greens are permanent features in terms of location in golf courses, but are highly managed and therefore disturbed habitats. Bryum argenteum is known to be adapted to ephemeral habitats, often appearing and disappearing from the same site over the course of just a few years (LR Stark, personal observation). Although the sample size is not high, only females have been found to date in putting greens, and there may be a link between the r and K argument and the female bias: females on average are more K type than males, and males are more r type. Putting greens present a more female-adapted habitat, where being a good competitor is selected for. Females of B. argenteum have greater shoot height, resulting in greater clump water-holding capacity than male clumps. This size difference was consistent with female and male functions of sperm capture and release, respectively, in B. argenteum (Moore et al. Reference Moore, Kollar and McLetchie2016).
Bryum argenteum is capable of dispersing via asexual (shoot fragments, bulbils, gemmae) and sexual (spores) structures, making it difficult to determine which of these microscopic entities is ultimately perpetuating the putting green colonization (invasion) process. Identifying the primary dispersal mechanism(s) is critical when attempting to develop a successful integrated weed management strategy at the most effective spatial scale (Fletcher and Westcott Reference Fletcher and Westcott2013). Interestingly, B. argenteum has separate sexes (dioecy), so spores from a sporophyte should have an offspring male-to-female sex ratio of 1:1; however, in the field, researchers observe a trend toward a greater number of female plants than male plants (70% vs. 30%, respectively) from native habitats (Stark et al. Reference Stark, McLetchie and Eppley2010). No males were identified in samples collected from putting greens; 14 genotypes expressed female, and 3 genotypes did not express sex. Two of the three nonexpressing genotypes expressed sex (also female) after the experiment had ended (the plants were maintained under experimental conditions). Putting greens are typically mowed daily to heights <3.5 mm, and this practice prevents the production of the long, stalk-like setae that eventually give rise to the spore-containing capsule of mosses; therefore, it is unlikely, if not impossible, for spores to be a dispersing agent from green to green. Spores could serve as the initial inoculum during the “introduction” phase of the invasion process, but this hypothesis is not supported from the research presented here, considering no males were identified from putting greens. An alternative perspective arguing the role of spore dispersal may pose that females are better adapted to life in a putting green, while males simply do not thrive and hence are not observed. Regardless, preventing the movement of spores onto golf course putting greens would be impossible considering wind currents are capable of dispersing spores miles from the parent population (Miles and Longton Reference Miles and Longton1992).
Findings of divergent traits between golf course genotypes and native genotypes of B. argenteum have important implications for management of this species. Bryum argenteum collected from putting greens exhibited a much greater vertical shoot growth rate compared with genotypes from native habitats, especially when cultures were supplied with nutrients (Figure 3B). Additionally, green genotypes had a 3- to 4-fold increase in shoot density compared with native genotypes, irrespective of nutrients (Figure 3A). These results have implications when attempting to understand and limit the invasion process in putting greens. For instance, golf course superintendents often foliarly apply nutrients every 7 to 14 d throughout the growing season to greens, and previous research has shown this spoon-feeding approach increases the size of an existing B. argenteum infestation (Raudenbush and Keeley Reference Raudenbush and Keeley2015; Thompson et al. Reference Thompson, Kennelly and Fry2011). Longer moss shoots are more likely to come into contact with the reel and/or bedknife, producing dislodged or severed shoot apices that can be dispersed by golfers, equipment, or precipitation. Furthermore, green genotypes exhibited much greater shoot density; more propagules per unit area will be dislodged from B. argenteum colonies in putting greens. Superintendents should be cognizant of any cultural practices that are manipulating the turfgrass canopy (e.g., grooming, brushing, aerifying, verticutting), because they are likely dislodging asexual propagules from established colonies. These practices may be important tools when attempting to control severe B. argenteum infestations by introducing available sites within the moss colony for the establishment of desirable turfgrasses. For example, Raudenbush and Keeley (Reference Raudenbush and Keeley2017) reported significant decreases in B. argenteum cover in putting greens using hollow-tine aerification and vertislicing on existing B. argenteum infestations. It is recommended that superintendents strategically implement chemical control strategies 7 d following any of the aforementioned cultural practices, because this time frame is when regrowth from asexual structures occurs (Horsley et al. Reference Horsley, Stark and McLetchie2011). Finally, superintendents struggling with B. argenteum infestations should consider changes to their cutting-unit setup. A lower effective height of cut will increase the likelihood of the reel and/or bedknife contacting B. argenteum shoots, so superintendents should combat this by raising the height of cut, using less aggressive rollers, reducing cutting-unit weight, reducing behind center distance, and decreasing bedknife thickness. Future studies should focus on the effectiveness of these proposed management strategies on controlling B. argenteum cover and spread in golf course greens.
Acknowledgments
The authors thank the U.S. Golf Association for funding this research and the National Science Foundation (DEB 1638943) for providing laboratory support during a portion of this project. John Brinda (Missouri Botanical Garden) confirmed the identity of Bryum specimens from golf course putting greens, Robin Riker provided assistance with Figure 1, and many golf course superintendents contributed samples of mosses for this study. No conflicts of interest have been declared.