Several studies have been conducted on the processing of milk using high hydrostatic pressure (HHP) for the manufacture of cheese (López-Fandiño, Reference López-Fandiño, Carrascosa and Olano1996; Drake et al. Reference Drake, Harrison, Asplund, Barbosa-Cánovas and Swanson1997; Trujillo et al. Reference Trujillo, Capellas, Buffa, Royo, Gervilla, Felipe, Sendra, Saldo, Ferragut and Guamis2000) and yogurt (Needs et al. Reference Needs, Capellas, Bland, Manoj, Macdougal and Paul2000a; Harte et al. Reference Harte, Amonte, Luedecke, Swanson and Barbosa-Cánovas2002a). HHP technology has been mainly targeted at food safety improvement (Hoover, Reference Hoover, Juneja and Sofos2002) and to the change of textural and yield properties of dairy products through whey protein denaturation (Panick et al. Reference Panick, Malessa and Winter1999; Yang et al. Reference Yang, Dunker, Powers, Clark and Swanson2001; Huppertz, Reference Huppertz, Kelly and Fox2002) and casein micelle disruption (Needs et al. Reference Needs, Capellas, Bland, Manoj, Macdougal and Paul2000a,Reference Needs, Stenning, Gill, Ferragut and Richb; Gebhardt et al. Reference Gebhardt, Doster and Kulozik2005; Huppertz & De Kruif, Reference Huppertz and Kruif2006).
Extensive research has been conducted on the denaturing effect of HHP on whey protein isolates in order to alter the functional properties of this by-product of the cheese-making industry (Schrader & Buchheim, Reference Schrader and Buchheim1998; Panick et al. Reference Panick, Malessa and Winter1999; Scollard et al. Reference Scollard, Beresford, Needs, Murphy and Kelly2000; Yang et al. Reference Yang, Dunker, Powers, Clark and Swanson2001; Huppertz et al. Reference Huppertz, Kelly and Fox2002, Reference Huppertz, Fox and Kelly2004a). Anema & Li (Reference Anema and Li2003a) and Gulbrandsen et al. (Reference Gulbrandsen, Jansen, Langsrud and Elisabeth2000) reported increase (15%) in casein micelle size for thermally heated skim milk without high pressure. The level of association of whey proteins with the casein micelles depends on the conditions under which milk was heated, pH of milk (Anema & Li, Reference Anema and Li2003a), and calcium ion activity (Gulbrandsen et al. Reference Gulbrandsen, Jansen, Langsrud and Elisabeth2000).
The other important protein components of milk, i.e. the caseins, have recently received as much attention as the whey proteins and most research on the effect of HHP on casein micelles has been conducted with skim milk using methods such as absorbance and lightness (Buchheim et al. Reference Buchheim, Schrader, Morr, Frede, Schutt and Fox1996; Gaucheron et al. Reference Gaucheron, Famelart, Mariette, Raulot, Michel and Le Graet1997), or by direct observation of micelles under transmission electron microscopy (TEM; Gaucheron et al. Reference Gaucheron, Famelart, Mariette, Raulot, Michel and Le Graet1997; Needs et al. Reference Needs, Stenning, Gill, Ferragut and Rich2000b; Keenan et al. Reference Keenan, Young, Tier, Jones and Underdown2001). The change in size induced by HHP has been measured by light scattering (Kelly et al. Reference Huppertz, Kelly and Fox2002) and analytical ultracentrifugation (Harte et al. Reference Harte, Luedecke, Swanson and Barbosa-Cánovas2002b). Most recently, the effect of HHP on casein micelles was studied by dynamic light scattering (Gebhardt et al. Reference Gebhardt, Doster and Kulozik2005) and photon correlation spectroscopy (Anema & Li, Reference Anema and Li2003a; Huppertz et al. Reference Huppertz, Fox and Kelly2004a,Reference Huppertz, Fox and Kellyb,Reference Huppertz, Grosman, Fox and Kellyc; Regnault et al. Reference Regnault, Thiebaud, Dumay and Cheftel2004; Anema et al. Reference Anema, Lowe and Stockmann2005).
Sedimentation velocity methods are particularly suitable for casein micelle size determination since casein micelles are considered as nearly spherical and non-interactive aggregates (Dalgleish, Reference Dalgleish1998). Different mechanisms have been suggested to explain the HHP induced increase in casein micelle size. Interaction of denatured β-lactoglobulin (β-lg) with the casein micelles by high pressure increased the casein micelle size (Schrader & Buchheim, Reference Schrader and Buchheim1998; Huppertz et al. Reference Huppertz, Fox and Kelly2004a). The thermal or HHP denaturation of whey proteins and their subsequent interaction with κ-casein may protect the micelle from being disrupted by high pressure (Harte et al. Reference Harte, Luedecke, Swanson and Barbosa-Cánovas2002b; Kelly et al. Reference Huppertz, Kelly and Fox2002) or may aggregate the casein micelles (Huppertz et al. Reference Huppertz, Fox and Kelly2004a). Treatments below 250 MPa increased micelle size but size was decreased above 300 MPa by 50% (Needs et al. Reference Needs, Stenning, Gill, Ferragut and Rich2000b; Huppertz et al. Reference Huppertz, Fox and Kelly2004a,Reference Huppertz, Fox and Kellyb).
Understanding the factors affecting the disruption of casein micelles is important because the tailoring of casein micelles size may improve textural, rheological, and whey retention properties of dairy products. Furthermore, the reversible dissociation of casein micelles is a promising functional property for the stabilization of hydrophobic compounds (such as flavours and drugs) in foods.
The objective of this research was to study the disruption effect of HHP on heated and non-heated casein micelle isolates with and without the presence of whey proteins.
Materials and Methods
Milk supply and preparation of casein micelle suspension
Raw whole milk was purchased from the WSU creamery and skimmed by centrifugation at 20 000×g for 15 min at 15°C. The skim milk samples were centrifuged at 100 000×g for 1 h 40 min at 25°C using an L8-70 ultracentrifuge and a 70Ti rotor (Beckman Instruments, Inc., Palo Alto, California, USA) to separate the whey and casein fractions. After centrifugation, the supernatant was discarded and the casein micelle clumps were suspended by stirring overnight at 5°C in milk permeate obtained from ultra-filtered raw milk using a 10 KDa molecular weight cut-off ultra filter (Romicon, model HF-LAB-5, Koch membrane systems, Inc., Wilmington, Massachusetts, USA).
An absorbance pattern master curve (not shown in this manuscript) was created and used to adjust the casein micelles concentration to keep a constant volume fraction of casein micelles. After the micelle fraction was re-suspended in milk permeate, the separation of the casein fraction from the whey proteins was confirmed by SDS-PAGE electrophoresis using an 8–16% acrylamide Tris-HCl ready gel (Bio-Rad, Hercules, California, USA).
High hydrostatic pressure treatment
The casein micelle isolates, with or without thermal treatment (85°C, 20 min), were subjected to HHP from 0 to 676 MPa (come-up time only, at room temperature) using an isostatic pressing system (Engineered Pressure Systems, Inc., Haverhill, Massachusetts, USA) having a cylindrical pressure chamber of 125 mm3 effective volume. The same experiments were conducted but with the addition of 5 g whey protein isolate/l (BiPRO®, Davisco Foods International, Inc., Le Sueur, Minnesota, USA) before any thermal or pressure treatment to study the effect of whey proteins on the disruption of casein micelles. To determine the effect of different levels of denatured whey proteins on the HHP induced disruption of casein micelles, 1 to 5 g whey protein isolate/l was added to the casein micelle isolate, which was then subjected to thermal treatment (85°C, 20 min) and HHP. The experiments were analysed as a randomized complete block design with two replicates.
Micelle size determination by sedimentation velocity
Casein micelle size determination was done by sedimentation velocity analytical ultracentrifugation using a Beckman ultracentrifuge (Beckman Coulter, Fullerton, California, USA). Three main forces act upon a micelle subjected to a strong centrifugal field (van Holde et al. Reference Van Holde, Johnson and Ho1998). The three force vectors are the buoyancy force (FB), centrifugal force (FC), and drag force (FD). These vectors add to zero in order to reach a constant terminal sedimentation velocity,
The centrifugal field is given by ![]() , where the mass of the casein micelle is the product of its volume (Vm) times its density (ρm) and the acceleration is given by the product of the centrifugal rotational speed (ω2) times the position (r) of the particle at a given time relative to the center of the spinning element. Similarly, the buoyancy force is given by
, where the mass of the casein micelle is the product of its volume (Vm) times its density (ρm) and the acceleration is given by the product of the centrifugal rotational speed (ω2) times the position (r) of the particle at a given time relative to the center of the spinning element. Similarly, the buoyancy force is given by ![]() , where the mass of fluid displaced by the micelle is the volume of the micelle times the fluid's density (ρf). If we assume the casein micelle is a solid sphere and laminar flow sedimentation in a Newtonian fluid, the drag force (FD) is expressed as
, where the mass of fluid displaced by the micelle is the volume of the micelle times the fluid's density (ρf). If we assume the casein micelle is a solid sphere and laminar flow sedimentation in a Newtonian fluid, the drag force (FD) is expressed as ![]() , where vt is the terminal velocity of the micelle, η is the viscosity of the medium, and R is the radius of the micelle. In this way, eqn (1) can be expressed as:
, where vt is the terminal velocity of the micelle, η is the viscosity of the medium, and R is the radius of the micelle. In this way, eqn (1) can be expressed as:
Expressing the Volume of the micelle in terms of its radius and rearranging eqn (2), then
where s is the Svedberg coefficient, which can be determined experimentally by:
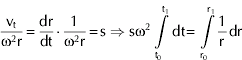
Solving the integral from time to to t1,
In this way, by knowing the position of the casein micelles at each time, the Svedberg coefficient can be found as the slope of the Ln(r) vs. t line (Van Holde et al. Reference Van Holde, Johnson and Ho1998). The position of the micelles is obtained from the mid point of successive absorbance curves during centrifugation of the sample containing the micelles Vs the reference, in this case the permeate from ultrafiltrated (10 kDa MWCO) milk. The samples were spun in a 4-place rotor at 25°C. Since the size of the micelles was greatly affected by the high pressure treatments, sedimentation velocity was done at various rotational speeds (2500 to 3000 rpm) and two wavelengths (400 and 500 nm).
Micelle size determination by Transmission Electron Microscopy
Casein micelles from selected treatments were observed under TEM. A mix of casein micelles suspension (1:1 (v/v) and 3% (w/v) agar in water at 43°C was prepared. After solidification by cooling to ~5°C, 1 mm3 cubes were cut and submerged in 0·05 m-PIPES buffer (pH 7·2) containing 12·5 g glutaraldehyde/l and 20 g paraformaldehyde/l for 24 h at 4°C for fixation. After three washes (10 min each) in PIPES buffer, the cubes were dehydrated through serial 10 min washes in 30, 50, 70, and 95% ethanol in distilled water. Dehydration was completed with three 10 min washes in 100% ethanol. The fixed samples were infiltrated using a 1:1 (v/v) medium grade LR White resin (London Resin Company, Ltd., Reading, England) in 100% ethanol for 12 h at 4°C. The cubes were left in 100% LR White for 12 h at 4°C (three times) and cured in 100% LR White for 12 h at 60°C. Ninety nanometer sections of the fixed and cured samples were obtained using a Reichert ultracut microtome (Leica Microsystems Inc., Chicago, Illinois, USA). The grids with samples were stained with 4% uranyl acetate and Sato's lead stain and examined with a transmission electron microscope, Joel 1200 EX JEM (Joel Ltd., Akishima, Japan) operating at 80 kV.
Results and Discussion
Heat and high pressure induced changes are increasingly becoming important in understanding the functional properties of dairy products for the commercialization of HHP processing technology. The results of this paper add to the current knowledge about the effects of high pressure on casein micelles in the presence of whey proteins. Significant differences in the Svedberg coefficient were not observed for casein micelle isolates after treatment with HHP up to 200 MPa (Fig. 1a). The observation that treatment at pressures up to 200 MPa had no significant effect on casein micelle size was in accordance with the results reported by Needs et al. (Reference Needs, Stenning, Gill, Ferragut and Rich2000b) and by Huppertz et al. (Reference Huppertz, Fox and Kelly2004a). However, a 10-fold reduction in the Svedberg coefficient was observed when casein micelle isolates were subjected to pressure above 310 MPa, with no further reduction even at maximum applied pressure (676 MPa). When the casein micelle isolates were subjected to thermal treatment (85°C, 20 min) before HHP, a small initial increase in size was observed, but the disruption pattern induced by the HHP treatment was similar to that of casein micelle isolates with no previous thermal treatment. Furthermore, Svedberg coefficient values were the same when the casein micelle isolates were subjected to pressures greater than 300 MPa, regardless of previously applied thermal treatment. Preliminary experiments showed no difference in Svedberg coefficient values for casein micelle isolates subjected to 400, 500, and 600 MPa, compared with those subjected to 676 MPa. These results were in good agreement with turbidity patterns in skim milk reported by Buchheim et al. (Reference Buchheim, Schrader, Morr, Frede, Schutt and Fox1996). The addition of 5 g whey protein/l to the casein micelle isolate did not affect the disruption pattern induced by the HHP treatment (Fig. 1b) when no previous thermal treatment was applied to the mixture. However, when the casein micelle isolates and whey protein mix was thermally treated (85°C, 20 min), an initial 3-fold increase in the Svedberg coefficient was observed and no significant reduction in the Svedberg coefficient was observed for pressure up to 250 MPa. Furthermore, in the 310 to 676 MPa range, the thermally treated casein micelle isolates containing whey proteins exhibited higher Svedberg coefficient values than the casein micelle isolates, with or without thermal treatment, or the casein micelle samples containing whey proteins but without thermal treatment.

Fig. 1. Effect of different high hydrostatic pressure treatments on casein micelle's Svedberg coefficient (S). (A) No whey protein added, (B) 5 g whey protein/l added. Bars are 95% confidence intervals for the mean.
Contrary to reports by Gaucheron et al. (Reference Gaucheron, Famelart, Mariette, Raulot, Michel and Le Graet1997) for skim milk at 20 to 40°C, no increase in size of the casein micelles was observed in the 200 to 300 MPa range. As reported by Huppertz et al. (Reference Huppertz, Fox and Kelly2004a), the final size of micelles pressurized in the 200 to 300 MPa range is both time and temperature dependant, and may be the result of whey protein denaturation and interaction with casein micelles occurring simultaneously to micelle disruption. Gaucheron et al. (Reference Gaucheron, Famelart, Mariette, Raulot, Michel and Le Graet1997) observed similar patterns in whey-free milk at 40°C, suggesting that hydrophobic interaction may also play a role in the pressure-induced increase in size of casein micelles. Since our experiments were done at both lower temperature (~25°C) and shorter pressure times (seconds), we were not able to observe this phenomenon.
Based on several transmission electron micrographs for casein micelle isolates, the average diameter of the casein micelles was determined as ~80 nm. This was smaller than values reported elsewhere, by Needs et al. (Reference Needs, Capellas, Bland, Manoj, Macdougal and Paul2000a,Reference Needs, Stenning, Gill, Ferragut and Richb; by TEM), Fox & McSweeney (Reference Fox and McSweeney1998), and Regnault et al. (Reference Regnault, Thiebaud, Dumay and Cheftel2004; by photon correlation microscopy and atomic force microscopy), and may be the result of (1) different milk, since casein micelle size varies depending on the milk source, (2) the high centrifugal force (20 000×g) used when skimming the milk causing a small precipitate of bigger micelles to be discarded, leaving an increased proportion of smaller micelles in suspension.
The Svedberg coefficient values were transformed to micelle diameter by solving for the variable radius (R) in the sedimentation velocity equation and assuming (1) initial mean diameter of micelles ~80 nm before treatment, (2) no changes in the density of micelles and permeate after treatments, and (3) no changes in viscosity of the permeate in all cases but the thermally treated permeate containing whey proteins. The estimated diameter values obtained based on the sedimentation equation (Fig. 2) was in close agreement with the casein micelle diameter observed in the transmission electron micrographs (Fig. 3a & b).

Fig. 2. Effect of different high hydrostatic pressure treatments on casein micelle calculated diameter. WP: whey protein. Bars are 95% confidence intervals for the mean.

Fig. 3. Transmission electron microscopy of (a) casein micelle isolates subjected to different levels of high hydrostatic pressure; (b) thermally (85°C, 20 min) treated casein micelle isolates subjected to different levels of high hydrostatic pressure; (c) thermally (85°C, 20 min) treated casein micelle isolates and whey protein isolate subjected to different levels of high hydrostatic pressure.
The diameter of casein micelle isolates was not markedly affected by thermal treatment but micelles were disrupted by pressure in the 250 to 310 range. Gulbrandson et al. (Reference Gulbrandsen, Jansen, Langsrud and Elisabeth2000), and Anema & Li (Reference Anema and Li2003a), observed only small changes (<15%) in casein micelle size when milk samples were heated to 90°C and above without HHP. The average diameter of casein micelles after HHP of 300 MPa or greater was ~21 nm, regardless of being previously subjected to thermal treatment. These casein micelles are larger than the micelles measured by dynamic light scattering (3 nm at 300 MPa; Gebhardt et al. Reference Gebhardt, Doster and Kulozik2005) and smaller than the micelles measured by atomic force microscopy (40 nm to 80 nm at 300 MPa; Regnault et al. Reference Regnault, Thiebaud, Dumay and Cheftel2004) or by photo correlation spectroscopy (⩽50 nm at 300 MPa; Regnault et al. Reference Regnault, Thiebaud, Dumay and Cheftel2004). Regnault et al. Reference Regnault, Thiebaud, Dumay and Cheftel2004 also reported that the micellar sub-units formed due to high pressure at 300 MPa are of 30 nm in size and are larger than the sub-micelles measured in unpressurized bovine milk by neutron scattering (13 nm; Stothart & Cebula, Reference Stothart and Cebula1982) or by TEM of freeze-fractured cow's milk samples (10 nm; Schmidt & Buchheim, Reference Schmidt and Buchheim1970). These differences in micelle size are may be due to the separation techniques (centrifugation 20 000×g for 15 min and 100 000×g for longer times (1 h 40 min), different temperatures, and/or the analysis techniques (TEM: procedure dehydrates the sample). Higher g forces increase the level of whey protein deposited with the pellet without increasing the levels of casein deposited, indicating that larger whey protein aggregates were being deposited (Anema & Li, Reference Anema and Li2003b). Addition of urea to milk dissociates the casein micelles, disrupting hydrophobic and hydrogen bonds in the micelles without rupturing phosphate linkages according to Huppertz et al. (Reference Huppertz, Fox and Kelly2004b) ; they reported considerable changes only in larger casein micelle fractions (220 to 150 nm) with high pressure treatment (250 MPa for 30 min) but not in small casein micelle fractions (118 nm). Huppertz & Kruif (Reference Huppertz and Kruif2006) also concluded that micelle stability against HHP induced disruption increases with increasing casein micelle concentration as micelle calcium phosphate increases. In the present study, the addition of whey proteins did not affect the HHP induced disruption provided there was no thermal treatment; however adding whey proteins after a thermal treatment increased the casein micelle size. The increase in casein micelle size (formation of casein micelle aggregates) could be due to (1) Extensive formation of hydrophobic bonds formed between submicellar particles and/or (2) Formation of complexes (hydrophobic, disulphide or covalent bonds) between whey proteins and caseins.
In the case of thermally treated casein micelles containing whey proteins, the diameter of the micelles was calculated correcting eqn 3 to show an increase in viscosity from 1 mPa.s in the medium with no whey proteins to 1·8 mPa.s in the medium containing thermally denatured whey proteins. Viscosity was measured in thermally treated whey containing milk permeate using a rheometer (Model MCR300, Paar-Physica, Ashland, Virginia, USA). Calculated diameter for thermally treated micelles containing whey proteins (Fig. 2) showed good agreement with observations under TEM for pressures up to 250 MPa (Fig. 3c). However, calculated diameters were smaller than observed under TEM for thermally treated micelles containing whey proteins and then disrupted by HHP higher than 310 MPa.
This deviation of calculated versus observed values was caused by three factors: (1) the increase in micelle roughness due to whey protein and κ-casein disulphide interaction promoted by thermal treatment, (2) deviations from sphericity of individual or coalesced micelles and micelles disrupted by HHP (Fig. 3c), and (3) sample dehydration during sample preparation for electron microscopy, which causes extensive reduction in size (Pierre et al. Reference Pierre, Michael and Le Graet1995). Factors (1) and (2) would decrease the sedimentation velocity of the casein micelles, promoting sub-estimation of the real casein micelle diameter. Other factors, such as individual caseins and CaPO3 liberation into the serum, might increase the viscosity and thus decrease the sedimentation velocity of the micelles. However, factors (2) and (3) were not considered as important since they would also affect non-heated micelles in which the diameters were well calculated.
In general two possible theories have been suggested to explain HHP induced increase in casein micelle size, the interaction of denatured β-lg with the casein micelles (Schrader & Buchheim, Reference Schrader and Buchheim1998; Huppertz et al. Reference Huppertz, Fox and Kelly2004a) or the aggregation of casein micelles (Huppertz et al. Reference Huppertz, Fox and Kelly2004a,Reference Huppertz, Fox and Kellyb). Results from this study support the former theory, i.e., interaction of denatured β-lg with the casein micelles with the application of heat before high pressure. Clearly, further studies on micelle structure and size are necessary to confirm the exact aggregation or disaggregating mechanisms of casein micelles in HHP processing, with or without heat.
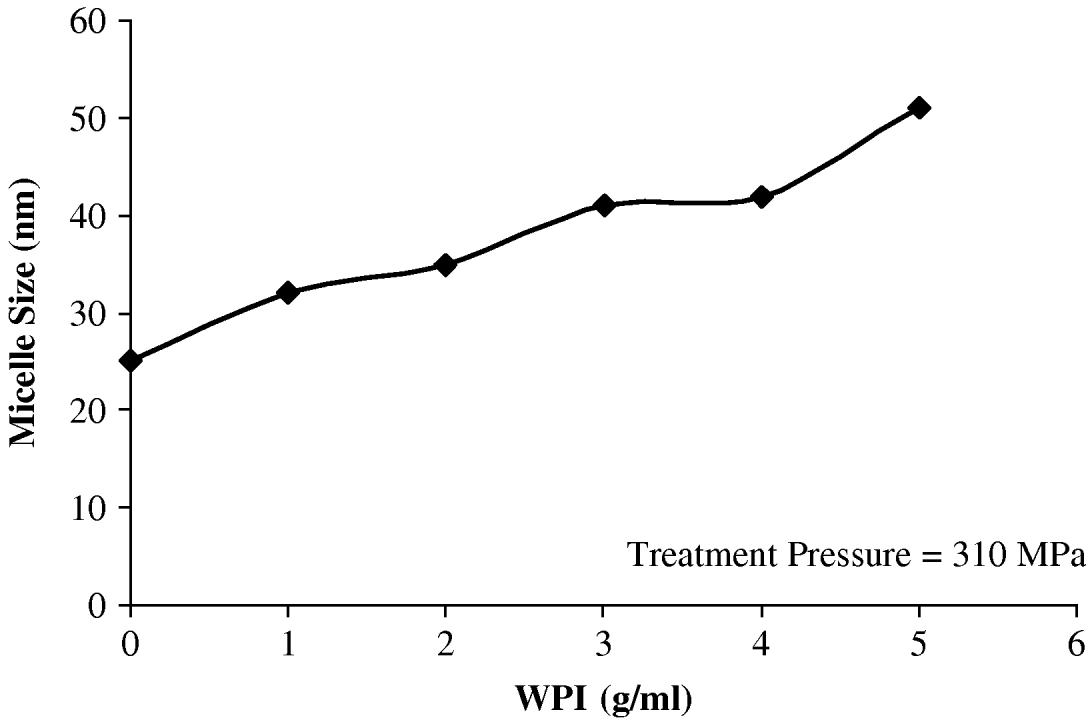
An intermediate pressure (310 MPa) was selected to study the protective effect of different levels of thermally denatured whey proteins on the disruption of casein micelles subjected to HHP. The increase in viscosity of the medium induced by increasing levels of denatured whey proteins was taken into consideration for calculation of the diameter based on the sedimentation velocity equation. As seen in Fig. 4, increased levels of denatured whey proteins interacting with the casein micelles, protected the micelles from disruption at intermediate pressure. This is in contrast with the claim that the process is primarily driven by the extent of solubilization of micelle calcium phosphate (Huppertz et al. Reference Huppertz and Kruif2006). Increase in roughness or deviations from sphericity may also affect the calculated diameters in such intermediate pressure. The calculated diameters should be considered as bottom line estimates of the real diameters of casein micelles since, as discussed before, values from the sedimentation equation may sub-estimate the real diameter of the micelles in systems containing thermally denatured whey proteins. Furthermore, the studies at different pressures are necessary to confirm the above conclusion, that by adding whey proteins to casein micelle isolate, the micelles are protected from disruption. It is worth investigating at different pressures and temperatures the utilization of whey proteins, which could yield unique product characteristics not achievable by any other means at present.
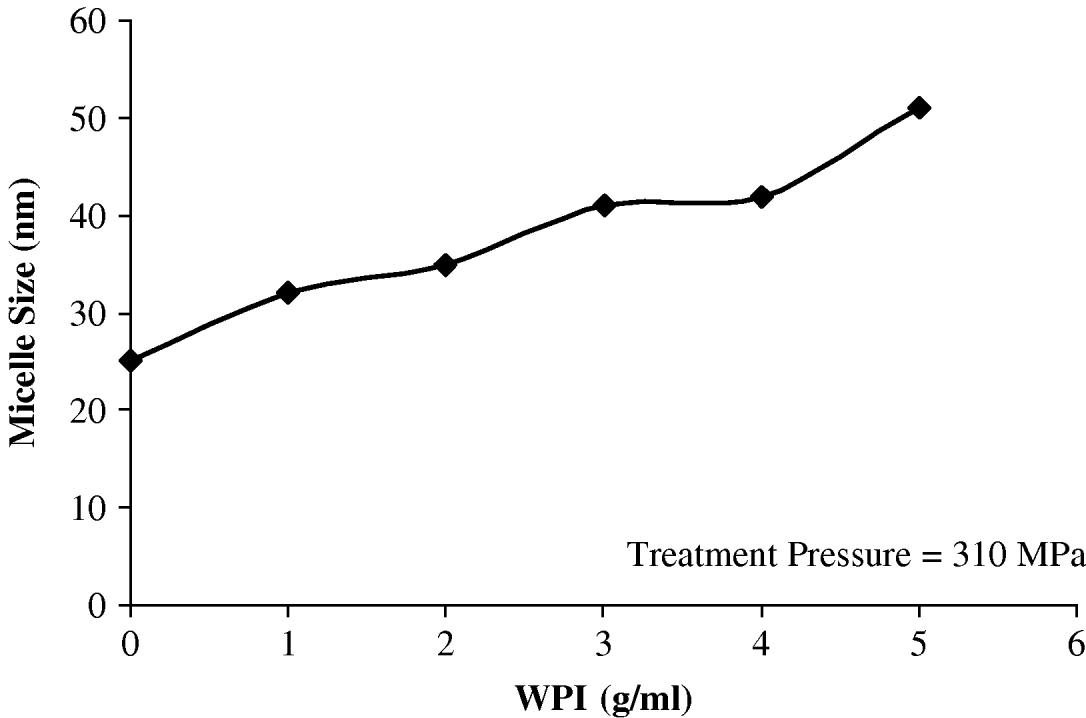
Fig. 4. Disrupting effect of high hydrostatic pressure on heated casein micelles isolate containing various levels of whey protein isolate (WPI).
Conclusions
HHP in the 250 to 310 MPa range promotes a rapid disruption or disaggregation of casein micelles isolates into small casein micelles. The addition of whey proteins and their interaction (promoted by thermal denaturation) with the casein micelles protects the micelle from extensive HHP induced disruption. Furthermore, the higher the concentration of whey proteins, the more the protection to disruption of casein micelles is observed. Therefore the change in functional properties of casein micelles induced by HHP cannot be considered as independent from other factors such as milk composition and temperature.